Carbon Fiber Recycling from Waste CFRPs via Microwave Pyrolysis: Gas Emissions Monitoring and Mechanical Properties of Recovered Carbon Fiber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
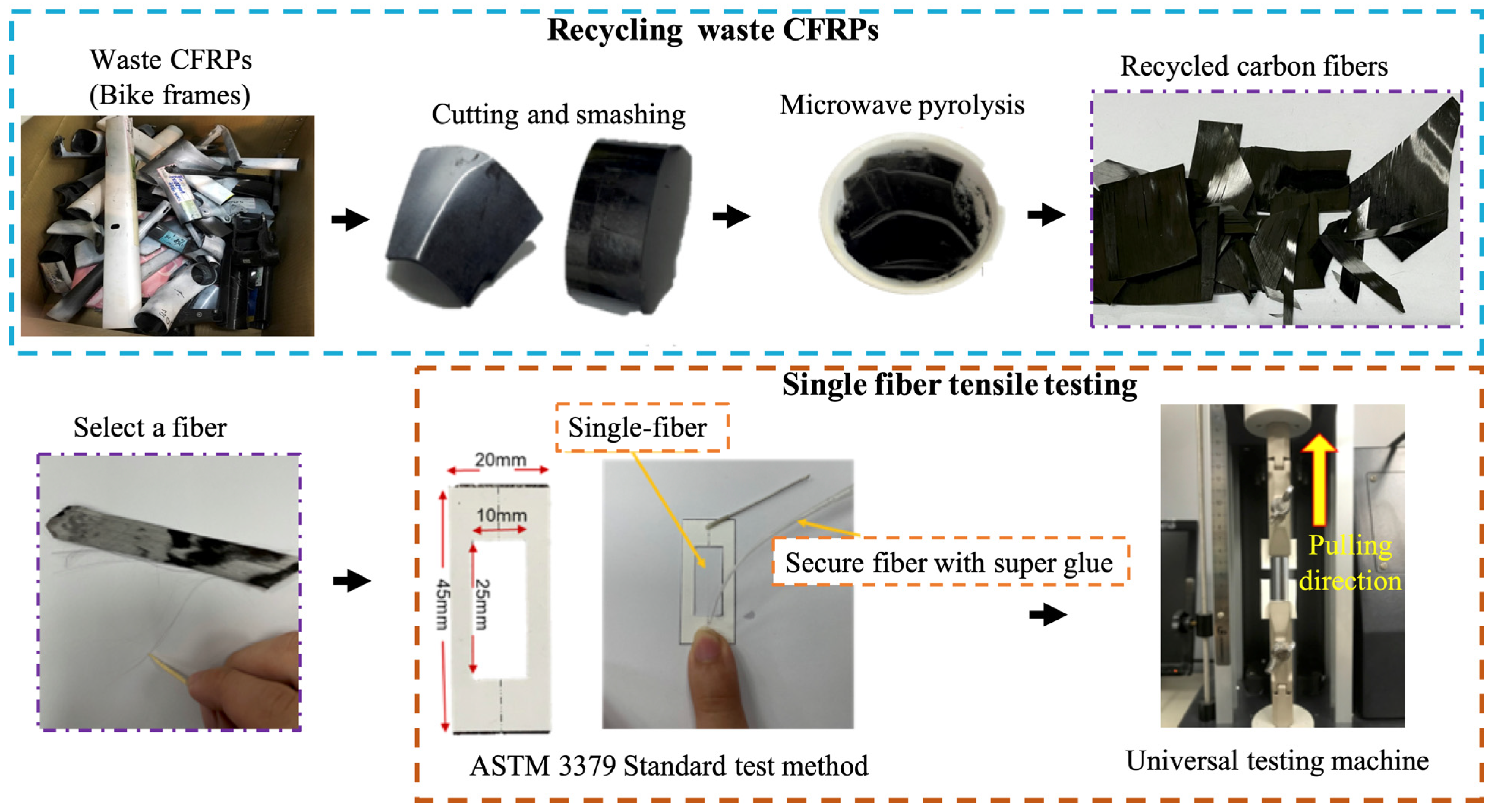
2.2. Pyrolysis
2.3. Characterization
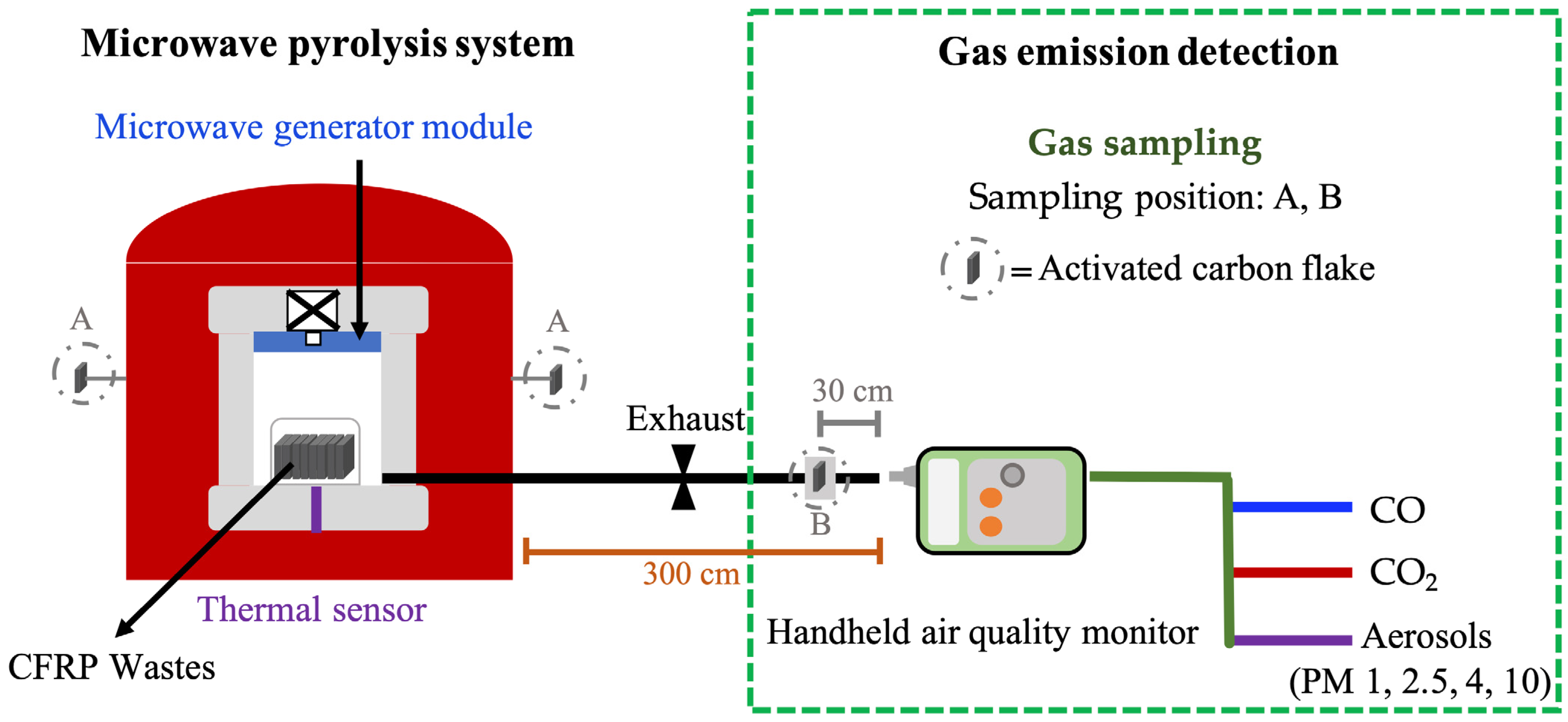
2.3.1. Emission Detection in the Pyrolysis Process of CFRP Waste
2.3.2. Composition of Gas Emissions in CFRP Waste Pyrolysis Process
2.3.3. The Single-Fiber Tensile Properties of Recycled Fibers
2.3.4. Fibers Structural Characterization
3. Results and Discussion
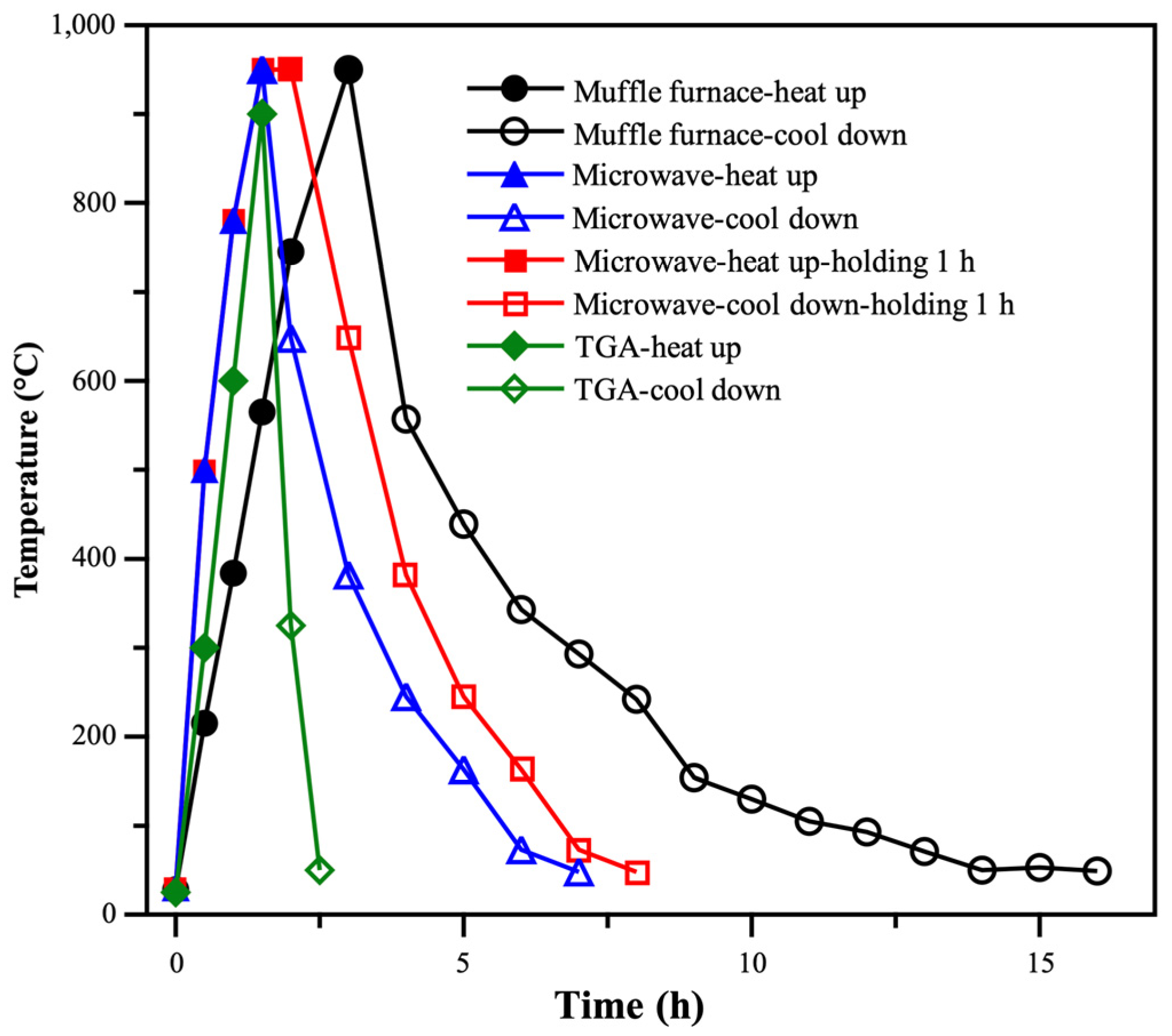
3.1. Time–Temperature Curves During the Pyrolysis of CFRP Waste
3.2. Emission in the Pyrolysis Process of CFRP Waste
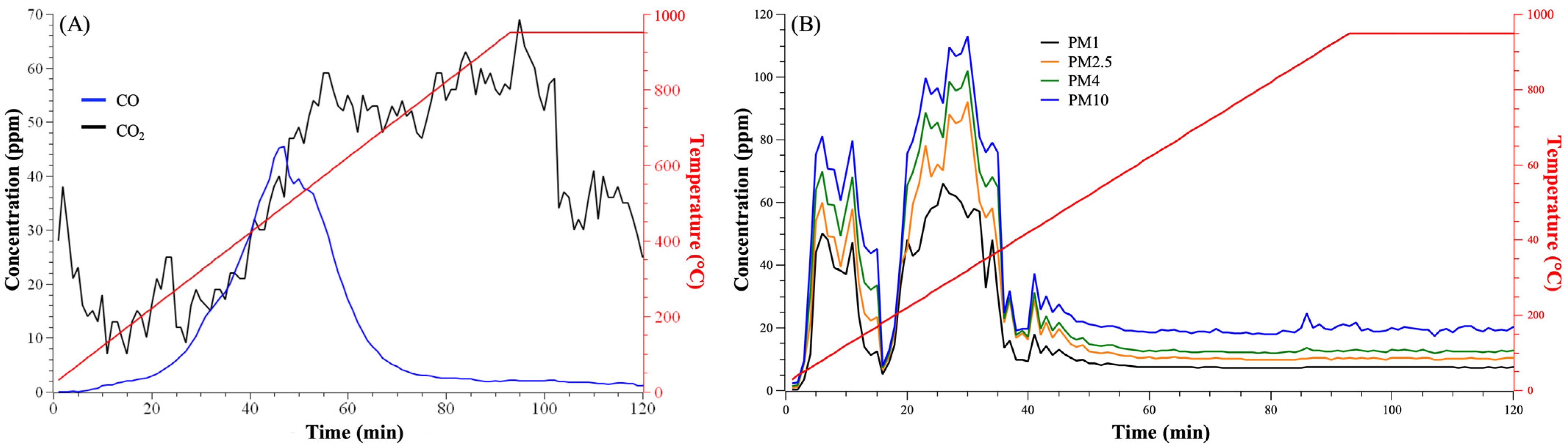
3.2.1. Derived Gas
3.2.2. Composition of Derived Gas Emissions
3.3. Properties of Recovered Carbon Fibers
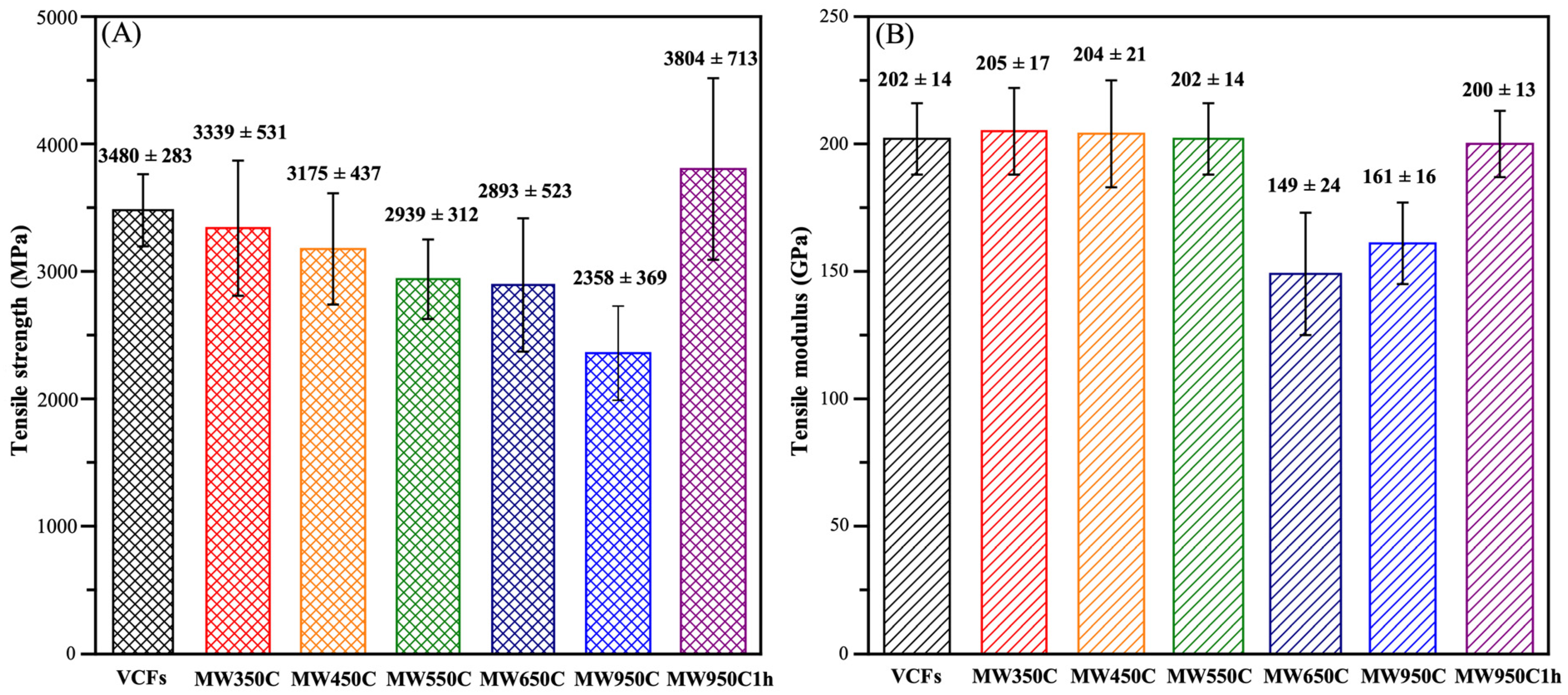
3.3.1. Mechanical Performance
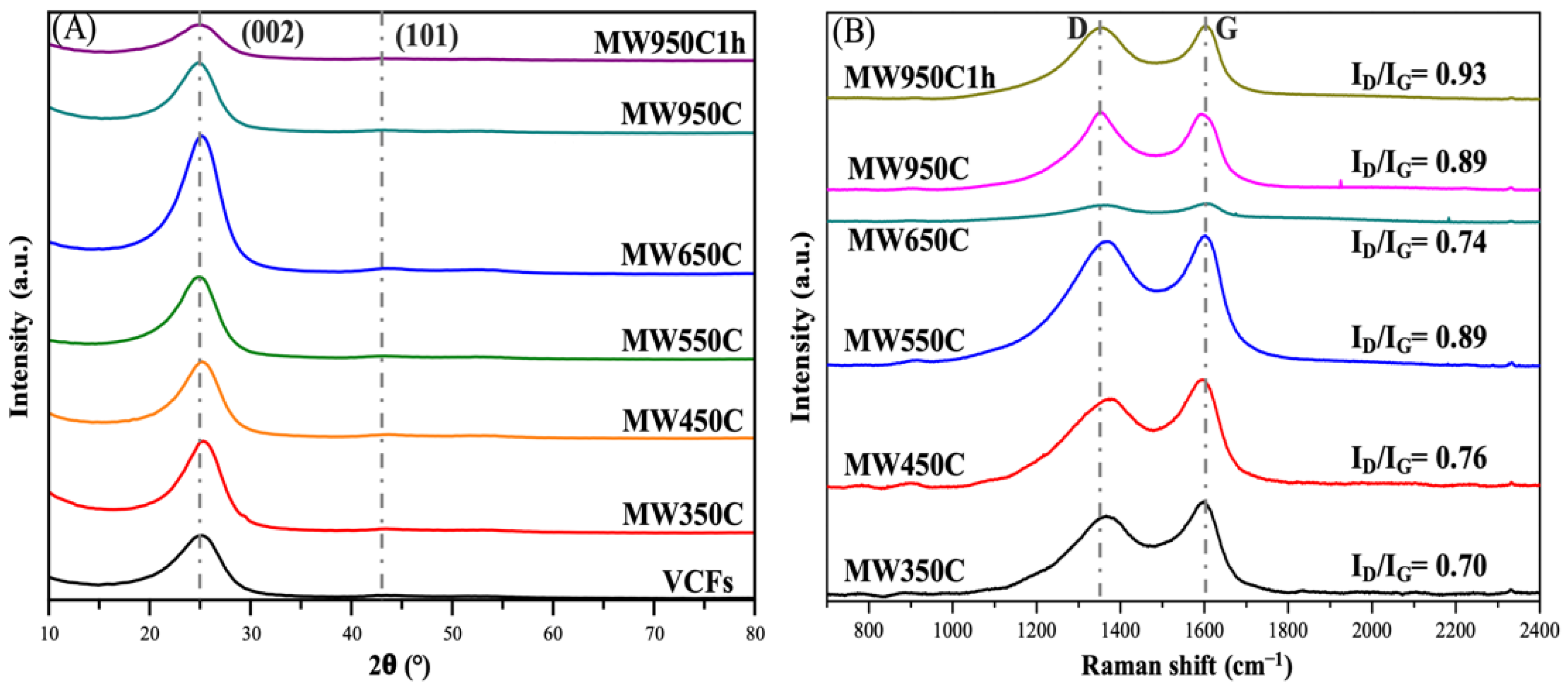
3.3.2. Structural Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, Y.; Hu, S.; Han, S.; Wang, H.; Wei, G.; Gu, Z.; Han, P. Multistage Rigid and Flexible Structure-Decorated Basalt Fibers for Epoxy Resin Composites with Synergistically Enhanced Strength and Toughness. Colloids Surf. A Physicochem. Eng. Asp. 2025, 704, 135478. [Google Scholar] [CrossRef]
- Han, P.; Hu, S.; Wei, G. Precisely Adjusting the Interface Adhesion of Carbon Fiber/Epoxy Composites by Regulating the Micro-Configuration of Graphene Oxide Nanosheets on the Fiber Surface. Appl. Surf. Sci. 2024, 665, 160327. [Google Scholar] [CrossRef]
- Barnett, P.R.; Hmeidat, N.S.; Zheng, B.; Penumadu, D. Toward a Circular Economy: Zero-Waste Manufacturing of Carbon Fiber-Reinforced Thermoplastic Composites. npj Mater. Sustain. 2024, 2, 3. [Google Scholar] [CrossRef]
- Jensen, J.P.; Skelton, K. Wind Turbine Blade Recycling: Experiences, Challenges and Possibilities in a Circular Economy. Renew. Sustain. Energy Rev. 2018, 97, 165–176. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Guo, X.; Liu, C.; Liu, T.; Xin, J.; Zhang, J. Mild Chemical Recycling of Aerospace Fiber/Epoxy Composite Wastes and Utilization of the Decomposed Resin. Polym. Degrad. Stab. 2017, 139, 20–27. [Google Scholar] [CrossRef]
- Liu, P.; Barlow, C.Y. Wind Turbine Blade Waste in 2050. Waste Manag. 2017, 62, 229–240. [Google Scholar] [CrossRef]
- Souppez, J.-B.; Pavar, G. Recycled Carbon Fibre Composites in Automotive Manufacturing. Int. J. Automot. Manuf. Mater. 2023, 2, 38–49. [Google Scholar] [CrossRef]
- Karuppannan Gopalraj, S.; Kärki, T. A Review on the Recycling of Waste Carbon Fibre/Glass Fibre-Reinforced Composites: Fibre Recovery, Properties and Life-Cycle Analysis. SN Appl. Sci. 2020, 2, 433. [Google Scholar] [CrossRef]
- Meng, F.; McKechnie, J.; Pickering, S.J. An Assessment of Financial Viability of Recycled Carbon Fibre in Automotive Applications. Compos. Part A Appl. Sci. Manuf. 2018, 109, 207–220. [Google Scholar] [CrossRef]
- Julian, I.; García-Jiménez, A.; Aguado, A.; Arenal, C.; Calero, A.; Campos, V.; Escobar, G.; López-Buendía, A.M.; Romero, D.; Verdejo, E.; et al. Advances in the Circularity of End-of-Life Fibre-Reinforced Polymers by Microwave Intensification. Chem. Eng. Process.-Process Intensif. 2022, 178, 109015. [Google Scholar] [CrossRef]
- Zhang, J.; Chevali, V.S.; Wang, H.; Wang, C.-H. Current Status of Carbon Fibre and Carbon Fibre Composites Recycling. Compos. Part B Eng. 2020, 193, 108053. [Google Scholar] [CrossRef]
- Li, Y.-F.; Li, J.-Y.; Syu, J.-Y.; Yang, T.-H.; Chang, S.-M.; Shen, M.-Y. Mechanical Behaviors of Microwave-Assisted Pyrolysis Recycled Carbon Fiber-Reinforced Concrete with Early-Strength Cement. Materials 2023, 16, 1507. [Google Scholar] [CrossRef]
- Muthukumarana, T.V.; Arachchi, M.A.V.H.M.; Somarathna, H.M.C.C.; Raman, S.N. A Review on the Variation of Mechanical Properties of Carbon Fibre-Reinforced Concrete. Constr. Build. Mater. 2023, 366, 130173. [Google Scholar] [CrossRef]
- De, B.; Bera, M.; Bhattacharjee, D.; Ray, B.C.; Mukherjee, S. A Comprehensive Review on Fiber-Reinforced Polymer Composites: Raw Materials to Applications, Recycling, and Waste Management. Prog. Mater. Sci. 2024, 146, 101326. [Google Scholar] [CrossRef]
- Pickering, S.J. Recycling Technologies for Thermoset Composite Materials—Current Status. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1206–1215. [Google Scholar] [CrossRef]
- Kuang, X.; Zhou, Y.; Shi, Q.; Wang, T.; Qi, H.J. Recycling of Epoxy Thermoset and Composites via Good Solvent Assisted and Small Molecules Participated Exchange Reactions. ACS Sustain. Chem. Eng. 2018, 6, 9189–9197. [Google Scholar] [CrossRef]
- Piñero-Hernanz, R.; García-Serna, J.; Dodds, C.; Hyde, J.; Poliakoff, M.; Cocero, M.J.; Kingman, S.; Pickering, S.; Lester, E. Chemical Recycling of Carbon Fibre Composites Using Alcohols under Subcritical and Supercritical Conditions. J. Supercrit. Fluids 2008, 46, 83–92. [Google Scholar] [CrossRef]
- Aldosari, S.M.; AlOtaibi, B.M.; Alblalaihid, K.S.; Aldoihi, S.A.; AlOgab, K.A.; Alsaleh, S.S.; Alshamary, D.O.; Alanazi, T.H.; Aldrees, S.D.; Alshammari, B.A. Mechanical Recycling of Carbon Fiber-Reinforced Polymer in a Circular Economy. Polymers 2024, 16, 1363. [Google Scholar] [CrossRef]
- Pakdel, E.; Kashi, S.; Varley, R.; Wang, X. Recent Progress in Recycling Carbon Fibre Reinforced Composites and Dry Carbon Fibre Wastes. Resour. Conserv. Recycl. 2021, 166, 105340. [Google Scholar] [CrossRef]
- Song, W.; Magid, A.; Li, D.; Lee, K.-Y. Application of Recycled Carbon-Fibre-Reinforced Polymers as Reinforcement for Epoxy Foams. J. Environ. Manag. 2020, 269, 110766. [Google Scholar] [CrossRef]
- Ateeq, M. A State of Art Review on Recycling and Remanufacturing of the Carbon Fiber from Carbon Fiber Polymer Composite. Compos. Part C Open Access 2023, 12, 100412. [Google Scholar] [CrossRef]
- Hadigheh, S.A.; Wei, Y.; Kashi, S. Optimisation of CFRP Composite Recycling Process Based on Energy Consumption, Kinetic Behaviour and Thermal Degradation Mechanism of Recycled Carbon Fibre. J. Clean. Prod. 2021, 292, 125994. [Google Scholar] [CrossRef]
- Deng, J.; Xu, L.; Zhang, L.; Peng, J.; Guo, S.; Liu, J.; Koppala, S. Recycling of Carbon Fibers from CFRP Waste by Microwave Thermolysis. Processes 2019, 7, 207. [Google Scholar] [CrossRef]
- Fresneda-Cruz, A.; Chaine, C.; Figueirêdo, M.B.; Murillo-Ciordia, G.; Sanz-Martinez, A.; Julian, I. Potentials and Limitations of Microwave-Assisted Chemical Recycling of Fiber-Reinforced Composites from Wind Blades. Sustain. Energy Fuels 2024, 8, 4752–4766. [Google Scholar] [CrossRef]
- Teltschik, J.; Matter, J.; Woebbeking, S.; Jahn, K.; Borja Adasme, Y.; Van Paepegem, W.; Drechsler, K.; Tallawi, M. Review on Recycling of Carbon Fibre Reinforced Thermoplastics with a Focus on Polyetheretherketone. Compos. Part A Appl. Sci. Manuf. 2024, 184, 108236. [Google Scholar] [CrossRef]
- Ren, Y.; Xu, L.; Shang, X.; Shen, Z.; Fu, R.; Li, W.; Guo, L. Evaluation of Mechanical Properties and Pyrolysis Products of Carbon Fibers Recycled by Microwave Pyrolysis. ACS Omega 2022, 7, 13529–13537. [Google Scholar] [CrossRef]
- Hao, S.; He, L.; Liu, J.; Liu, Y.; Rudd, C.; Liu, X. Recovery of Carbon Fibre from Waste Prepreg via Microwave Pyrolysis. Polymers 2021, 13, 1231. [Google Scholar] [CrossRef]
- Lopez-Urionabarrenechea, A.; Gastelu, N.; Acha, E.; Caballero, B.M.; Orue, A.; Jiménez-Suárez, A.; Prolongo, S.G.; De Marco, I. Reclamation of Carbon Fibers and Added-Value Gases in a Pyrolysis-Based Composites Recycling Process. J. Clean. Prod. 2020, 273, 123173. [Google Scholar] [CrossRef]
- Kawajiri, K.; Kobayashi, M. Cradle-to-Gate Life Cycle Assessment of Recycling Processes for Carbon Fibers: A Case Study of Ex-Ante Life Cycle Assessment for Commercially Feasible Pyrolysis and Solvolysis Approaches. J. Clean. Prod. 2022, 378, 134581. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Shiue, A.; Wu, Y.-J.; Chang, S.-M.; Li, Y.-F.; Shen, M.-Y.; Leggett, G. Studies on Recycling Silane Controllable Recovered Carbon Fiber from Waste CFRP. Sustainability 2022, 14, 700. [Google Scholar] [CrossRef]
- Li, Y.-F.; Li, J.-Y.; Ramanathan, G.K.; Chang, S.-M.; Shen, M.-Y.; Tsai, Y.-K.; Huang, C.-H. An Experimental Study on Mechanical Behaviors of Carbon Fiber and Microwave-Assisted Pyrolysis Recycled Carbon Fiber-Reinforced Concrete. Sustainability 2021, 13, 6829. [Google Scholar] [CrossRef]
- Li, Y.-F.; Hung, J.-Y.; Syu, J.-Y.; Chen, S.-H.; Huang, C.-H.; Chang, S.-M.; Kuo, W.-S. Effect of the Sizing Removal Methods of Fiber Surface on the Mechanical Performance of Basalt Fiber-Reinforced Concrete. Fibers 2024, 12, 10. [Google Scholar] [CrossRef]
- Li, Y.-F.; Hung, J.-Y.; Syu, J.-Y.; Chang, S.-M.; Kuo, W.-S. Influence of Sizing of Basalt Fiber on the Mechanical Behavior of Basalt Fiber Reinforced Concrete. J. Mater. Res. Technol. 2022, 21, 295–307. [Google Scholar] [CrossRef]
- Baloyi, R.B.; Sithole, B.B. Pyrolysis-Gas Chromatography/Mass Spectrometry Analysis as a Useful Tool in Identifying Fibre Composition in Mixed Post-Consumer Textile Waste. J. Anal. Appl. Pyrolysis 2024, 183, 106740. [Google Scholar] [CrossRef]
- Picó, Y.; Barceló, D. Pyrolysis Gas Chromatography-Mass Spectrometry in Environmental Analysis: Focus on Organic Matter and Microplastics. TrAC Trends Anal. Chem. 2020, 130, 115964. [Google Scholar] [CrossRef]
- ASTN Test Method D3379–75; Standard Test Method for Tensile Strength and Young’s Modulus for High-Modulus Single-Filament Materials. American Society for Testing and Materials: Philadelphia, PA, USA, 1993.
- Qiao, Y.; Das, O.; Zhao, S.-N.; Sun, T.-S.; Xu, Q.; Jiang, L. Pyrolysis Kinetic Study and Reaction Mechanism of Epoxy Glass Fiber Reinforced Plastic by Thermogravimetric Analyzer (TG) and TG–FTIR (Fourier-Transform Infrared) Techniques. Polymers 2020, 12, 2739. [Google Scholar] [CrossRef]
- Vázquez-Santos, M.B.; Geissler, E.; László, K.; Rouzaud, J.-N.; Martínez-Alonso, A.; Tascón, J.M.D. Comparative XRD, Raman, and TEM Study on Graphitization of PBO-Derived Carbon Fibers. J. Phys. Chem. C 2012, 116, 257–268. [Google Scholar] [CrossRef]
- Peng, S.; Shao, H.; Hu, X. Lyocell Fibers as the Precursor of Carbon Fibers. J Appl. Polym. Sci. 2003, 90, 1941–1947. [Google Scholar] [CrossRef]
- Nunna, S.; Maghe, M.; Rana, R.; Varley, R.J.; Knorr, D.B.; Sands, J.M.; Creighton, C.; Henderson, L.C.; Naebe, M. Time Dependent Structure and Property Evolution in Fibres during Continuous Carbon Fibre Manufacturing. Materials 2019, 12, 1069. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. WJNSE 2012, 02, 154–160. [Google Scholar] [CrossRef]
- Hallam, K.R.; Darnbrough, J.E.; Paraskevoulakos, C.; Heard, P.J.; Marrow, T.J.; Flewitt, P.E.J. Measurements by X-Ray Diffraction of the Temperature Dependence of Lattice Parameter and Crystallite Size for Isostatically-Pressed Graphite. Carbon Trends 2021, 4, 100071. [Google Scholar] [CrossRef]
- Khanna, R.; Ikram-Ul-Haq, M.; Cayumil, R.; Rajarao, R.; Sahajwalla, V. Novel Carbon Micro Fibers and Foams from Waste Printed Circuit Boards. Fuel Process. Technol. 2015, 134, 473–479. [Google Scholar] [CrossRef]
- Toyoda, M.; Kaburagi, Y.; Yoshida, A.; Inagaki, M. Acceleration of Graphitization in Carbon Fibers through Exfoliation. Carbon 2004, 42, 2567–2572. [Google Scholar] [CrossRef]
- Nemanich, R.J.; Solin, S.A. First- and Second-Order Raman Scattering from Finite-Size Crystals of Graphite. Phys. Rev. B 1979, 20, 392–401. [Google Scholar] [CrossRef]
- Katagiri, G.; Ishida, H.; Ishitani, A. Raman Spectra of Graphite Edge Planes. Carbon 1988, 26, 565–571. [Google Scholar] [CrossRef]
- Torres-Canas, F.; Bentaleb, A.; Föllmer, M.; Roman, J.; Neri, W.; Ly, I.; Derré, A.; Poulin, P. Improved Structure and Highly Conductive Lignin-Carbon Fibers through Graphene Oxide Liquid Crystal. Carbon 2020, 163, 120–127. [Google Scholar] [CrossRef]
- Dervishi, E.; Ji, Z.; Htoon, H.; Sykora, M.; Doorn, S.K. Raman Spectroscopy of Bottom-up Synthesized Graphene Quantum Dots: Size and Structure Dependence. Nanoscale 2019, 11, 16571–16581. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman Spectroscopy of Graphene and Graphite: Disorder, Electron–Phonon Coupling, Doping and Nonadiabatic Effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Salas, A.; Berrio, M.E.; Martel, S.; Díaz-Gómez, A.; Palacio, D.A.; Tuninetti, V.; Medina, C.; Meléndrez, M.F. Towards Recycling of Waste Carbon Fiber: Strength, Morphology and Structural Features of Recovered Carbon Fibers. Waste Manag. 2023, 165, 59–69. [Google Scholar] [CrossRef]
- Park, M.; Jang, D.; Endo, M.; Lee, S.; Lee, D.S. The Effect of Heat Treatment on Temperature-Dependent Transport and Magnetoresistance in Polyacrylonitrile-Based Carbon Fibers. Mater. Des. 2022, 222, 111071. [Google Scholar] [CrossRef]
- Lu, J.; Li, W.; Kang, H.; Feng, L.; Xu, J.; Liu, R. Microstructure and Properties of Polyacrylonitrile Based Carbon Fibers. Polym. Test. 2020, 81, 106267. [Google Scholar] [CrossRef]
| Locations | Major Compounds | |
|---|---|---|
| Microwave * | Position A | Toluene Octane |
| Exhaust vent | Position B | Toluene Octane Ethylbenzene Styrene Phenol o-Cymene p-Cumenol 5,6,7,8-Tetrahydro-2-acetonaphthone Tetrahydrofuran |
| Sample | * Lc(002) (Å) | ID/IG | ** TS (MPa) | *** TM (GPa) |
|---|---|---|---|---|
| VCFs | 0.256 | - | 3480 ± 283 | 202 ± 14 |
| MW350C | 0.283 | 0.70 | 3339 ± 531 | 205 ± 17 |
| MW450C | 0.279 | 0.76 | 3175 ± 437 | 204 ± 21 |
| MW550C | 0.275 | 0.89 | 2939 ± 312 | 202 ± 14 |
| MW650C | 0.292 | 0.74 | 2893 ± 523 | 149 ± 24 |
| MW950C | 0.297 | 0.89 | 2358 ± 369 | 161 ± 16 |
| MW950C1H | 0.222 | 0.93 | 3804 ± 713 | 200 ± 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, K.-Y.; Shiue, A.; You, J.-L.; Wu, Y.-J.; Cheng, K.-Y.; Chang, S.-M.; Li, Y.-F.; Tseng, C.-H.; Leggett, G. Carbon Fiber Recycling from Waste CFRPs via Microwave Pyrolysis: Gas Emissions Monitoring and Mechanical Properties of Recovered Carbon Fiber. Fibers 2024, 12, 106. https://doi.org/10.3390/fib12120106
Chin K-Y, Shiue A, You J-L, Wu Y-J, Cheng K-Y, Chang S-M, Li Y-F, Tseng C-H, Leggett G. Carbon Fiber Recycling from Waste CFRPs via Microwave Pyrolysis: Gas Emissions Monitoring and Mechanical Properties of Recovered Carbon Fiber. Fibers. 2024; 12(12):106. https://doi.org/10.3390/fib12120106
Chicago/Turabian StyleChin, Kai-Yen, Angus Shiue, Jhu-Lin You, Yi-Jing Wu, Kai-Yi Cheng, Shu-Mei Chang, Yeou-Fong Li, Chao-Heng Tseng, and Graham Leggett. 2024. "Carbon Fiber Recycling from Waste CFRPs via Microwave Pyrolysis: Gas Emissions Monitoring and Mechanical Properties of Recovered Carbon Fiber" Fibers 12, no. 12: 106. https://doi.org/10.3390/fib12120106
APA StyleChin, K.-Y., Shiue, A., You, J.-L., Wu, Y.-J., Cheng, K.-Y., Chang, S.-M., Li, Y.-F., Tseng, C.-H., & Leggett, G. (2024). Carbon Fiber Recycling from Waste CFRPs via Microwave Pyrolysis: Gas Emissions Monitoring and Mechanical Properties of Recovered Carbon Fiber. Fibers, 12(12), 106. https://doi.org/10.3390/fib12120106








