1. Introduction
Cellulose, consisting of D-glucose units linked by β-1,4-glycosidic linkages, is the most abundant linear polymer in the world. Together with hemicellulose and lignin (40–60% cellulose, 20–40% hemicellulose and 10–25% lignin), it is the major component of plant cell walls [
1,
2]. The isolation of cellulose requires complex chemical processes that can cause irreversible changes in the structure of plants. Moreover, the process causes ecological pollution [
3,
4,
5]. As the world’s population grows, the need for more cellulose is increasing every day, and to meet this growing demand, more wood and cotton are needed. Utilizing wood and cotton resources negatively affects the global carbon (C) cycle [
6]. Therefore, alternative methods are being sought to limit or replace the use of plant-based cellulose. On the other hand, the high purity of bacterial cellulose is possible with fewer steps, lower costs and less waste at the end of the process. The main advantage is that bio-cellulose fibers can have a higher degree of polymerization due to the simple processing steps [
7,
8].
Bacterial cellulose is a microorganism-based homopolymer of β-(1,4)-glucose (C
6H
10O
5)
n [
9]. Bacteria synthesize cellulose fibrils to form strands, and biofilm formation occurs during synthesis [
10,
11,
12,
13]. In some bacterial species, BC can be produced inversely by synthesizing cellulose and forming nanofibril bundles instead of forming nanocellulose via fibrillating fibers [
14].
In order to achieve maximum yield and cost-effectiveness in BC production, it is crucial to optimize production parameters such as time, pH and temperature [
15,
16,
17]. Some authors have described different methods to optimize the BC yield from different species. For example, Bagewadi et al. [
18] used Plackett–Burmann’s design to optimize the culture medium for producing BC by
Enterobacter hormaechei. In another study, Calderón-Toledo et al. [
19] supplemented HS medium with mango extracts and optimized BC production by
Komagataeibacter sp. SU12 using Plackett–Burmann’s design and compared the purified BCs produced by standard and mango-modified media. However, no report is available on the optimization of BC production by
Komagataeibacter rhaeticus using the Taguchi methods and pH, temperature, incubation time and inoculum concentration parameters. Genichi Taguchi developed the Taguchi methods, which comprise statistical techniques for the improvement of the quality of manufactured products. More recently, these techniques have been applied to engineering [
20], biotechnology [
21] and marketing and advertising [
22]. Professional statisticians have praised Taguchi’s methods for their advances and goals, particularly the designs that he used to analyze variation. The practice of modifying a process to maximize a given set of parameters within certain constraints is known as process optimization. Minimizing costs, maximizing benefits and/or maximizing efficiency are the most typical objectives. It is also one of the most important quantitative tools for business decision-making. When optimizing a process, one or more process specifications should be maximized while others are kept within their limits. The core principle of the Taguchi methods is the application of parameter design, an engineering method for product or process design that focuses on the identification of parameter (factor) settings that produce the best levels of a quality characteristic (performance measure) with the least amount of variation [
23].
The aim of this study was to identify and evaluate the optimum parameters for BC production by K. rhaeticus isolated from Kombucha tea which is identified by 16S rRNA gene sequence analysis. In this study, the Taguchi methods were used for the first time to optimize the parameters for maximum BC production by Komagataeibacter rhaeticus, and the experiments were conducted with one-fourth of the normally required number of trials, achieving the targeted results with 16 experiments instead of the usual 64. The effects of experimental factors on BC production were studied using analysis of variance (ANOVA) for BC production at different temperatures, pH values, inoculum concentrations and incubation time. The morphology and structural properties of BC produced by K. rhaeticus in Hestrin‒Schramm medium with optimized parameters were characterized by FTIR, SEM, water-holding capacity and mechanical tests.
2. Materials and Methods
2.1. Materials
Kombucha tea was kindly provided by Dr Melih Guzel, Gumushane University. D-glucose (Applichem, Darmstadt, Germany), yeast extract (Merck, Darmstadt, Germany), peptone (Sigma, MO, USA), Na2HPO4 (Sigma, MO, USA) and citric acid (Sigma, MO, USA) were used for Hestrin‒Schramm medium preparation. Sodium hydroxide (ISOLAB, Wertheim, Germany) and acetic acid (ISOLAB, Wertheim, Germany) were used for purification and neutralization, respectively. All chemicals used were of analytical grade.
2.2. Isolation of BC Producer Bacteria
Kombucha tea was used as the starter culture. Inocula were cultured at 30 °C for 7 days in 250 mL Erlenmeyer flasks containing 50 mL of Hestrin‒Schramm (HS) medium (20 g·L
−1 glucose, 5 g·L
−1 yeast extract, 5 g·L
−1 peptone, 2.7 g·L
−1 Na
2HPO
4 and 1.15 g·L
−1 citric acid) as previously reported by Hestrin and Schramm [
24]. The inoculum was subcultured in fresh medium at a ratio of 1:10 and incubated for 72 h. A total of 1 mL of suspension was taken from the culture and serial dilutions were made with 0.9% (
w/
w) sterile NaCl solution. Aliquots of each dilution were plated on Petri dishes containing solid HS medium. The plates were then incubated at 30 °C for 72 h. The solid-medium-grown colonies were isolated according to their morphological characteristics. In order to assess cellulose formation at the top of the liquid, each colony isolated from the solid medium was inoculated into HS broth and incubated for 72 h. Then, BC-forming colonies were seeded on HS agar and incubated at 30 °C for 48 h. The bacterial cells presenting cellulose formation were Gram stained, and catalase tests were performed. Finally, the cellulose-producing pure isolate was cryopreserved in 1.5 mL of 20% glycerol solution and stored at −80 °C until use.
2.3. Identification of Bacterial Isolate via DNA Sequencing
The bacterial isolate shown to produce BC was identified by sequencing the 16S rRNA gene (BM Labosis, Ankara, Turkey). cDNA templates were prepared by employing the gene amplification technique using the PCR method. The 16S rRNA gene was amplified using universal primers (27F 5′-AGAGTTTGATCCTGGCTCAG-3′ and 1492R 5′-GGTTACCTTGTTACGACTT-3′) [
25,
26]. PCR reactions for 16S rRNA gene amplification were performed under the following conditions: 5 min initial denaturation at 95 °C followed by 30 cycles as recorded previously by Manjeet Sharan Pankaj Dhaka, [
27]; denaturation at 95 °C for 45 s, annealing at 57 °C for 45 s, extension at 72 °C for 60 s and final extension at 72 °C for 5 min. The PCR product was analyzed by means of the sizes, quantity and purity via electrophoresis on a 1.5% (
w/
v) agarose gel in Tris-acetate-EDTA buffer. The agarose gel was then stained with ethidium bromide (EtBr) and visualized under UV light. Sequence similarity was then determined using the BLAST program in the GenBank database “
https://www.ncbi.nlm.nih.gov/ (accessed on 13 April 2023)”.
2.4. Culture Conditions and Cellulose Formation
The inoculation medium and all the culture media were sterilized at 121 °C for 15 min, and the pH of the medium was adjusted with diluted HCl and NaOH (
Table 1). Static conditions for the incubation time (7, 14, 15, 16, 17, 21 and 28 days) and temperatures (26 °C, 28 °C, 30 °C and 32 °C) were used for BC production in Erlenmeyer flasks (250 mL) containing 50 mL of HS medium. After incubation under defined conditions, BC membranes, formed at the air‒medium interface, were harvested and centrifuged at 4000 rpm for 10 min then purified in 4% (
w/
v) NaOH aqueous solution at boiling temperature to inactivate bacterial cells and other contaminants. The BC membrane was then placed in 2% acetic acid and washed with distilled water to neutral pH. At the final stage, the films were oven dried to constant weight at 37 °C for 24 h.
2.5. Optimization Methodology
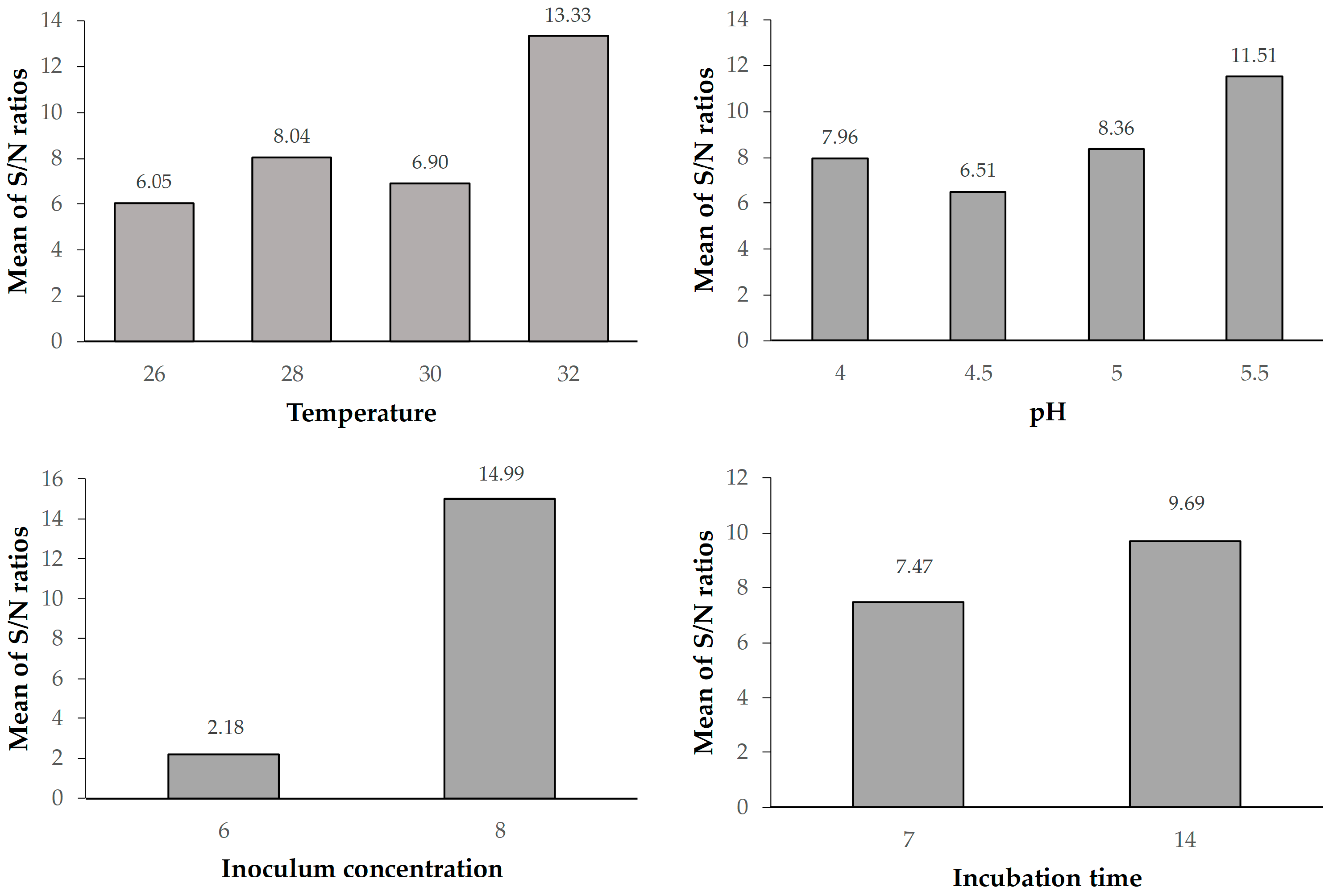
The Taguchi design is a powerful and effective technique for the design of processes that will operate consistently and optimally under a wide variety of conditions. It requires the use of a carefully planned experiment that exposes the process to multiple levels of design parameters to determine the ideal design. The signal to noise ratio (S/N) was chosen by Taguchi as the preferred quality criterion. The standard deviation is not used as a quantitative value because as the mean decreases, so does the standard deviation, and vice versa. The use of the Taguchi methods in the study was justified by the fact that the methods offer advantages such as a wide range of applications, time savings by reducing the number of experiments and the ability to obtain results at a lower cost.
Experiments were conducted using an orthogonal design (SPSS, software version: 28.0) to evaluate four factors affecting BC production, namely inoculum concentration (colony-forming units (CFU) per milliliter), incubation time, incubation temperature and pH (
Table 1). By studying the primary effects of each of these factors, it is possible to identify the general tendencies of the selected factors that influence the cellulose production process. The characteristics can be set to produce the desired effect with smaller or larger values, depending on the particular influence. As a result, it is possible to predict the values of the factors that might give the desired results.
An appropriately singled-out S/N ratio was used to assess response variance using the Taguchi methods. The phrase “S/N” is typically used to denote the proportion between the average value (signal) and the standard deviation (noise). The experiments indicated in
Table 2 were carried out in at least three replicates.
Since the aim of the study was to maximize the amount of bacterial cellulose, the production “larger is better” type of quality characteristic was used. The typical formula for calculating the S/N ratio for this kind of response is:
where i is the number of the first trial, Y
ij is the measured value of the quality characteristic for the i process and j experiment, and n is the number of times that the experimental combination was repeated. Equation (1) was used to calculate the S/N ratios for each of the sixteen experiments listed in
Table 2. The S/N average value was computed for each of the factors, and levels after the S/N ratios for each experiment were determined.
Following the Taguchi experiments, to further investigate the incubation time and inoculum concentrations, more experiments for the inoculum concentrations of 8.5, 9, 9.5, 10 and 10.5 log CFU·mL−1 and the incubation times of 15, 16, 17, 18, 19, 20, 21 and 28 days were evaluated in addition.
2.6. Characterization of Bacterial Cellulose
2.6.1. Scanning Electron Microscopy (SEM)
The BC membranes were coated with a 15 nm layer of gold/palladium. The interior morphology of BC membranes was observed using a SEM (ZEISS, Oberkochen, Germany) at an accelerating voltage of 5 kV.
2.6.2. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectroscopy Analysis
The FT-IR spectra of the dried BC membrane were acquired using a IRTracer-100 Fourier Infrared Spectrometer (Shimadzu, Kyoto, Japan) in ATR mode. The scanning range was from 500 to 4000 cm−1 with a resolution of 4 cm−1.
2.6.3. Water-Holding Capacity (WHC) of BC
The wet BC membranes produced by
K. rhaeticus under optimum conditions were weighed and wet BC membranes were dried until constant weight at 37 °C for 24 h in an oven. Six replicates were analyzed by calculating the following formula [
28,
29]:
where W
wet and W
dry were the wet and dry weight of BC cultivated at the 14th day. The data were presented as the means of six replicates and ±95% confidence interval.
2.6.4. Mechanical Properties
The tensile strength of the BC membrane was measured using a AGS-X Tensile-Compression Tester (Shimadzu, Japan) at room temperature and a crosshead speed of 5 mm/min. Samples were cut into strip-shaped specimens which were 30 mm in length, 5 mm in width and 0.02 mm in thickness. The experiments were repeated at least three times, and average values were taken.
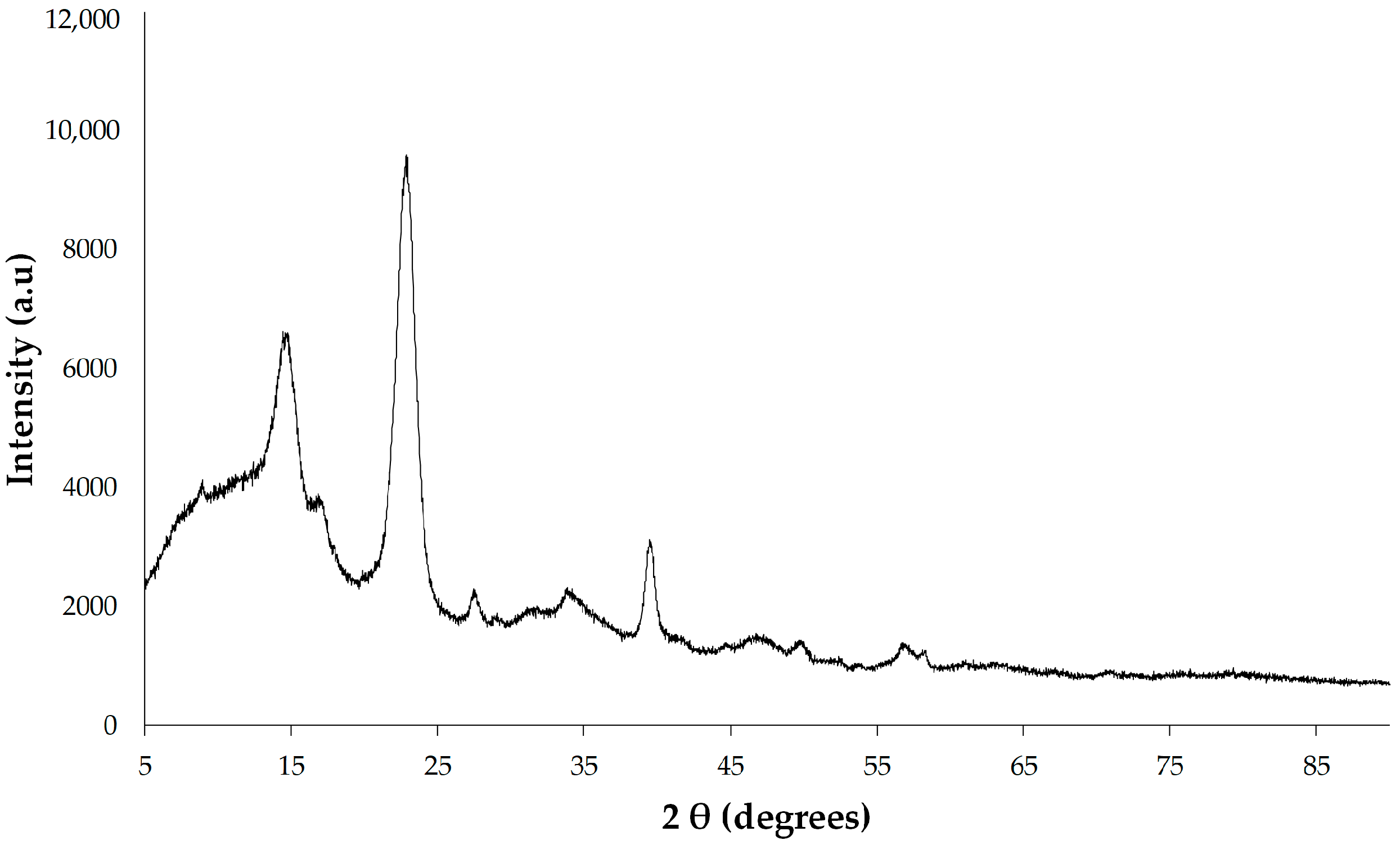
2.6.5. X-ray Diffractometry (XRD)
The X-ray diffractometry of BC membrane was conducted by using an XRD instrument (Bruker, Karlsruhe, Germany) with a 1.8 kW copper-anode-powered X-ray tube. Samples were scanned from 5° to 90° (2θ range) at a scanning rate of 4° min
−1. The degree of crystallinity of BC samples was calculated based on peak intensity using the Segal method [
30].
2.7. Statistical Analysis
Data obtained from the Taguchi experiments were determined by ANOVA analysis using Minitab Statistical Software Version 21.1.0 and SPSS Version 28.0. p values less than 0.05 were considered significant.
4. Discussion
In this study, it was aimed to determine the optimal conditions for the production of bacterial cellulose (BC) by the Komagataeibacter rhaeticus K23 strain isolated from Kombucha tea. The Taguchi methods have been used effectively in the determination of these optimal conditions. The results revealed that K. rhaeticus K23 strain has a high potential for BC production. The Taguchi experiment design has been useful for quickly determining the interactions of these factors and the optimal conditions. On the other hand, the Taguchi methods have the advantage of quickly determining optimal conditions by reducing the number of experiments. This provides an optimization strategy that is effective in terms of cost and time.
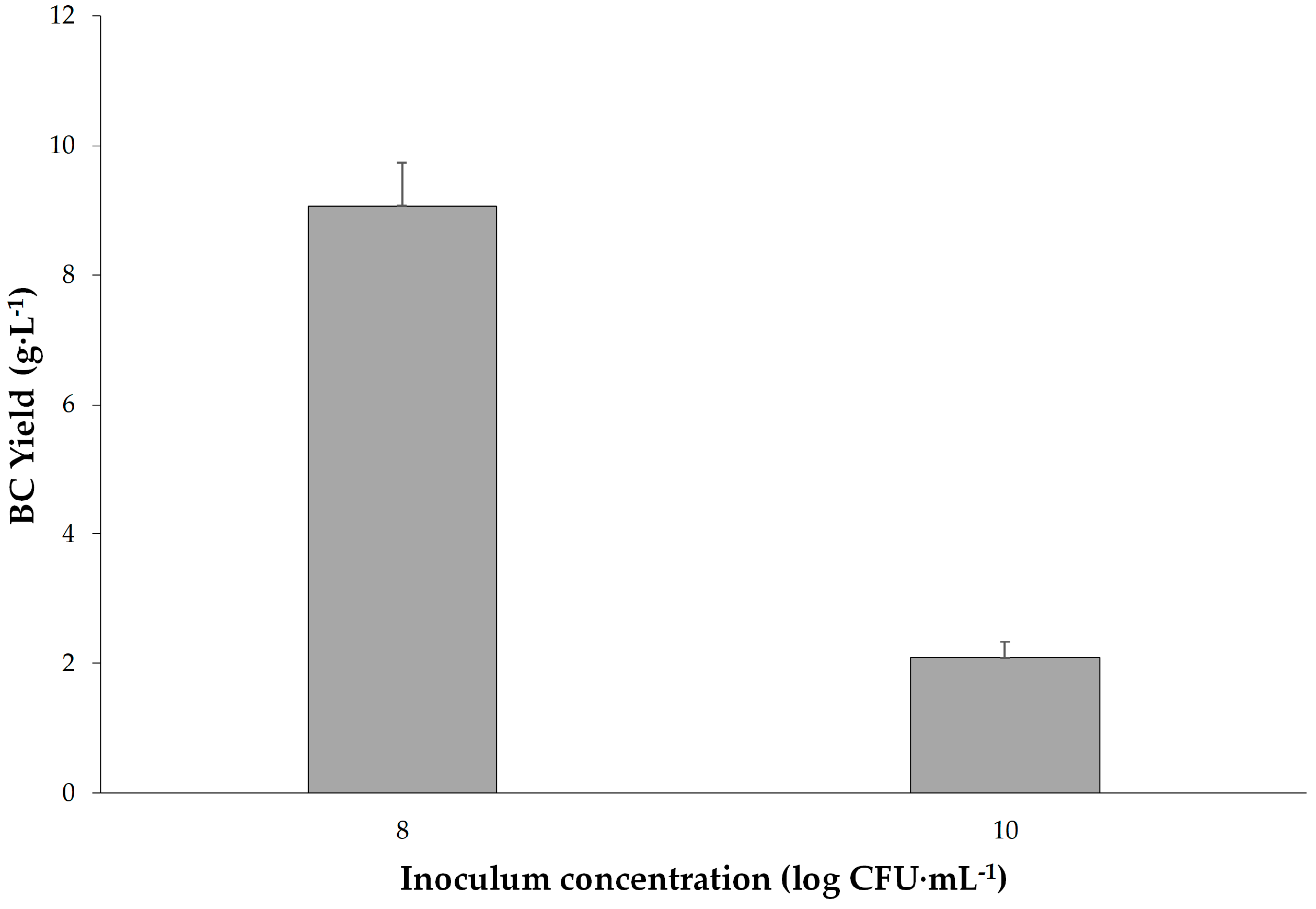
The optimal conditions for maximum BC production were found to be 32 °C, pH 5.5, 8 log CFU·mL
−1 inoculum concentration and 14 days of incubation. Under these conditions, a remarkable BC production of 9.1 ± 0.66 g·L
−1 (dry weight) was achieved. This BC yield obtained in this study significantly surpasses yields reported for other strains of
K. rhaeticus and even outperforms various BC producer strains. This suggests the high potential of
K. rhaeticus K23 for large-scale BC production, presenting economic and environmental advantages over traditional methods of cellulose extraction [
3,
4,
5,
6,
7,
8].
Temperature and pH are critical factors affecting bacterial growth and cellulose synthesis. The highest BC production occurred at 32 °C, aligning with previous studies suggesting the optimal temperature range for K. rhaeticus. Similarly, pH 5.5 was identified as the most favorable condition. The incubation time significantly impacted BC production, with the highest yield observed at 14 days. Extended incubation periods beyond this optimal point led to a decline in BC production, possibly due to the accumulation of metabolic byproducts inhibiting cellulose synthesis. This finding aligns with literature reports suggesting that BC production reaches a plateau after a certain period, emphasizing the importance of efficient harvesting.
Inoculum concentration (Factor C) was determined as the most significant factor affecting BC production (F = 49.63). Higher inoculum concentrations were associated with increased BC yield up to a certain point, after which diminishing returns were observed. This highlights the importance of balancing microbial population density for optimal cellulose synthesis in order to improve the efficiency of biological production. These results could be a fundamental step for the industrial-scale production of bacterial cellulose. Further research could explore downstream processing methods to enhance and add various properties to BC such as antibacterial and explore its diverse applications in pharmaceuticals, membranes, drug carriers, and nanotechnology.
Temperature was an important parameter in BC production by
K. rhaeticus. The lowest BC production by
K. rhaeticus K23 was recorded at 26 °C and the maximum production was obtained at 32 °C (
Figure 3). Many researchers have found that the optimum growth temperature for biocellulose production is 30 °C [
38,
39,
40,
41]. However, this may be due to the strains which they used in their studies (
Acetobacter sp. and
K. saccharivorans) and the tested ranges of temperature. Ye et al. [
26] reported an optimal range of BC production by
K. rhaeticus to be 28 to 32 °C, in parallel with our findings. As a result, the impact of temperature on
K. rhaeticus K23’s ability to manufacture BC is consistent with previous findings that suggested that the BC producers have a comparable degree of environmental flexibility.
The pH value of growth medium also plays an important role in the production of BC, since it effects enzyme activity, influencing the bacterial growth [
42]. The lowest production by
K. rhaeticus K23 was recorded at pH 4.5 and the largest amount of BC yield was obtained at pH 5.5. These results are similar to the findings of Ye et al. [
26] who reported the optimum pH for BC production by
K. rhaeticus K23 to be pH 5.5.
The yield of BC by
K. rhaeticus K23 in this study, which was 9.1 g·L
−1, is not only higher than by other strains of
K. rhaeticus, but also much higher than by other BC producer strains. For example, another variant of the BC producer strains belonging to the same genus, namely
K. saccharivorans BC-1, has been reported to give the concentration of cellulose as 5.1 g·L
−1 [
41]. After seven days of incubation, the amounts of BC produced by five strains of
K. xylinus from different kinds of carbon sources were measured [
43]. According to Singhsa et al. [
43], the bacterial strains
K. xylinus KX (1.14–1.84 g·L
−1) and
K. xylinus K975 (1.11–1.55 g·L
−1) were similarly able to produce the greatest amount of BC membranes in all carbon sources, followed by
K. xylinus K1011 (0.57–1.46 g·L
−1). Similarly, Volova et al. [
44] found the highest BC yield to be 2.2 g·L
−1 which was obtained with
K. xylinus B-12068 cells cultivated for 7 days in HS medium containing glucose. In another study,
K. hansenii was used as a BC producer strain and the maximum obtained BC production was 4.46 g·L
−1 [
45]. Although it is difficult to precisely compare the yield between a variety of experimental designs, considering different strains and incubation conditions including pH, temperature and incubation time, comparison of BC production (g·L
−1) using HS medium revealed that our strain provides the highest yield among these closely related published data.
Frequently, reports indicate that the greatest increase in the weight of BC takes place after 7–8 days [
46,
47,
48]. According to Raghunathan [
49], BC production begins after 24 h of incubation and reaches its highest level after 10 days. Similar results were also obtained by Ye et al. [
26] who have tested BC production over different time periods by
K. rhaeticus K23 and showed that the BC production reached a plateau between 10 and 12 days. However, the results of most researchers’ efforts to determine the maximum production time are not very reliable because incubation periods longer than 10 days have generally not been studied. The properties of BC produced by
K. rhaeticus K23 were evaluated by means of morphology, chemical structure, water-holding capacity and mechanical properties. The fibers in the BC network are homogenous with fibrils oriented in the three-dimensional network. The fibers are intertwined with each other, leading to the formation of interconnected porous networks. The chemical structure of the BC was revealed by ATR-FTIR spectra which showed very similar absorption peaks to those in previous reports [
30,
32]. Tests on water-holding capacity revealed similar results with He et al. [
32] (100 ± 4%). The water-holding capacity was slightly higher than found in Rouhi et al. [
50] (96.6 g/g) and Machado et al. [
28] (70 g/g). The high water-holding capacity of BC is valuable since it is necessary for several application fields, especially in biomedical applications including wound dressing and tissue engineering [
51,
52]. The results of tensile strength of BC were slightly higher than generated by He et al. [
32] (tensile strength 70.4 ± 6.9). On the other hand, Young’s modulus of BC produced in HS medium by
K. rhaeticus K23 was almost 25 times and tensile strength was 2.5 times higher than BC produced in studies of [
53] (tensile strength 17.09 MPa and Young’s modulus 217.77 MPa). The results showed lower tensile strength (62 MPa) and higher Young’s modulus (1.06 GPa) compared to studies by Rouhi et al. [
50]. Multiple research studies have documented the variability in bacterial cellulose (BC) crystallinity, ranging from 46.7 to 91.62%, with its degree often influenced by the culture medium, particularly the carbon and nitrogen sources employed [
44]. The results suggest that BC produced by
K. rhaeticus K23 exhibits a typical crystalline form of cellulose I in agreement with the previously reported other
Komagataeibacter strains [
26,
30].
In conclusion, this study demonstrates the successful optimization of BC production by K. rhaeticus K23, shedding light on the importance of cultivation parameters. The findings contribute to the ongoing efforts to harness the potential of BC as a sustainable and versatile biomaterial.
5. Conclusions
In this study, a Gram-negative bacterial strain “Komagataeibacter rhaeticus strain K23”, isolated from Kombucha tea, was investigated for its bio-cellulose polymer production ability. Moreover, the culture conditions of the bacterium for maximum BC production were also optimized. For the first time, the Taguchi methods were used for investigating the optimum parameters for a potential BC-producing strain, K. rhaeticus K23. The hypothesis of the study was, “pH, temperature, inoculum concentration and incubation period have equal levels of effect on BC production”. It has been observed that among the tested parameters, the most significant parameter is the inoculum concentration, followed by temperature and pH. The dry weight of BC obtained with parameters optimized by the Taguchi design in HS medium was 9.1 ± 0.66 g·L−1, significantly higher than that reported previously. On the other hand, the chemical structure, water-holding capacity and mechanical properties of the biosynthesized BC were similar to previously reported data. The physicochemical properties of the BC membrane produced by K. rhaeticus with HS medium were analyzed via FTIR, XRD, SEM, water-holding capacity and mechanical analysis techniques. The results for morphology, chemical structure, water-holding capacity and mechanical properties displayed similar features to those obtained from using conventional Hestrin‒Schramm medium. Morphological analysis showed a three-dimensional network of BC nanofibers. Our results strongly indicate that this strain could give rise to valuable biomedical products of commercial importance. Further studies could be carried out using supplementary additives, and new properties can be imparted by combining them with various materials tailored to the intended application.