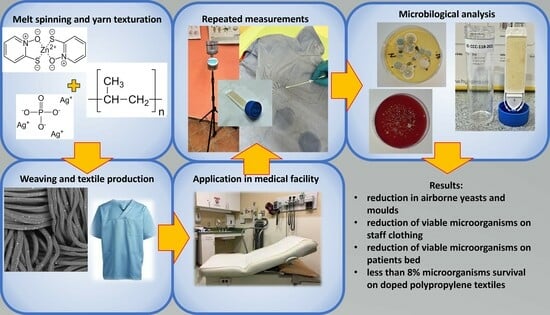
Field Study of Activity of Antimicrobial Polypropylene Textiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Textile Samples Preparation
2.2.2. Replacement of Staff Clothing and Bedding in a Small Surgery Department and Frequency of Measurements
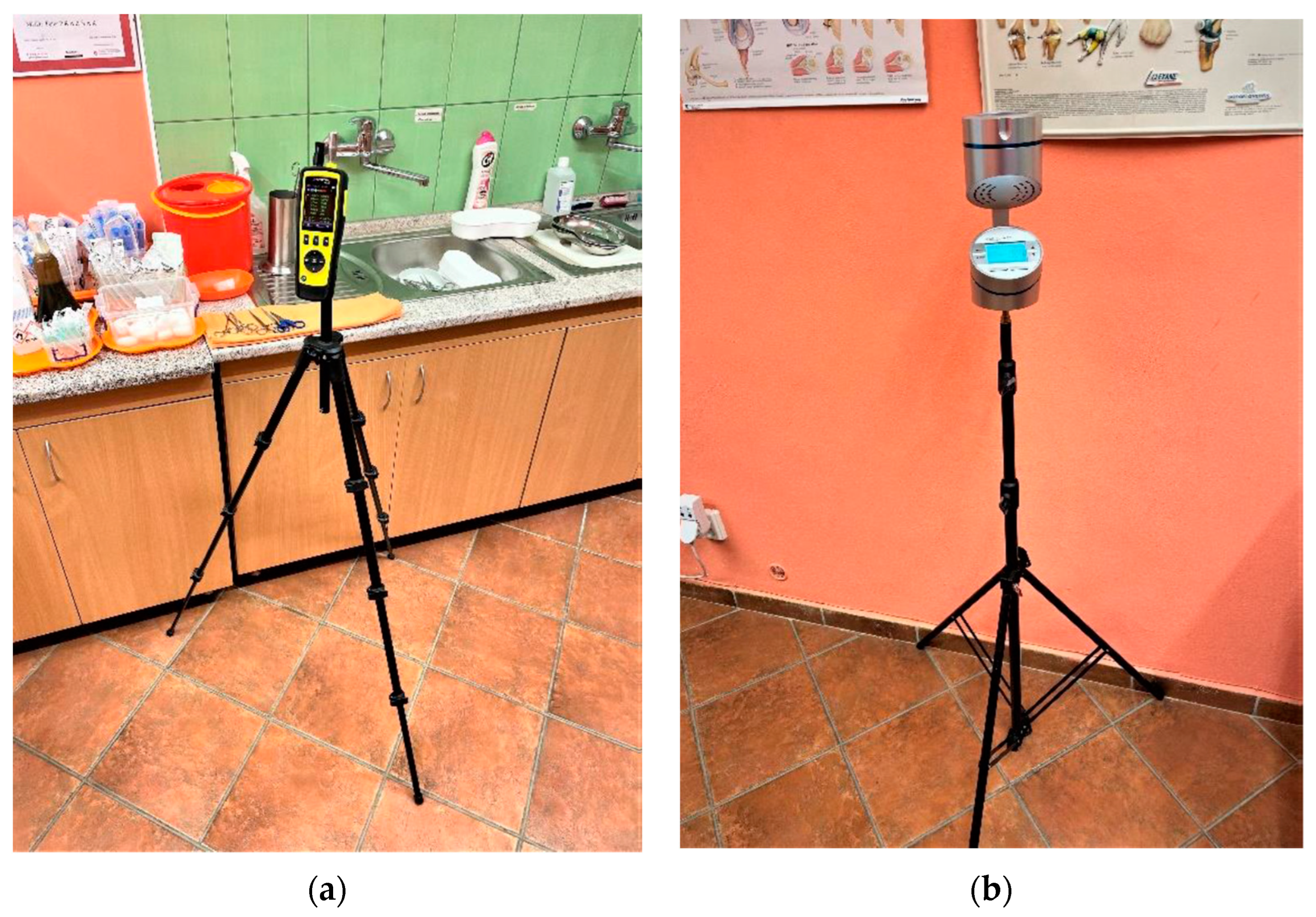
2.2.3. Evaluation of Impact on Airborne Particles, Airborne Microorganisms, and Viable Microorganisms on Textile Samples
3. Results
3.1. Preparation of Yarns and Vowen Textile
3.2. Evaluation of Airborne Particles and Airborne Microorganisms
3.3. Quantification and Identification of Microorganisms Present on Textile Surfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cummings, K.L.; Anderson, D.J.; Kaye, K.S. Hand hygiene noncompliance and the cost of hospital-acquired methicillin-resistant Staphylococcus aureus infection. Infect. Control Hosp. Epidemiol. 2010, 31, 357–364. [Google Scholar] [CrossRef]
- Stewart, S.; Robertson, C.; Pan, J.; Kennedy, S.; Dancer, S.; Haahr, L.; Reilly, J. Epidemiology of healthcare-associated infection reported from a hospital-wide incidence study: Considerations for infection prevention and control planning. J. Hosp. Infect. 2021, 114, 10–22. [Google Scholar] [CrossRef]
- Weiner-Lastinger, L.M.; Abner, S.; Edwards, J.R.; Kallen, A.J.; Karlsson, M.; Magill, S.S.; Dudeck, M.A. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control. Hosp. Epidemiol. 2020, 41, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Suetens, C.; Kärki, T.; Plachouras, D. ECDC surveillance report. In Point Prevalence Survey of Healthcare Associated Infections and Antimicrobial Use in European Acute Care Hospitals 2016–2017; ECDC: Stockholm, Sweden, 2023; pp. 9–13. ISBN 978-92-9498-636-8. [Google Scholar]
- Štefkovičová, M.; Litvová, S.; Mikas, J.; Kopilec Garabášová, M.; Jamrichová, M.; Prostináková, Z. Second Point Prevalence Survey of Healthcare-Associated Infections in The Slovak Republic as a Part of The European Survey. Zdr. Listy 2019, 7, 12–18. [Google Scholar]
- Kampf, G. How long can nosocomial pathogens survive on textiles? A systematic review. GMS Hyg. Infect. Control. 2020, 15, 1–15. [Google Scholar] [CrossRef]
- Ljungqvist, B.; Reinmüller, B. Some observations on protective efficacy of surgical clothing systems with additional clothing components concerning airborne bacteria-carrying particles measured during ongoing surgery. Eur. J. Parenter. Pharm. Sci. 2021, 26, 1–14. [Google Scholar] [CrossRef]
- Ullmann, C.; Ljungqvist, B.; Reinmüller, B. Some aspects of protective efficacy of surgical clothing systems concerning airborne microorganisms based on results from measurements in a dispersal chamber and during surgical procedures. Eur. J. Parenter. Pharm. Sci. 2017, 22, 51–58. [Google Scholar]
- Mitchell, A.; Spencer, M.; Edmiston, C., Jr. Role of healthcare apparel and other healthcare textiles in the transmission of pathogens: A review of the literature. J. Hosp. Infect. 2015, 90, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Botelho, C.M.; Fernandes, M.M.; Souza, J.M.; Dias, N.; Sousa, A.M.; Teixeira, J.A.; Fangueiro, R.; Zille, A. New Textile for Personal Protective Equipment—Plasma Chitosan/Silver Nanoparticles Nylon Fabric. Fibers 2021, 9, 5:1–5:13. [Google Scholar] [CrossRef]
- Gomes, L.; Monteiro, P.; Cotas, J.; Gonçalves, A.M.; Fernandes, C.; Gonçalves, T.; Pereira, L. Seaweeds’ pigments and phenolic compounds with antimicrobial potential. Biomol. Concepts 2022, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Staneva, D.; Atanasova, D.; Angelova, D.; Grozdanov, P.; Nikolova, I.; Grabchev, I. Antimicrobial Properties of Chitosan-Modified Cotton Fabric Treated with Aldehydes and Zinc Oxide Particles. Materials 2023, 16, 5090. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Regulation (Ec) No 1272/2008 of the European Parliament and of the Council: Regulation on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006; Official Journal of the European Union: Brussels, Belgium, 2008. [Google Scholar]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef]
- Rajski, L.; Juda, M.; Los, A.; Witun, E.; Malm, A. Medical textiles with silver/nanosilver and their potential application for the prevention and control of healthcare-associated infections–mini-review. Curr. Issues Pharm. Med. Sci. 2019, 32, 104–107. [Google Scholar] [CrossRef]
- van Arkel, A.; Willemsen, I.; Kluytmans, J. The correlation between ATP measurement and microbial contamination of inanimate surfaces. Antimicrob. Resist. Infect. Control. 2021, 10, 116. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Lee, M.-J. Poly (Methyl Methacrylate)-Containing Silver-Phosphate Glass Exhibits Potent Antimicrobial Activity without Deteriorating the Mechanical and Biological Properties of Dental Prostheses. Polymers 2023, 15, 297. [Google Scholar] [CrossRef] [PubMed]
- Novi, V.T.; Gonzalez, A.; Brockgreitens, J.; Abbas, A. Highly efficient and durable antimicrobial nanocomposite textiles. Sci. Rep. 2022, 12, 17332. [Google Scholar] [CrossRef] [PubMed]
- Kumah, E.A.; Fopa, R.D.; Harati, S.; Boadu, P.; Zohoori, F.V.; Pak, T. Human and environmental impacts of nanoparticles: A scoping review of the current literature. BMC Public Health 2023, 23, 1059. [Google Scholar] [CrossRef] [PubMed]
- Official Journal of the European Union. Commission Regulation (EU) 2021/1902 of 29 October 2021 Amending Annexes II, III and V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council as Regards the Use in Cosmetic Products of Certain Substances Classified as Carcinogenic, Mutagenic or Toxic for Reproduction (Text with EEA Relevance); Official Journal of the European Union: Brussels, Belgium, 2021. [Google Scholar]


| Yarn Color | Additive | Additive Masterbatch | Pigment Masterbatch | Polypropylene |
|---|---|---|---|---|
| Purple | SPG | 250 | 1560 | 8190 |
| ZP | 200 | 1560 | 8240 | |
| Light blue | SPG | 250 | 1200 | 8550 |
| ZP | 200 | 1200 | 8600 | |
| Beige | SPG | 250 | 1560 | 8190 |
| ZP | 200 | 1560 | 8240 |
| Yarn Color | Additive | Relative Tenacity (cN/dtex) | Strain at Break (%) |
|---|---|---|---|
| Purple | SPG | 2.61 | 82.1 |
| ZP | 2.60 | 81.6 | |
| Light blue | SPG | 2.79 | 35.8 |
| ZP | 2.71 | 34.2 | |
| Beige | SPG | 2.87 | 39.8 |
| ZP | 2.63 | 39.3 |
| Particle Size | |||||||
|---|---|---|---|---|---|---|---|
| No. | 0.3 µm | 0.5 µm | 1.0 µm | 2.5 µm | 5.0 µm | 10 µm | Conditions |
| 0 | 72,613 | 22,688 | 6119 | 1296 | 247 | 129 | 21.9 °C/47.9% |
| 1 | 109,189 | 29,491 | 5425 | 971 | 168 | 76 | 22.1 °C/49.3% |
| 2 | 137,560 | 36,561 | 6226 | 1080 | 165 | 83 | 22.3 °C/48.1% |
| 3 | 106,454 | 29,580 | 5923 | 1116 | 193 | 96 | 22.1 °C/47.7% |
| Particle Size | |||||||
|---|---|---|---|---|---|---|---|
| No. | 0.3 µm | 0.5 µm | 1.0 µm | 2.5 µm | 5.0 µm | 10 µm | Conditions |
| 0 | 90,911 | 27,366 | 6053 | 1055 | 150 | 83 | 21.9 °C/47.9% |
| 1 | 117,653 | 32,873 | 6273 | 1124 | 171 | 85 | 22.1 °C/49.3% |
| 2 | 130,963 | 35,359 | 6450 | 1133 | 157 | 84 | 22.3 °C/48.1% |
| 3 | 56,692 | 16,954 | 3766 | 661 | 93 | 49 | 22.1 °C/47.7% |
| No. of Measurement | Yeasts and Molds | Cultivated on Blood Medium | Cultivated on GTK Medium |
|---|---|---|---|
| 0 | 52 | >535 | >535 |
| 1 | 24 | >535 | >535 |
| 2 | 55 | >535 | >535 |
| 3 | 55 | >535 | >535 |
| No. of Measurement | Yeasts and Molds | Cultivated on Blood Medium | Cultivated on GTK Medium |
|---|---|---|---|
| 0 | 57 | >535 | >535 |
| 1 | 23 | >535 | >535 |
| 2 | 37 | >535 | >535 |
| 3 | 32 | >535 | >535 |
| No.* | Bedding in Room No. 1 | Bedding in Room No. 2 | Tunic, Nurse | Tunic, Physician | Conditions ** |
|---|---|---|---|---|---|
| 0 | 400 | 106 | 67 | 72 | 21.9 °C/47.9% |
| 1 | 54 | 1 | 19 | 40 | 22.1 °C/49.3% |
| 2 | 16 | 3 | 21 | 14 | 22.3 °C/48.1% |
| 3 | 8 | 13 | 57 | 9 | 22.1 °C/47.7% |
| Result | Bedding in Room No. 1 | Bedding in Room No. 2 | Tunic, Nurse | Tunic, Physician |
|---|---|---|---|---|
| 0 | 400 | 106 | 67 | 72 |
| Average 1–3 | 26 | 6 | 32 | 21 |
| Reduction | −94% | −94% | −52% | −71% |
| Bedding in Room No. 1 | Bedding in Room No. 2 | Tunic—Nurse | Tunic—Physician | |||||
|---|---|---|---|---|---|---|---|---|
| No. of Sampling | Yeasts and Bacteria | Molds | Yeasts and Bacteria | Molds | Yeasts and Bacteria | Molds | Yeasts and Bacteria | Molds |
| 0 | 3 | 3 | 4 | 4 | 4 | 3 | 4 | 3 |
| 1 | 3 | 2 | 3 | 3 | 3 | 2 | 4 | 3 |
| 2 | 3 | 2 | 3 | 0 | 3 | 2 | 3 | 2 |
| 3 | 3 | 0 | 3 | 2 | 3 | 2 | 3 | 0 |
| 4 | 3 | 0 | 3 | 2 | 3 | 0 | 3 | 2 |
| No. of Measurement | CFU per Contact Plate | Identified Organisms | Conditions ** |
|---|---|---|---|
| 0 | 60 | aerobic sporulants, saprophytic staphylococci, micrococci, and two CFU micromycetes | 21.9 °C/47.9% |
| 1 | 47 | aerobic sporulants, saprophytic staphylococci, micrococci, and bacillus cereus | 22.1 °C/49.3% |
| 2 | 22 | aerobic sporulants, saprophytic staphylococci, and micrococci | 22.3 °C/48.1% |
| 3 | 2 | aerobic sporulants | 22.1 °C/47.7% |
| 4 | 3 * | saprophytic staphylococci | 21.8 °C/47.8% |
| No. of Measurement | CFU per Contact Plate | Identified Organisms | Conditions ** |
|---|---|---|---|
| 0 | 96 | aerobic sporulants, saprophytic staphylococci, and micrococci | 21.9 °C/47.9% |
| 1 | 3 * | saprophytic staphylococci | 22.1 °C/49.3% |
| 2 | 5 * | micrococci | 22.3 °C/48.1% |
| 3 | 1 * | micrococci | 22.1 °C/47.7% |
| 4 | 14 | aerobic sporulants, saprophytic staphylococci, and micrococci | 21.8 °C/47.8% |
| No. of Measurement | CFU per Contact Plate | Identified Organisms | Conditions *** |
|---|---|---|---|
| 0 | 100 ** | aerobic sporulants, saprophytic staphylococci, micrococci, and one CFU micromycetes | 21.9 °C/47.9% |
| 1 | 100 ** | aerobic sporulants, saprophytic staphylococci, micrococci, Streptococcus sp., and two CFU micromycetes | 22.1 °C/49.3% |
| 2 | 100 ** | aerobic sporulants, saprophytic staphylococci, and micrococci | 22.3 °C/48.1% |
| 3 | 100 ** | aerobic sporulants, saprophytic staphylococci, and micrococci | 22.1 °C/47.7% |
| 4 | 30 | aerobic sporulants, saprophytic staphylococci, micrococci, Acinetobacter sp., and Pantoea sp. | 21.8 °C/47.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balogová, A.; Bizubová, B.; Kleščík, M.; Zatroch, T. Field Study of Activity of Antimicrobial Polypropylene Textiles. Fibers 2023, 11, 97. https://doi.org/10.3390/fib11110097
Balogová A, Bizubová B, Kleščík M, Zatroch T. Field Study of Activity of Antimicrobial Polypropylene Textiles. Fibers. 2023; 11(11):97. https://doi.org/10.3390/fib11110097
Chicago/Turabian StyleBalogová, Alena, Bibiána Bizubová, Michal Kleščík, and Tomáš Zatroch. 2023. "Field Study of Activity of Antimicrobial Polypropylene Textiles" Fibers 11, no. 11: 97. https://doi.org/10.3390/fib11110097
APA StyleBalogová, A., Bizubová, B., Kleščík, M., & Zatroch, T. (2023). Field Study of Activity of Antimicrobial Polypropylene Textiles. Fibers, 11(11), 97. https://doi.org/10.3390/fib11110097