A Comparison of Chitosan Adhesion to KOH and H2O2 Pre-Treated Electrospun Poly(3-Hydroxybutyrate) Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparing PHB and CS Solutions
2.3. Fabrication of PHB Electrospun Fibers
2.4. Surface Functionalization of Electrospun Fibrous PHB Membranes with DA and GA
2.5. Scanning Electron Microscopy (SEM)
2.6. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.7. Thermalgravimetric Analysis (TGA)
2.8. Differential Scanning Calorimetry (DSC)
2.9. Contact Angle Analysis
2.10. BET Analysis of Porosity
2.11. Stability Analysis of CS on KOH and H2O2-Treated PHB-DA-GA-CS Electrospun Fibers
2.12. Mechanical Tests
2.13. Statistical Analysis
3. Results and Discussion
3.1. Morphology and Size of Electrospun Membranes with Varying CS Concentrations (0.2–2 w/v%) in PHB-KOH/H2O2
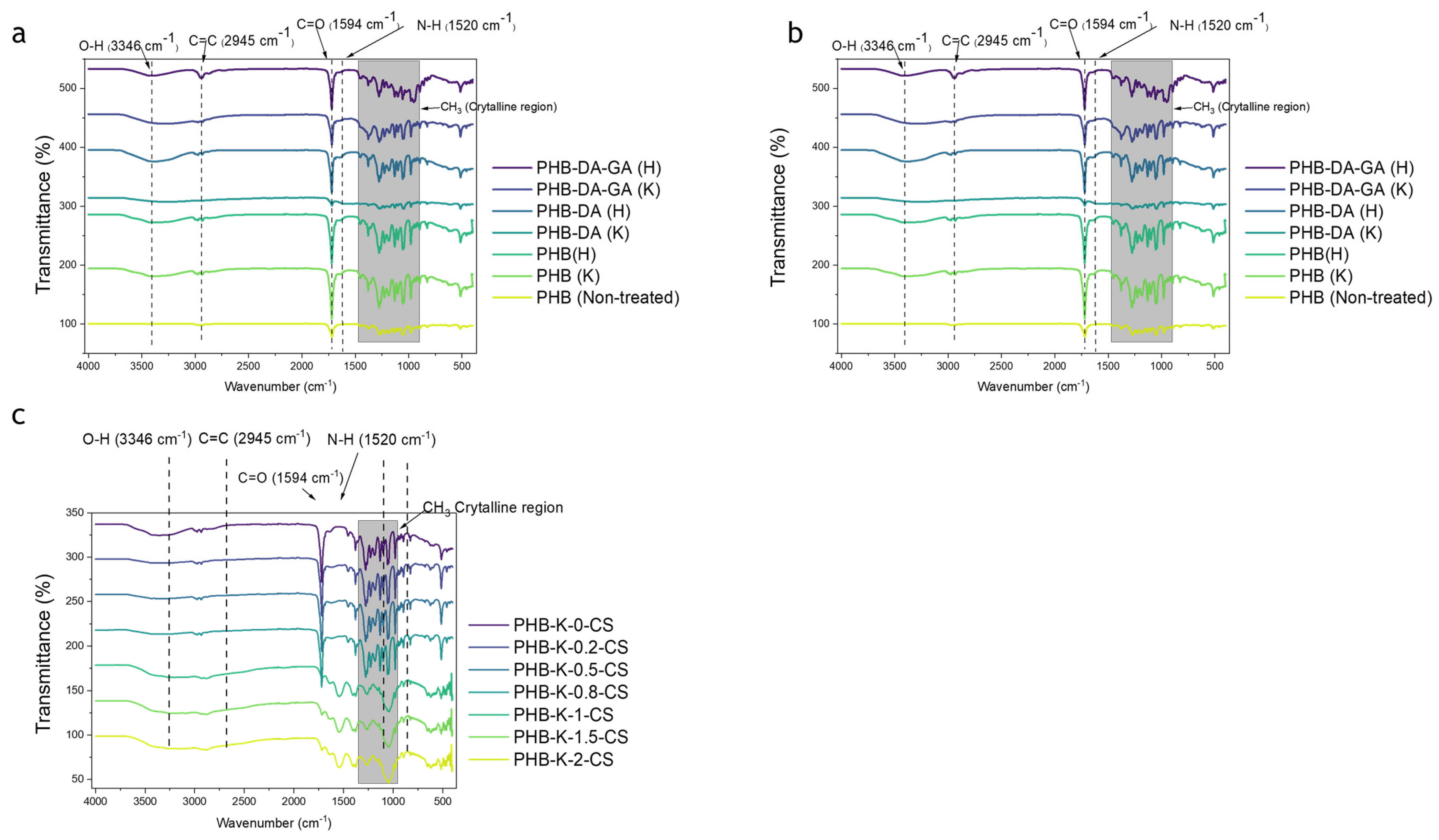
3.2. Total Reflectance-Fourier Transform Infrared (ATR-FTIR)
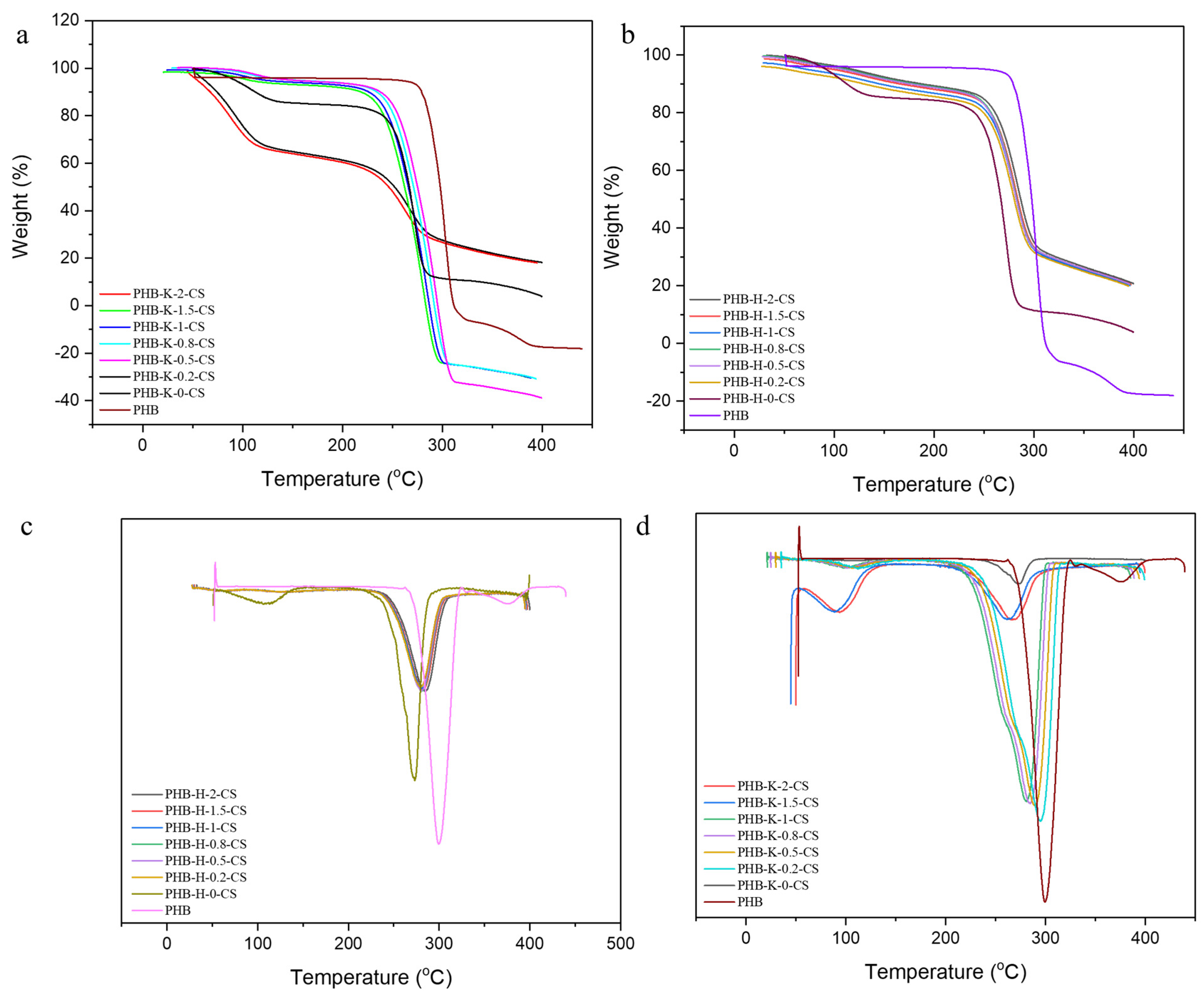
3.3. TGA and DTA Analysis of PHB-CS Electrospun Fibers
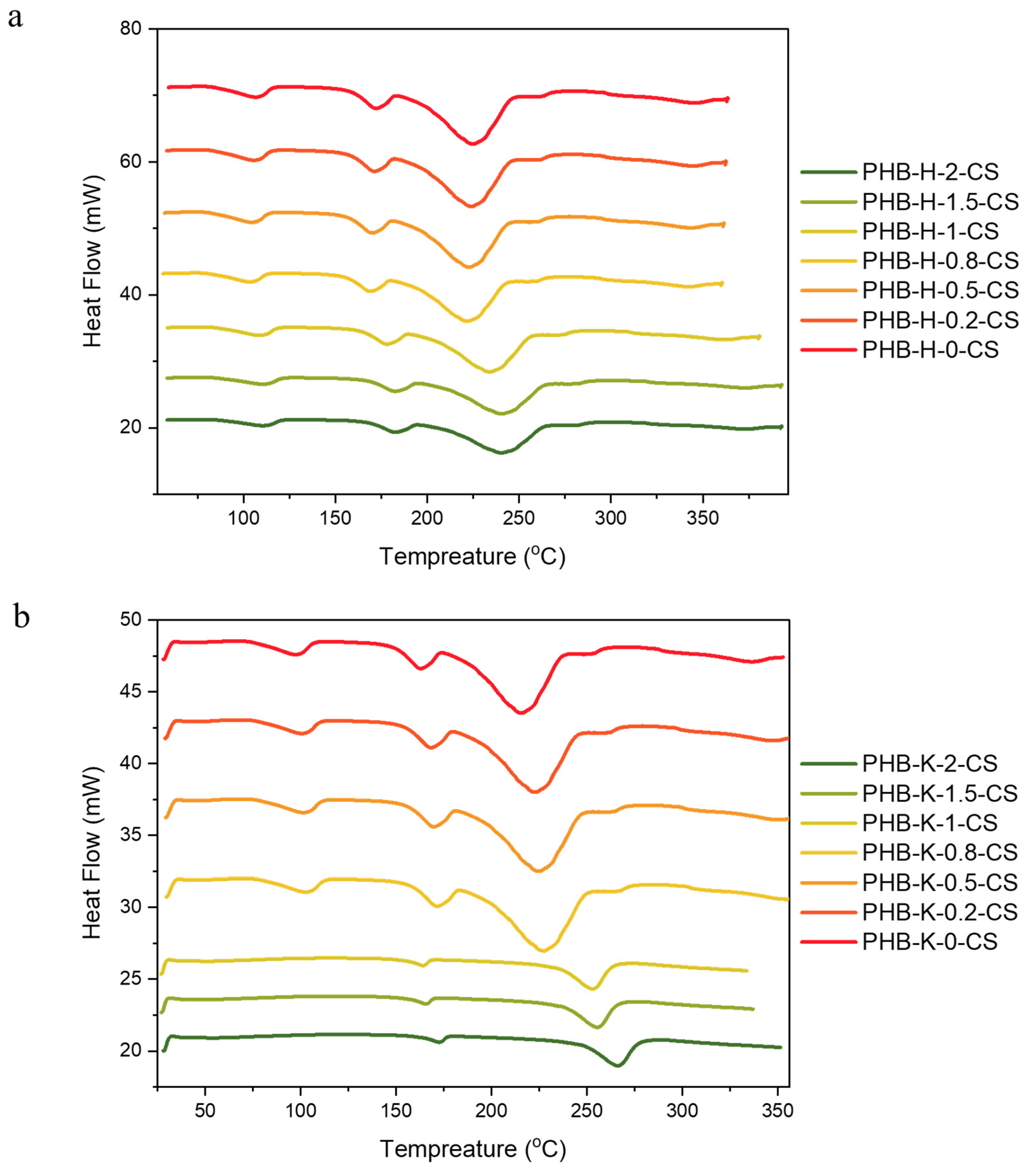
3.4. DSC Analysis of PHB-CS Electrospun Fibers
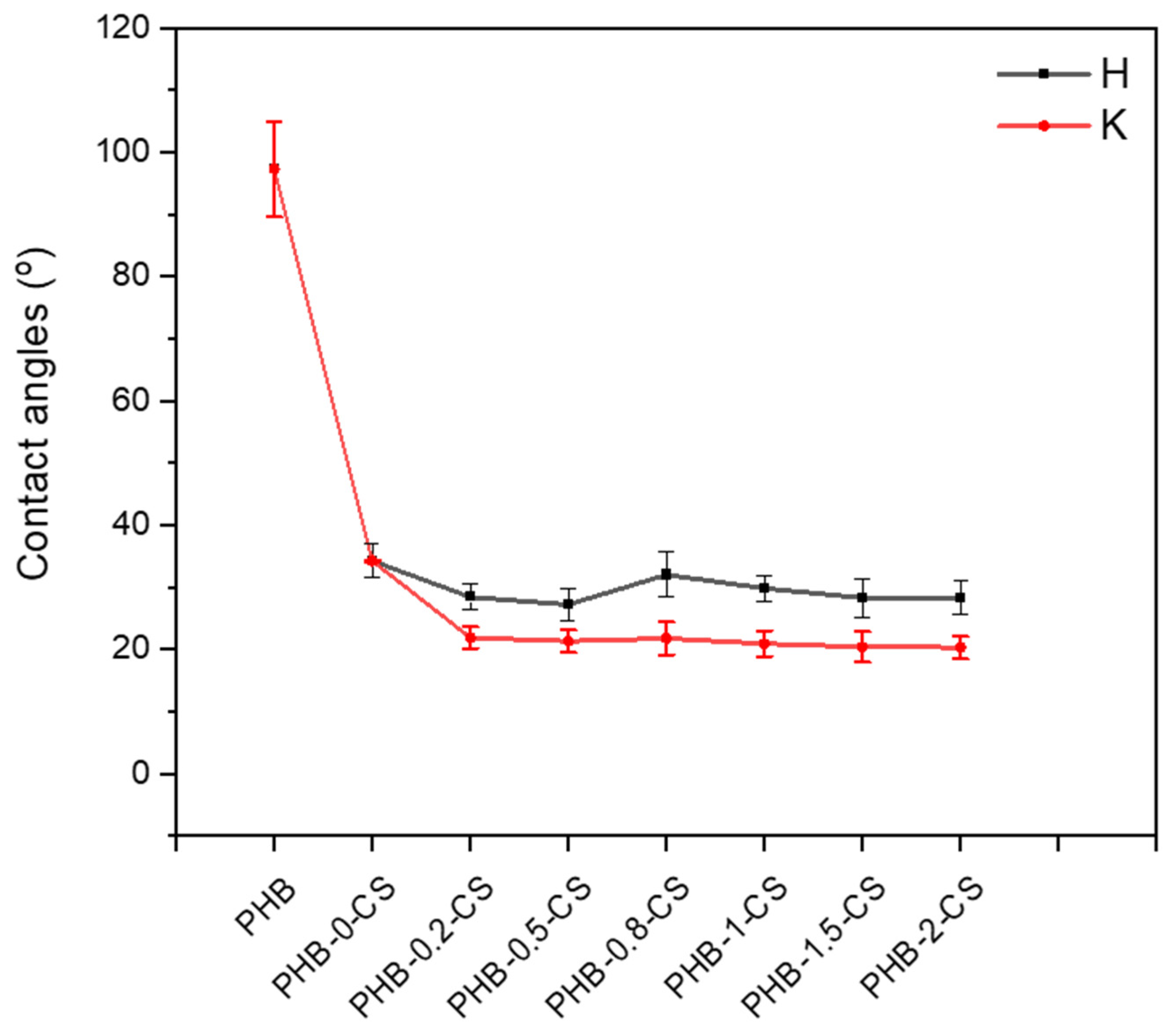
3.5. Hydrophilicity Analysis of PHB-CS Electrospun Fibers with Contact Angle Measurements
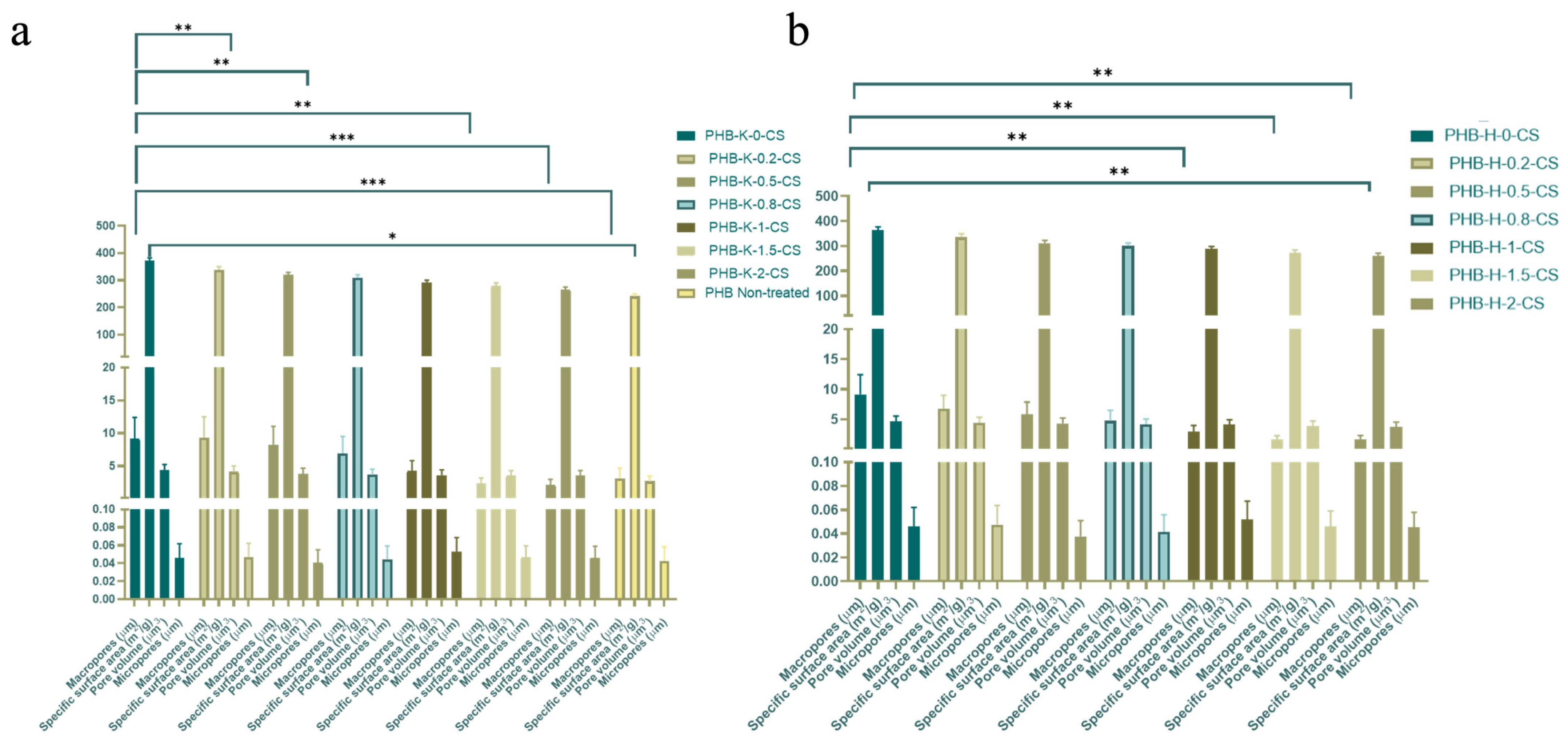
3.6. Analysis of Porosity and Specific Surface Area of PHB-CS Electrospun Fibers Using the BET Method
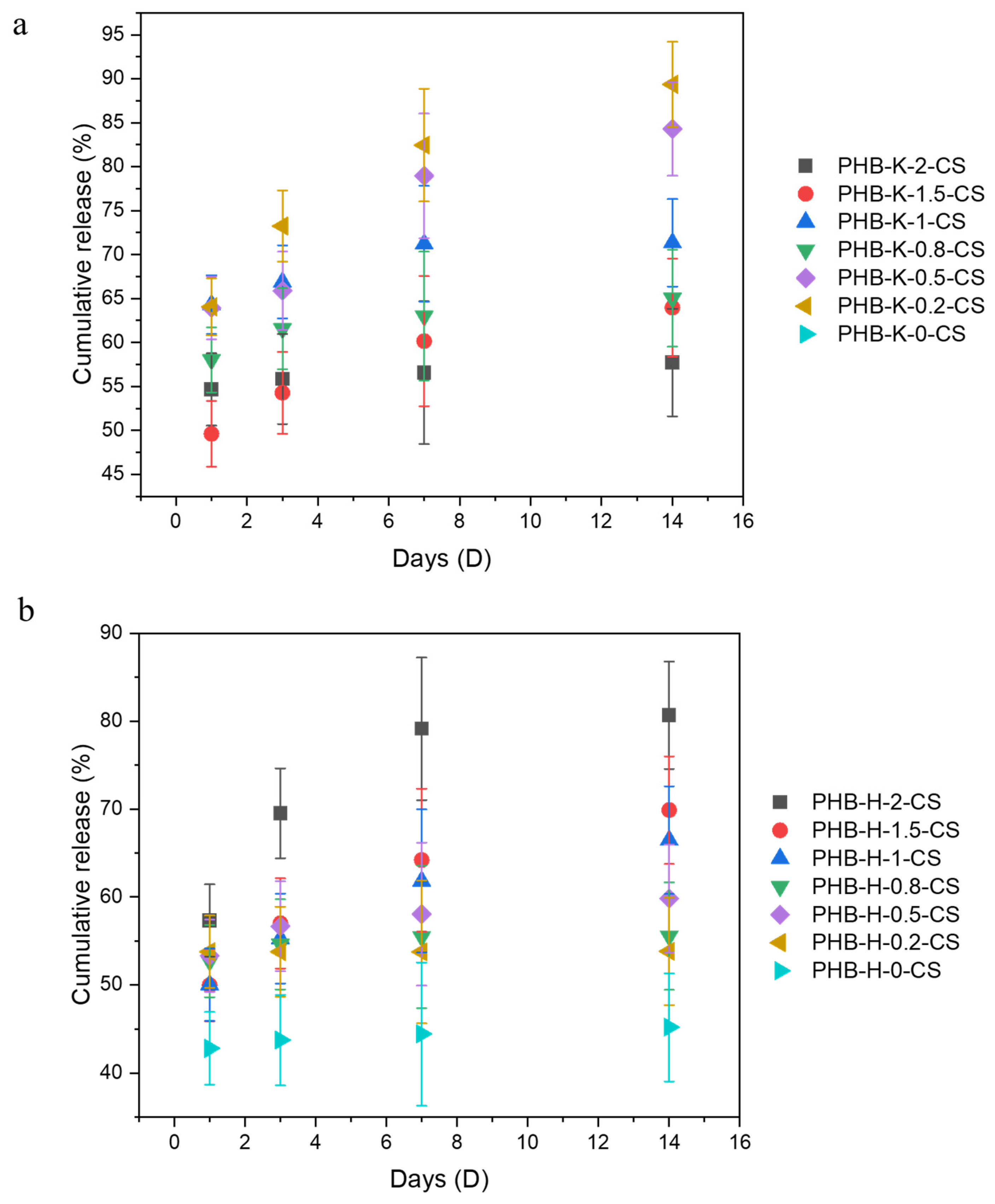
3.7. Stability Assessment of KOH/H2O2 PHB-DA-GA-CS Electrospun Samples: UV-Vis and CBR Analytical Techniques
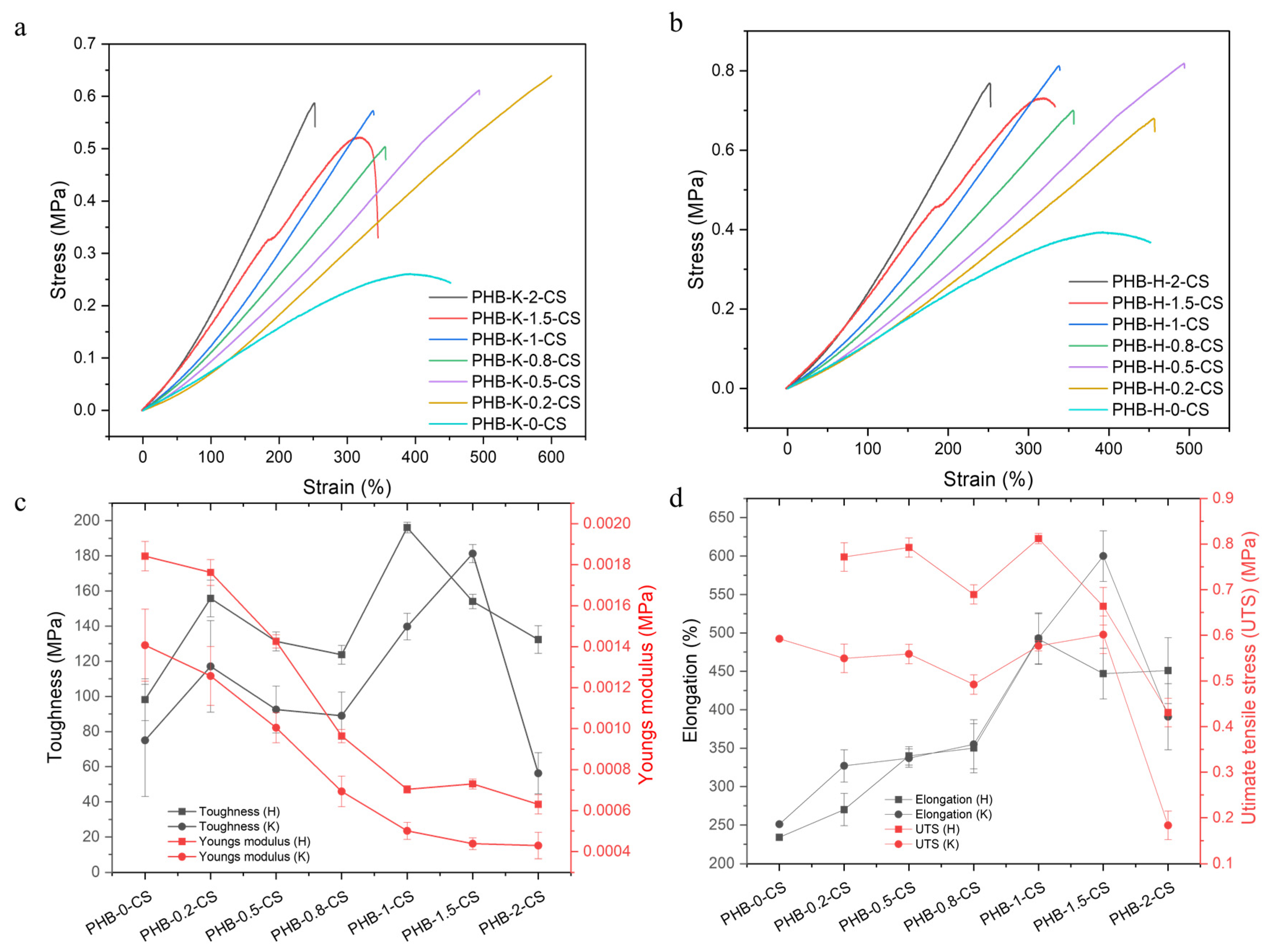
3.8. Mechanical Properties of KOH- and H2O2-Treated PHB Electrospun Nanofibers with Varying CS Concentrations: Tensile Strength, Toughness, Young’s Modulus, and Elongation at Break
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sasidharan, R.S.; Bhat, S.G.; Chandrasekaran, M. Biocompatible polyhydroxybutyrate (PHB) production by marine Vibrio azureus BTKB33 under submerged fermentation. Ann. Microbiol. 2014, 65, 455–465. [Google Scholar] [CrossRef]
- Mohammadalipour, M.; Karbasi, S.; Behzad, T.; Mohammadalipour, Z.; Zamani, M. Effect of cellulose nanofibers on polyhydroxybutyrate electrospun scaffold for bone tissue engineering applications. Int. J. Biol. Macromol. 2022, 220, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Demcisakova, Z.; Luptakova, L.; Tirpakova, Z.; Kvasilova, A.; Medvecky, L.; De Spiegelaere, W.; Petrovova, E. Evaluation of Angiogenesis in an Acellular Porous Biomaterial Based on Polyhydroxybutyrate and Chitosan Using the Chicken Ex Ovo Chorioallantoic Membrane Model. Cancers 2022, 14, 4194. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, M.; Karbasi, S. Electrospun halloysite nanotube loaded polyhydroxybutyrate-starch fibers for cartilage tissue engineering. Int. J. Biol. Macromol. 2022, 214, 301–311. [Google Scholar] [CrossRef]
- Lezcano, M.F.; Álvarez, G.; Chuhuaicura, P.; Godoy, K.; Alarcón, J.; Acevedo, F.; Gareis, I.; Dias, F.J. Polyhydroxybutyrate (PHB) Scaffolds for Peripheral Nerve Regeneration: A Systematic Review of Animal Models. Biology 2022, 11, 706. [Google Scholar] [CrossRef]
- Guo, W.; Yang, K.; Qin, X.; Luo, R.; Wang, H.; Huang, R. Polyhydroxyalkanoates in tissue repair and regeneration. Eng. Regen. 2022, 3, 24–40. [Google Scholar] [CrossRef]
- Trujillo-Miranda, M.; Apsite, I.; Agudo, J.A.R.; Constante, G.; Ionov, L. 4D Biofabrication of Mechanically Stable Tubular Constructs Using Shape Morphing Porous Bilayers for Vascularization Application. Macromol. Biosci. 2022, 23, e2200320. [Google Scholar] [CrossRef]
- Hetemi, D.; Pinson, J. Surface functionalisation of polymers. Chem. Soc. Rev. 2017, 46, 5701–5713. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Li, D.; Yin, Y.; Zhou, F. Electrospun PHB/Chitosan Composite Fibrous Membrane and Its Degradation Behaviours in Different pH Conditions. J. Funct. Biomater. 2022, 13, 58. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Abdelhakim, H.E. Drug Delivery Applications of Coaxial Electrospun Nanofibres in Cancer Therapy. Molecules 2022, 27, 1803. [Google Scholar] [CrossRef]
- Righetti, M.C.; Cinelli, P.; Aliotta, L.; Bianchi, E.; Tricoli, F.; Seggiani, M.; Lazzeri, A. Immiscible PHB/PB S and PHB/PBSA blends: Morphology, phase composition and modelling of elastic modulus. Polym. Int. 2022, 71, 47–56. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Veiga, M.D. Applications of Chitosan in Surgical and Post-Surgical Materials. Mar. Drugs 2022, 20, 396. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Yun, S.I. Chitosan-reinforced PHB hydrogel and aerogel monoliths fabricated by phase separation with the solvent-exchange method. Carbohydr. Polym. 2022, 284, 119184. [Google Scholar] [CrossRef] [PubMed]
- Seddighian, A.; Ganji, F.; Baghaban-Eslaminejad, M.; Bagheri, F. Electrospun PCL scaffold modified with chitosan nanoparticles for enhanced bone regeneration. Prog. Biomater. 2021, 10, 65–76. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, W.; Tremblay, P.L.; Zhang, T. Electrostimulation of fibroblast proliferation by an electrospun poly (lactide-co-glycolide)/polydopamine/chitosan membrane in a humid environment. Colloids Surf. B Biointerfaces 2022, 220, 112902. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, K.; Chen, G.-Q. Effect of surface treatment on the biocompatibility of microbial polyhydroxyalkanoates. Biomaterials 2001, 23, 1391–1397. [Google Scholar] [CrossRef]
- Jiang, Y.; Mikova, G.; Kleerebezem, R.; van der Wielen, L.A.; Cuellar, M.C. Feasibility study of an alkaline-based chemical treatment for the purification of polyhydroxybutyrate produced by a mixed enriched culture. AMB Express 2015, 5, 5. [Google Scholar] [CrossRef]
- Rouxhet, L.; Duhoux, F.; Borecky, O.; Legras, R.; Schneider, Y.J. Adsorption of albumin, collagen, and fibronectin on the surface of poly (hydroxybutyrate-hydroxyvalerate) (PHB/HV) and of poly (ε-caprolactone) (PCL) films modified by an alkaline hydrolysis and of poly (ethylene terephtalate) (PET) track-etched membranes. J. Biomater. Sci. Polym. Ed. 1998, 9, 1279–1304. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, M.; Tao, Q.; Zhu, K.; Liu, X.; Wan, C.; Harder, M.K.; Yan, Q.; Liang, B.; Ntaikou, I.; et al. Recovery of polyhydroxyalkanoates (PHAs) polymers from a mixed microbial culture through combined ultrasonic disruption and alkaline digestion. J. Environ. Manag. 2023, 326, 116786. [Google Scholar] [CrossRef]
- Charpentier, P.A.; Maguire, A.; Wan, W.-K. Surface modification of polyester to produce a bacterial cellulose-based vascular prosthetic device. Appl. Surf. Sci. 2006, 252, 6360–6367. [Google Scholar] [CrossRef]
- Ke, Y.; Liu, C.; Zhang, X.; Xiao, M.; Wu, G. Surface Modification of Polyhydroxyalkanoates toward Enhancing Cell Compatibility and Antibacterial Activity. Macromol. Mater. Eng. 2017, 302, 1700258. [Google Scholar] [CrossRef]
- Morshed, M.N.; Behary, N.; Bouazizi, N.; Guan, J.; Chen, G.; Nierstrasz, V. Surface modification of polyester fabric using plasma-dendrimer for robust immobilization of glucose oxidase enzyme. Sci. Rep. 2019, 9, 15730. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, J.-M.; Lee, S.J.; Lee, S.G.; Jeong, Y.-K.; Kim, S.E.; Lee, S.C. Surface hydrolysis of fibrous poly(ε-caprolactone) scaffolds for enhanced osteoblast adhesion and proliferation. Macromol. Res. 2007, 15, 424–429. [Google Scholar] [CrossRef]
- Choong, C.; Yuan, S.; Thian, E.S.; Oyane, A.; Triffitt, J. Optimization of poly(ε-caprolactone) surface properties for apatite formation and improved osteogenic stimulation. J. Biomed. Mater. Res. Part A 2012, 100A, 353–361. [Google Scholar] [CrossRef]
- Chong, M.S.K.; Teoh, S.-H.; Teo, E.Y.; Zhang, Z.-Y.; Lee, C.N.; Koh, S.; Choolani, M.; Chan, J. Beyond Cell Capture: Antibody Conjugation Improves Hemocompatibility for Vascular Tissue Engineering Applications. Tissue Eng. Part A 2010, 16, 2485–2495. [Google Scholar] [CrossRef]
- Rajan, R.; Sreekumar, P.A.; Joseph, K.; Skrifvars, M. Thermal and mechanical properties of chitosan reinforced polyhydroxybutyrate composites. J. Appl. Polym. Sci. 2012, 124, 3357–3362. [Google Scholar] [CrossRef]
- Young, R.; Terenghi, G.; Wiberg, M. Poly-3-hydroxybutyrate (PHB): A resorbable conduit for long-gap repair in peripheral nerves. Br. J. Plast. Surg. 2002, 55, 235–240. [Google Scholar] [CrossRef]
- Ikejima, T.; Inoue, Y. Crystallization behavior and environmental biodegradability of the blend films of poly(3-hydroxybutyric acid) with chitin and chitosan. Carbohydr. Polym. 2000, 41, 351–356. [Google Scholar] [CrossRef]
- Kühn, S.; van Werven, B.; van Oyen, A.; Meijboom, A.; Rebolledo, E.L.B.; van Franeker, J.A. The use of potassium hydroxide (KOH) solution as a suitable approach to isolate plastics ingested by marine organisms. Mar. Pollut. Bull. 2017, 115, 86–90. [Google Scholar] [CrossRef]
- Mothes, G.; Schnorpfeil, C.; Ackermann, J.-U. Production of PHB from Crude Glycerol. Eng. Life Sci. 2007, 7, 475–479. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Lin, T.T.; Koh, J.J.; Low, L.W.; Tan, B.H.; Li, Z.; He, C. Insights into the nucleation and crystallization analysis of PHB-rubber toughened PLA biocomposites. Compos. Commun. 2021, 27, 100894. [Google Scholar] [CrossRef]
- Saratale, G.D.; Oh, M.-K. Characterization of poly-3-hydroxybutyrate (PHB) produced from Ralstonia eutropha using an alkali-pretreated biomass feedstock. Int. J. Biol. Macromol. 2015, 80, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Schaub, N.J.; Le Beux, C.; Miao, J.; Linhardt, R.J.; Alauzun, J.G.; Laurencin, D.; Gilbert, R.J. The Effect of Surface Modification of Aligned Poly-L-Lactic Acid Electrospun Fibers on Fiber Degradation and Neurite Extension. PLoS ONE 2015, 10, e0136780. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Koh, J. Physiochemical, Optical and Biological Activity of Chitosan-Chromone Derivative for Biomedical Applications. Int. J. Mol. Sci. 2012, 13, 6102–6116. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G.H.; Park, H.J. Preparation and characterization of drug-loaded chitosan-tripolyphosphate microspheres by spray drying. Drug Dev. Res. 2005, 64, 114–128. [Google Scholar] [CrossRef]
- Foroughi, M.R.; Karbasi, S.; Khoroushi, M.; Khademi, A.A. Polyhydroxybutyrate/chitosan/bioglass nanocomposite as a novel electrospun scaffold: Fabrication and characterization. J. Porous Mater. 2017, 24, 1447–1460. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, X.; Tan, R.; Hu, W.; Wang, X.; Zhang, P.; Zhang, T. Fabrication and properties of a porous chitin/chitosan conduit for nerve regeneration. Biotechnol. Lett. 2004, 26, 1793–1797. [Google Scholar] [CrossRef]
- Karbasi, S.; Alizadeh, Z.M. Effects of multi-wall carbon nanotubes on structural and mechanical properties of poly(3-hydroxybutyrate)/chitosan electrospun scaffolds for cartilage tissue engineering. Bull. Mater. Sci. 2017, 40, 1247–1253. [Google Scholar] [CrossRef]



| Sample Name | Concentration of PHB (w/v%) | Concentration of CS (w/v%) | Reagent for Surface Treatment |
|---|---|---|---|
| PHB-H-0-CS | 15 | 0 | H2O2 |
| PHB-H-0.2-CS | 15 | 0.2 | |
| PHB-H-0.5-CS | 15 | 0.5 | |
| PHB-H-0.8-CS | 15 | 0.8 | |
| PHB-H-1-CS | 15 | 1 | |
| PHB-H-1.5-CS | 15 | 1.5 | |
| PHB-H-2-CS | 15 | 2 | |
| PHB-K-0-CS | 15 | 0 | KOH |
| PHB-K-0.2-CS | 15 | 0.2 | |
| PHB-K-0.5-CS | 15 | 0.5 | |
| PHB-K-0.8-CS | 15 | 0.8 | |
| PHB-K-1-CS | 15 | 1 | |
| PHB-K-1.5-CS | 15 | 1.5 | |
| PHB-K-2-CS | 15 | 2 |
| Groups | Fiber Diameter (µm) | Standard Deviation |
|---|---|---|
| PHB (non-treated) | 5.22 | 0.05 |
| PHB-H-0-CS | 2.78 | 0.14 |
| PHB-H-0.2-CS | 2.90 | 0.03 |
| PHB-H-0.5-CS | 3.01 | 0.11 |
| PHB-H-0.8-CS | 3.00 | 0.20 |
| PHB-H-1-CS | 4.10 | 0.33 |
| PHB-H-1.5-CS | 4.32 | 0.37 |
| PHB-H-2-CS | 4.41 | 0.34 |
| PHB-K-0-CS | 1.90 | 0.12 |
| PHB-K-0.2-CS | 1.93 | 0.18 |
| PHB-K-0.5-CS | 1.98 | 0.19 |
| PHB-K-0.8-CS | 2.31 | 0.18 |
| PHB-K-1-CS | 2.78 | 0.31 |
| PHB-K-1.5-CS | 2.98 | 0.35 |
| PHB-K-2-CS | 3.14 | 0.42 |
| Temperature | First Melting Temperature (T1) | Second Melting Temperature (T2) | p Value (Compared to PHB-KOH-DA-GA-0 w/v% CS) |
|---|---|---|---|
| PHB-K-0-CS | 113 ± 4.14 | 280 ± 13.2 | / |
| PHB-K-0.2-CS | 98.7 ± 5.43 | 290 ± 12.2 | 0.712 |
| PHB-K-0.5-CS | 93.7 ± 7.32 | 289 ± 6.62 | 0.691 |
| PHB-K-0.8-CS | 100 ± 6.12 | 285 ± 8.34 | 0.681 |
| PHB-K-1-CS | 102 ± 4.12 | 281 ± 7.12 | <0.05 |
| PHB-K-1.5-CS | 88.8 ±6.13 | 263 ± 5.12 | <0.05 |
| PHB-K-2-CS | 92.9 ± 8.12 | 269 ± 5.32 | <0.05 |
| Temperature | First Melting Temperature (T1) | Second Melting Temperature (T2) | p Value (Compared to PHB-H2O2-DA-GA-0 w/v% CS) |
|---|---|---|---|
| PHB | 309 ± 3.41 | 368 ± 5.12 | <0.05 |
| PHB-H-0-CS | 110 ± 8.12 | 275 ± 11.1 | / |
| PHB-H-0.2-CS | 128 ± 5.123 | 282 ±9.77 | 0.555 |
| PHB-H-0.5-CS | 128 ± 4.53 | 282 ± 6.99 | 0.781 |
| PHB-H-0.8-CS | 128 ± 6.21 | 282 ± 4.51 | 0.841 |
| PHB-H-1-CS | 128 ± 5.12 | 281 ± 6.34 | 0.813 |
| PHB-H-1.5-CS | 128 ± 3.53 | 282 ± 5.32 | <0.05 |
| PHB-H-2-CS | 129 ± 5.12 | 284 ± 6.54 | <0.05 |
| Enthalpy of Fusion | Crystallinity | ||
|---|---|---|---|
| ΔHfusion (J/g) | % | ||
| H1 | H2 | ||
| PHB-K-0-CS | 4.87 | 95.31 | 70.40 |
| PHB-K-0.2-CS | 3.83 | 42.60 | 31.81 |
| PHB-K-0.5-CS | 4.03 | 41.52 | 31.12 |
| PHB-K-0.8-CS | 4.32 | 38.24 | 29.14 |
| PHB-K-1-CS | 4.34 | 37.01 | 28.31 |
| PHB-K-1.5-CS | 4.51 | 35.13 | 27.12 |
| PHB-K-2-CS | 4.52 | 32.81 | 25.62 |
| Groups | Enthalpy of Fusion | Crystallinity | |
|---|---|---|---|
| ΔHfusion (J/g) | % | ||
| H1 | H2 | ||
| PHB-H-0-CS | 6.80 | 117.92 | 85.21 |
| PHB-H-0.2-CS | 5.91 | 72.31 | 53.62 |
| PHB-H-0.5-CS | 6.27 | 71.23 | 53.13 |
| PHB-H-0.8-CS | 7.02 | 70.82 | 53.32 |
| PHB-H-1-CS | 7.16 | 65.21 | 49.63 |
| PHB-H-1.5-CS | 7.19 | 62.82 | 47.91 |
| PHB-H-2-CS | 7.39 | 58.73 | 45.34 |
| Groups | Specific Surface Area (m2/g) |
|---|---|
| PHB-K-0-CS | 371.00 ± 11.30 |
| PHB-K-0.2-CS | 338.00 ± 12.30 |
| PHB-K-0.5-CS | 319.00 ± 10.20 |
| PHB-K-0.8-CS | 309.00 ± 11.20 |
| PHB-K-1-CS | 291.00 ± 8.92 |
| PHB-K-1.5-CS | 280.00 ± 10.20 |
| PHB-K-2-CS | 264.00 ± 12.10 |
| PHB (Non-treated) | 276.00 ± 10.20 |
| Groups | Specific Surface Area (m2/g) |
|---|---|
| PHB-H-0-CS | 371.00 ± 11.30 |
| PHB-H-0.2-CS | 338.00 ± 12.30 |
| PHB-H-0.5-CS | 319.00 ± 10.20 |
| PHB-H-0.8-CS | 309.00 ± 11.20 |
| PHB-H-1-CS | 291.00 ± 8.92 |
| PHB-H-1.5-CS | 280.00 ± 10.20 |
| PHB-H-2-CS | 260.00 ± 11.10 |
| Groups | Elongation at Break (%) | Young’s Modulus (MPa) | Toughness (MPa) | Ultimate Tensile Stress (UTS) (MPa) |
|---|---|---|---|---|
| PHB-K-0-CS | 390 | 0.00043 | 56 | 0.18 |
| PHB-K-0.2-CS | 600 | 0.00044 | 180 | 0.6 |
| PHB-K-0.5-CS | 490 | 0.0005 | 140 | 0.58 |
| PHB-K-0.8-CS | 360 | 0.00069 | 89 | 0.49 |
| PHB-K-1-CS | 340 | 0.001 | 93 | 0.56 |
| PHB-K-1.5-CS | 330 | 0.0013 | 120 | 0.55 |
| PHB-K-2-CS | 250 | 0.0014 | 75 | 0.59 |
| Groups | Elongation at Break (%) | Young’s Modulus (MPa) | Toughness (MPa) | Ultimate Tensile Stress (UTS) (MPa) |
|---|---|---|---|---|
| PHB-H-0-CS | 450 | 0.00063 | 130 | 0.43 |
| PHB-H-0.2-CS | 450 | 0.00073 | 150 | 0.66 |
| PHB-H-0.5-CS | 490 | 0.0007 | 200 | 0.81 |
| PHB-H-0.8-CS | 350 | 0.00096 | 120 | 0.69 |
| PHB-H-1-CS | 340 | 0.0014 | 130 | 0.79 |
| PHB-H-1.5-CS | 270 | 0.0018 | 160 | 0.77 |
| PHB-H-2-CS | 230 | 0.0018 | 98 | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Li, D.; Li, X.; Li, Y.; Li, B.; Zhou, F. A Comparison of Chitosan Adhesion to KOH and H2O2 Pre-Treated Electrospun Poly(3-Hydroxybutyrate) Nanofibers. Fibers 2023, 11, 91. https://doi.org/10.3390/fib11110091
Zhou Y, Li D, Li X, Li Y, Li B, Zhou F. A Comparison of Chitosan Adhesion to KOH and H2O2 Pre-Treated Electrospun Poly(3-Hydroxybutyrate) Nanofibers. Fibers. 2023; 11(11):91. https://doi.org/10.3390/fib11110091
Chicago/Turabian StyleZhou, Yansheng, Daqing Li, Xin Li, Ying Li, Bing Li, and Fenglei Zhou. 2023. "A Comparison of Chitosan Adhesion to KOH and H2O2 Pre-Treated Electrospun Poly(3-Hydroxybutyrate) Nanofibers" Fibers 11, no. 11: 91. https://doi.org/10.3390/fib11110091
APA StyleZhou, Y., Li, D., Li, X., Li, Y., Li, B., & Zhou, F. (2023). A Comparison of Chitosan Adhesion to KOH and H2O2 Pre-Treated Electrospun Poly(3-Hydroxybutyrate) Nanofibers. Fibers, 11(11), 91. https://doi.org/10.3390/fib11110091








