Effect of Cross-Linkers on the Processing of Lignin/Polyamide Precursors for Carbon Fibres
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Melt Compounding and Melt Spinning into Continuous Filaments
2.3. Characterisation of Compounded Pellets
2.4. Characterisation of Melt-Spun Filaments from Pellet-Coated Blends Regarding Mechanical and Morphological Properties
2.5. Pre-Treatment of TcC/PA1010 with Cross-Linkers and Thermal Stabilisation
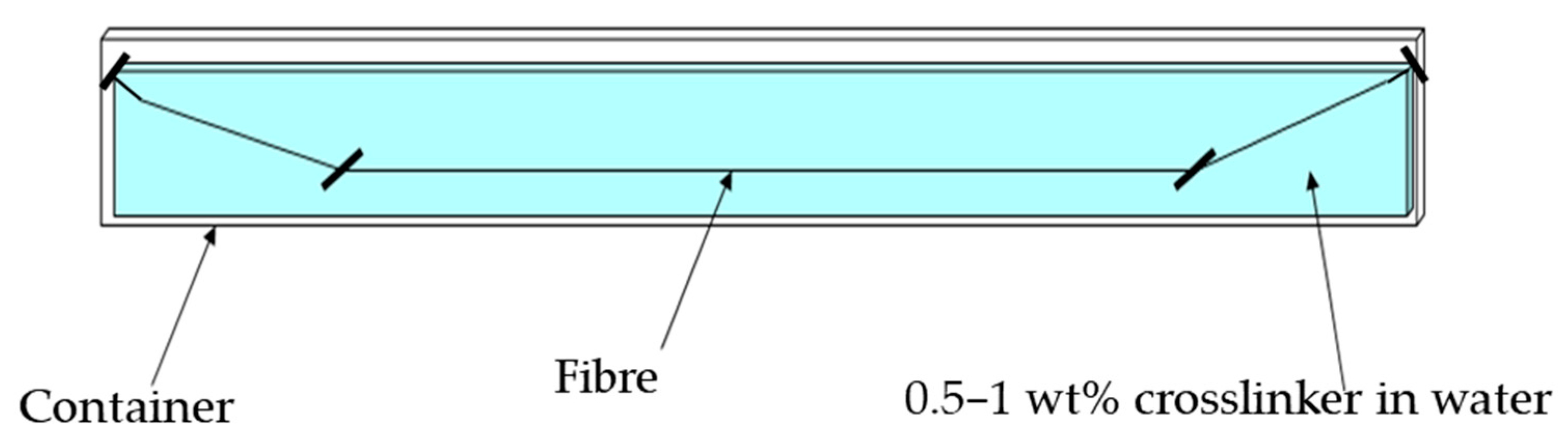
2.5.1. Filament Extrusion and Immersion
2.5.2. Thermal Stabilisation
3. Results
3.1. Effect of Cross-Linkers on Physico-Chemical Changes in Lignin/PA1010 Blends (Melt Compounded)
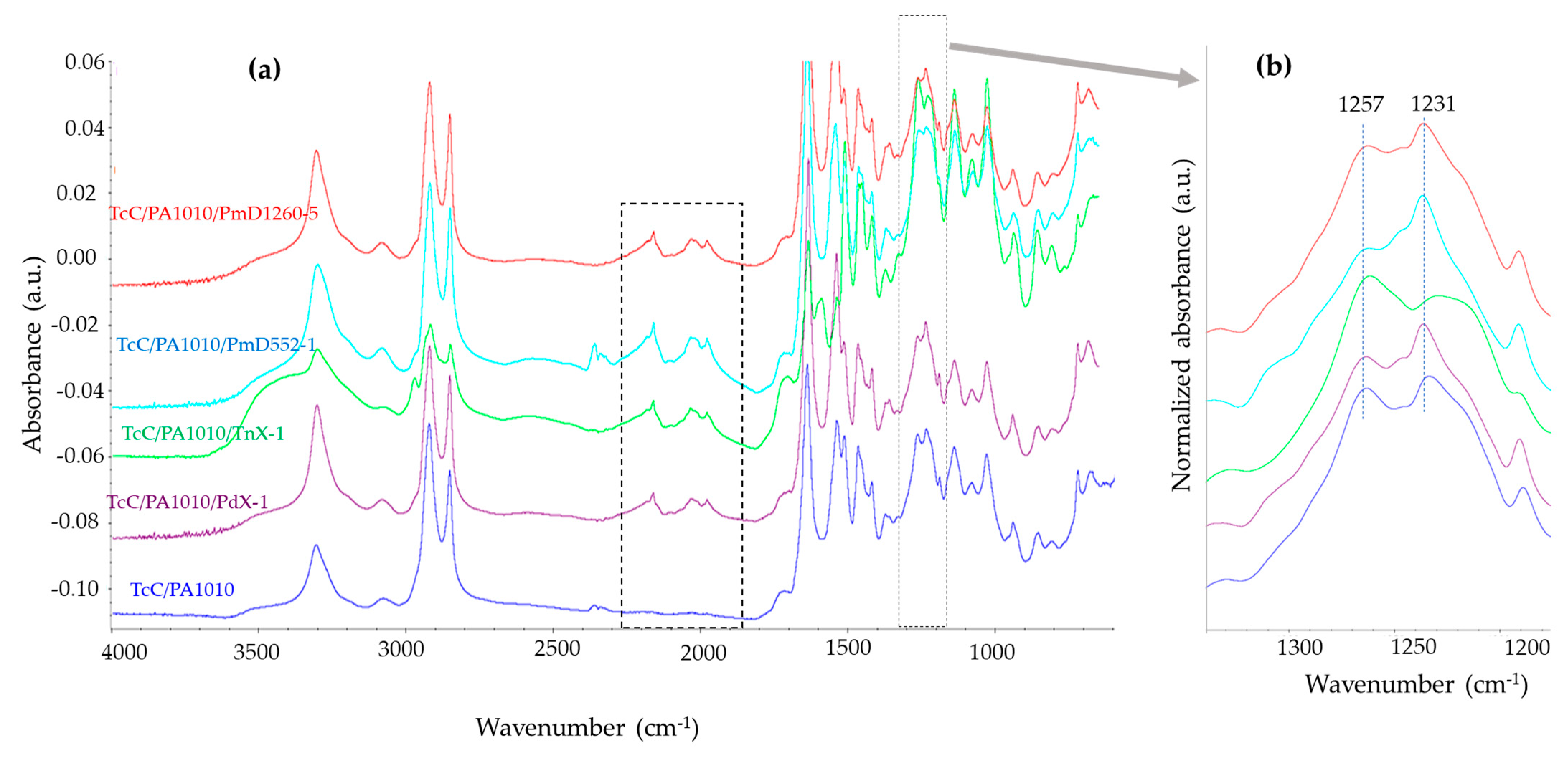
3.1.1. FTIR Spectroscopic Analysis
3.1.2. Differential Scanning Calorimetric Analysis
3.1.3. Dynamic Mechanical Analysis (DMA)
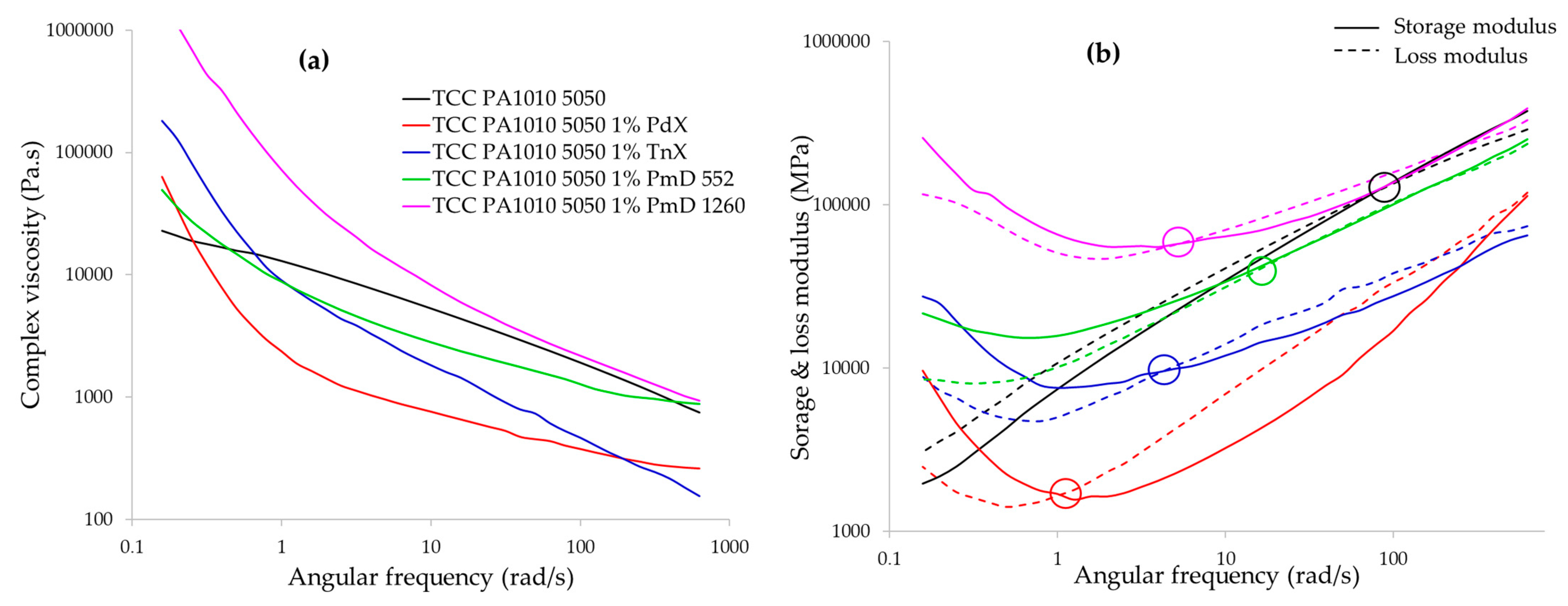
3.1.4. Rheological Behaviour
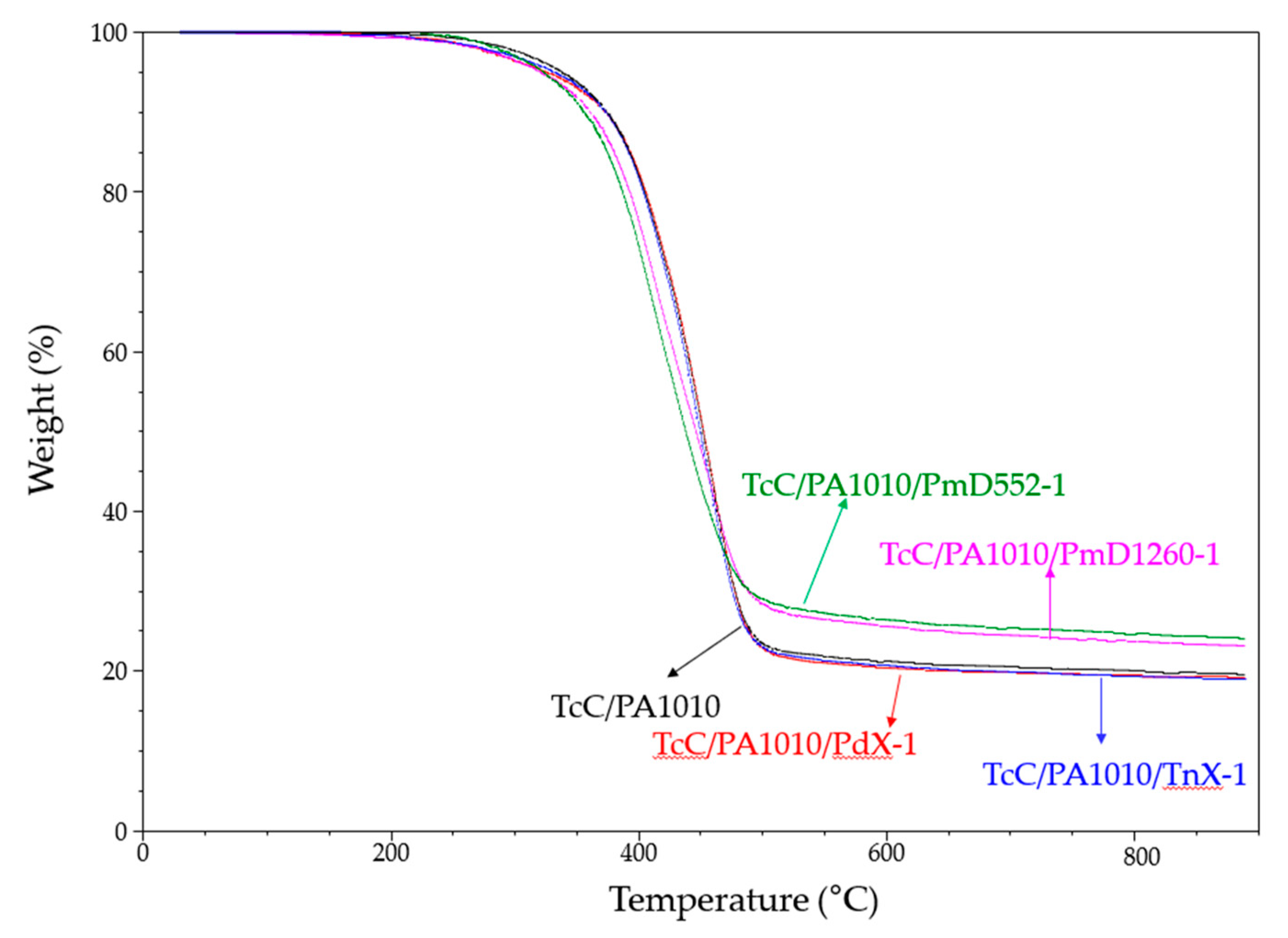
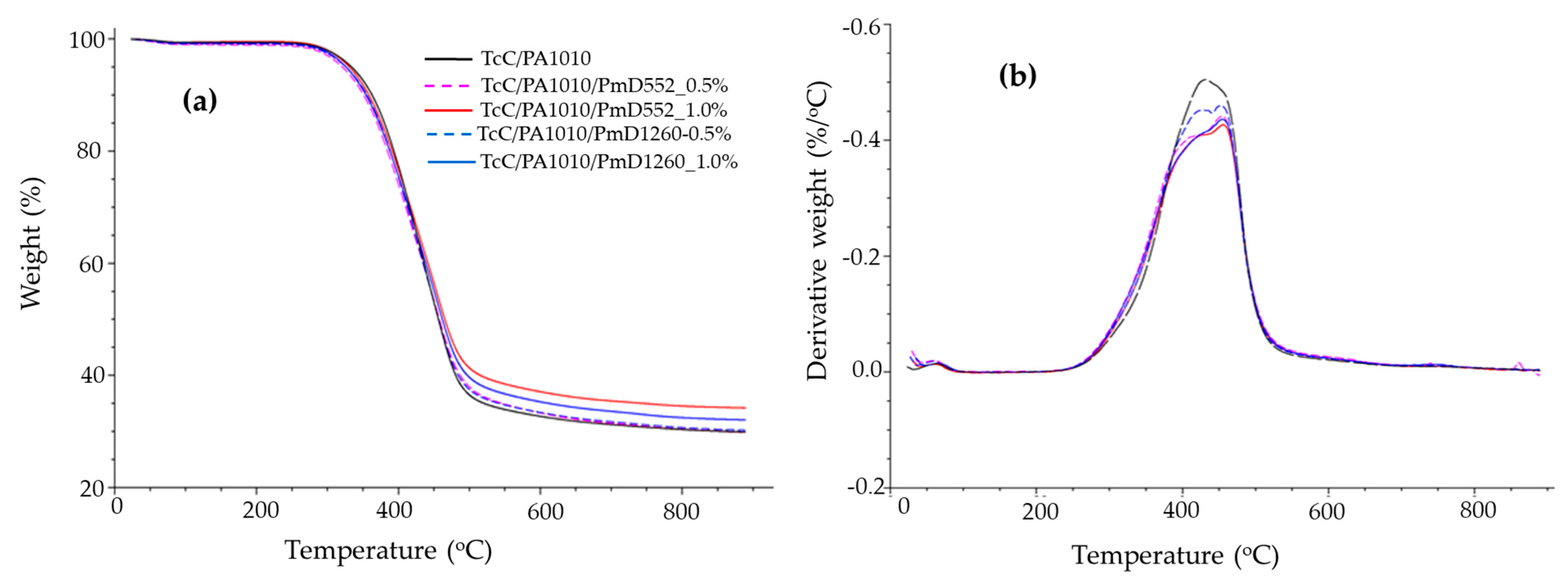
3.1.5. Thermogravimetric Analysis
3.2. Effect of Cross-Linkers Introduced Using Pellet-Coating TcC/PA1010 Compounded Pellets on the Physico-Mechanical Properties of Derived Filaments
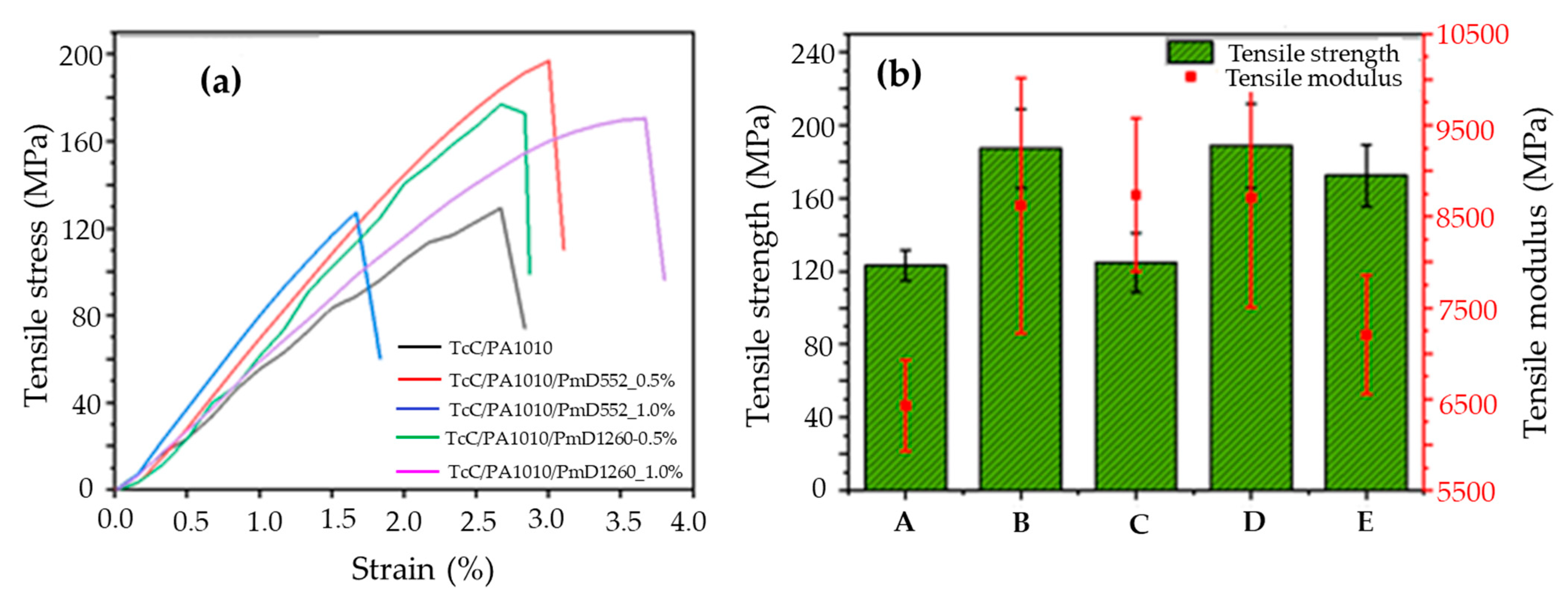
3.3. Effect of Cross-Linkers Introduced by Pre-Treating TcC/PA1010 Filaments on the Mechanical Properties of Thermally Stabilised Filaments
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogale, A.A.; Zhang, M.; Jin, J. Recent advances in carbon fibers derived from biobased precursors. J. Appl. Polym. Sci. 2016, 133, 43794. [Google Scholar] [CrossRef]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Spörl, J.M.; Buchmeiser, M.R. Carbon fibers: Precursor systems, processing, structure, and properties. Angew. Chem Int. Ed. Engl. 2014, 53, 5262–5298. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J. Precursors and Manufacturing of Carbon Fibers. In Carbon Fibers; Springer Series in Materials Science; Springer: Singapore, 2018; Volume 210. [Google Scholar] [CrossRef]
- Le, N.-D.; Varley, R.J.; Hummel, M.; Trogen, M.; Byrne, N. A review of future directions in the development of sustainable carbon fiber from bio-based precursors. Mater Today Sustain. 2022, 20, 100251. [Google Scholar] [CrossRef]
- Brown, K.R.; Harrell, T.M.; Skrzypczak, L.; Scherschel, A.; Wu, H.F.; Li, X. Carbon fibers derived from commodity polymers: A review. Carbon 2022, 196, 422–439. [Google Scholar] [CrossRef]
- Thunga, M.; Chen, K.; Grewell, D.; Kessler, M.R. Bio-renewable precursor fibers from lignin/polylactide blends for conversion to carbon fibers. Carbon 2014, 68, 159–166. [Google Scholar] [CrossRef]
- Baker, D.A.; Rials, T.G. Recent advances in low-cost carbon fiber manufacture from lignin. J. Appl. Polym. Sci. 2013, 130, 713–728. [Google Scholar] [CrossRef]
- Bengtsson, A.; Bengtsson, J.; Sedin, M.; Sjöholm, E. Carbon fibers from lignin-cellulose precursors: Effect of stabilization conditions. ACS Sustain. Chem. Eng. 2019, 7, 8440–8448. [Google Scholar] [CrossRef]
- Muthuraj, R.; Horrocks, A.R.; Kandola, B.K. Hydroxypropyl-modified and organosolv lignin/bio-based polyamide blend filaments as carbon fibre precursors. J. Mater. Sci. 2020, 55, 7066–7083. [Google Scholar] [CrossRef]
- Beaucamp, A.; Wang, Y.; Culebras, M.; Collins, M.N. Carbon fibres from renewable resources: The role of the lignin molecular structure in its blendability with biobased poly(ethylene terephthalate). Green Chem. 2019, 21, 5063–5072. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. Kraft lignin/poly (ethylene oxide) blends: Effect of lignin structure on miscibility and hydrogen bonding. J. Appl. Polym. Sci. 2005, 98, 1437–1444. [Google Scholar] [CrossRef]
- Muthuraj, R.; Hajee, M.; Horrocks, A.R.; Kandola, B.K. Biopolymer blends from hardwood lignin and bio-polyamides: Compatibility and miscibility. Int. J. Biol. Macromol. 2019, 132, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Hosseinaei, O.; Harper, D.P.; Bozell, J.J.; Rials, T.G. Improving processing and performance of pure lignin carbon fibers through hardwood and herbaceous lignin blends. Int. J. Mol. Sci. 2017, 18, 1410. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Kadla, J.F. The formation of strong intermolecular interactions in immiscible blends of poly(vinyl alcohol) (PVA) and lignin. Biomacromolecules 2003, 4, 61–567. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. Lignin-based carbon fibers: Effect of synthetic polymer blending on fiber properties. J. Polym. Environ. 2005, 13, 97–105. [Google Scholar] [CrossRef]
- Muthuraj, R.; Hajee, M.; Horrocks, A.R.; Kandola, B.K. Effect of compatibilizers on lignin/bio-polyamide blend carbon precursor filament properties and their potential for thermostabilisation and carbonization. Polym. Test 2021, 95, 107133. [Google Scholar] [CrossRef]
- Culebras, M.; Beaucamp, A.; Wang, Y.; Clauss, M.; Frank, E.; Collins, M.N. Bio-based structurally compatible polymer blends based on lignin and thermoplastic elastomer polyurethane as carbon fiber precursors. ACS Sustain. Chem. Eng. 2018, 6, 8816–8825. [Google Scholar] [CrossRef]
- Yusof, N.; Ismail, A.F. Post spinning and pyrolysis processes of polyacrylonitrile (PAN)-based carbon fiber and activated carbon fiber: A review. J. Anal. Appl. Pyrol. 2012, 93, 1–13. [Google Scholar] [CrossRef]
- Souto, F.; Calado, V.; Pereira, N. Lignin-based carbon fiber: A current overview. Mater. Res. Express 2018, 5, 072001. [Google Scholar] [CrossRef]
- Brodin, I.; Ernstsson, M.; Gellerstedt, G.; Sjöholm, E. Oxidative stabilisation of kraft lignin for carbon fibre production. Holzforschung 2012, 66, 141–147. [Google Scholar] [CrossRef]
- Kleinhans, H. Evaluation of the Carbonization of Thermo-Stabilized Lignin Fibers into Carbon Fibers. Master’s Thesis, Linköping University,, Linköping, Sweden, 2015. [Google Scholar]
- Bova, T.; Tran, C.D.; Balakshin, M.Y.; Chen, J.; Capanema, E.A.; Naskar, A.K. An approach towards tailoring interfacial structures and properties of multiphase renewable thermoplastics from lignin–nitrile rubber. Green Chem. 2016, 18, 5423–5437. [Google Scholar] [CrossRef]
- Toriz, G.; Denes, F.; Young, R.A. Lignin-polypropylene composites. Part 1: Composites from unmodified lignin and polypropylene. Polym. Comp. 2002, 23, 806–813. [Google Scholar] [CrossRef]
- Nam, B.-U.; Son, Y. Enhanced impact strength of compatibilized poly(lactic acid)/polyamide 11 blends by a crosslinking agent. J. Appl. Polym. Sci. 2020, 137, 49011. [Google Scholar] [CrossRef]
- Bondan, F.; Soares, M.R.F.; Bianchi, O. Effect of dynamic crosslinking on phase morphology and mechanical properties of polyamide 6,12/ethylene vinyl acetate copolymer blends. Polym. Bull. 2014, 71, 151–166. [Google Scholar] [CrossRef]
- Culebras, M.; Sanchis, M.J.; Beaucamp, A.; Carsí, M.; Kandola, B.K.; Horrocks, A.R.; Panzetti, G.; Birkinshaw, C.; Collins, M.N. Understanding the thermal and dielectric response of organosolv and modified kraft lignin as a carbon fibre precursor. Green Chem. 2018, 20, 4461–4472. [Google Scholar] [CrossRef]
- Available online: https://www.yumpu.com/en/document/view/11795302/initiators-and-reactor-additives-for-thermoplastics-akzonobel (accessed on 1 December 2022).
- EMS-CHEMIE AG. Primid ® XL-552. Datasheet:1-2. Available online: https://www.emsgriltech.com/fileadmin/ems-griltech/documents/Datasheet/Technical_Datasheet_XL-552.pdf (accessed on 1 December 2022).
- EMS-CHEMIE AG. Primid ® QM-1260 Datasheet:1-2. Available online: https://www.emsgriltech.com/fileadmin/ems-griltech/documents/Datasheet/Technical_Datasheet_QM-1260.pdf (accessed on 1 December 2022).
- Bol, C. Cross-Linking of Polyamide Materials. MSc Thesis, Politecnico Di Milano, Milano, Italy, 2016. [Google Scholar]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro- and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Luo, S.; Cao, J.; McDonald, A.G. Esterification of industrial lignin and its effect on the resulting poly(3-hydroxybutyrate-co-3-hydroxyvalerate) or polypropylene blends. Ind. Crop. Prod. 2017, 97, 281–291. [Google Scholar] [CrossRef]




| Sample | Cross-Linker in TcC/PA1010 50:50 wt% | Processability | |
|---|---|---|---|
| Type | Conc (pph) | ||
| TcC/PA1010 | - | - | Easy |
| TcC/PA1010/PdX-1 | Perkadox 30 | 1 | Moderate |
| TcC/PA1010/PdX-2 | Perkadox 30 | 2 | Moderate |
| TcC/PA1010/PdX-3 | Perkadox 30 | 3 | Moderate |
| TcC/PA1010/TnX-1 | Triganox 311 | 1 | Moderate |
| TcC/PA1010/TnX-2 | Triganox 311 | 2 | Difficult |
| TcC/PA1010/TnX-3 | Triganox 311 | 3 | Difficult |
| TcC/PA1010/PmD552-1 | Primid XL-552 | 1 | Easy |
| TcC/PA1010/PmD552-5 | Primid XL-552 | 5 | Easy |
| TcC/PA1010/PmD552-10 | Primid XL-552 | 10 | Moderate |
| TcC/PA1010/PmD1260-1 | Primid QM-1260 | 1 | Easy |
| TcC/PA1010/PmD1260-5 | Primid QM-1260 | 5 | Easy |
| TcC/PA1010/PmD1260-10 | Primid QM-1260 | 10 | Moderate |
| Sample | Spinnability | Brittleness/Strength |
|---|---|---|
| TcC/PA1010 | Good | Strong |
| TcC/PA1010/PdX-0.5_PC | Poor | Very brittle |
| TcC/PA1010/TnX-0.5_PC | Good | Strong |
| TcC/PA1010/PmD552-0.5_PC | Good | Strong |
| TcC/PA1010/PmD1260-0.5_PC | Good | Strong |
| Samples | DSC | DMA | TGA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | Tg (°C) | TOnset (°C) | TMax (°C) | CY* at 880 °C (%) | |||
| TcC/PA1010 | 186,197 | 33.6 | 174 | 34.6 | 68, 146 | 337 | 457 | 19 (17.0) | ||
| TcC/PA1010/PdX-1 | 184,194 | 37.7 | 174 | 35.2 | *, 147 | 328 | 458 | 19 (16.8) | ||
| TcC/PA1010/PdX -2 | 184,194 | 35.3 | 174 | 37.5 | *, 145 | 317 | 456 | 20 (16.7) | ||
| TcC/PA1010/PdX-3 | 183,193 | 33.8 | 172 | 34.8 | *, 144 | 299 | 454 | 18 (16.5) | ||
| TcC/PA1010/TnX-1 | 183,193 | 34.4 | 171 | 35.1 | *, 146 | 331 | 449 | 19 (17.0) | ||
| TcC/PA1010/TnX-2 | 184,193 | 33.8 | 172 | 34.1 | *, 145 | 311 | 456 | 18 (16.7) | ||
| TcC/PA1010/TnX-3 | 183,193 | 32.5 | 171 | 33.9 | *, 146 | 310 | 449 | 21 (16.5) | ||
| TcC/PA1010/PmD552-1 | 185,196 | 35.9 | 175 | 33.6 | 66, 152 | 322 | 412 | 24 (16.8) | ||
| TcC/PA1010 /PmD552-5 | 186,195 | 28.6 | 175 | 27.8 | 67, 126 | 330 | 447 | 23 (16.2) | ||
| TcC/PA1010/PmD552-10 | 187,194 | 26.6 | 175 | 26.4 | 68, 109 | 325 | 452 | 21 (15.3) | ||
| TcC/PA1010 /PmD1260-1 | 184,194 | 33.1 | 173 | 31.3 | 68, 150 | 322 | 416 | 23 (16.8) | ||
| TcC/PA1010/PmD1260-5 | 185,195 | 32.6 | 173 | 29.8 | 69, 134 | 325 | 455 | 19 (16.2) | ||
| TcC/PA1010/PmD1260-10 | 187,195 | 28.9 | 174 | 29.1 | 70, 112 | 306 | 441 | 21 (15.3) | ||
| Sample | Tensile Strength (MPa) | Tensile Modulus (GPa) | Strain-at-Break (%) |
|---|---|---|---|
| TcC/PA1010 | 39 ± 9 | 2.0 ± 0. 6 | 25 |
| TcC/PA1010/TnX-0.5_PC | 42 ± 10 | 2.3 ± 0.3 | 3 |
| TcC/PA1010/PmD552-0.5_PC | 34 ± 9 | 1.9 ± 0.3 | 2 |
| TcC/PA1010/PmD1260-0.5_PC | 33 ± 8 | 2.3 ± 0.3 | 8 |
| Sample | TOnset (°C) | TMax (°C) | Char Yield at 880 °C (%) |
|---|---|---|---|
| TcC/PA1010 | 316 | 427 | 30.7 |
| TcC/PA1010-PmD 552_0.5% | 320 | 405,453 | 30.4 |
| TcC/PA1010-PmD 552_1% | 331 | 420,456 | 34.4 |
| TcC/PA1010-PmD 1260_0.5% | 326 | 422,451 | 30.7 |
| TcC/PA1010-PmD 1260_1% | 327 | 426,456 | 32.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandola, B.K.; Hewage, T.A.M.; Hajee, M.; Horrocks, A.R. Effect of Cross-Linkers on the Processing of Lignin/Polyamide Precursors for Carbon Fibres. Fibers 2023, 11, 16. https://doi.org/10.3390/fib11020016
Kandola BK, Hewage TAM, Hajee M, Horrocks AR. Effect of Cross-Linkers on the Processing of Lignin/Polyamide Precursors for Carbon Fibres. Fibers. 2023; 11(2):16. https://doi.org/10.3390/fib11020016
Chicago/Turabian StyleKandola, Baljinder K., Trishan A. M. Hewage, Muhammed Hajee, and A. Richard Horrocks. 2023. "Effect of Cross-Linkers on the Processing of Lignin/Polyamide Precursors for Carbon Fibres" Fibers 11, no. 2: 16. https://doi.org/10.3390/fib11020016
APA StyleKandola, B. K., Hewage, T. A. M., Hajee, M., & Horrocks, A. R. (2023). Effect of Cross-Linkers on the Processing of Lignin/Polyamide Precursors for Carbon Fibres. Fibers, 11(2), 16. https://doi.org/10.3390/fib11020016








