Preparation, Characterization, and Surface Modification of Cellulose Nanocrystal from Lignocellulosic Biomass for Immobilized Lipase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Alkali Pretreatment
2.3. Synthesis of CNC
2.4. Surface Modification of CNC
2.5. Immobilization of Lipase on CNC Matrix
2.6. Characterization
2.6.1. X-ray Diffraction
2.6.2. Transmission Electron Microscopy
2.6.3. Fourier Transform Infrared Spectroscopy
2.6.4. Degree of Hydrolysis
3. Results
3.1. Synthesis of Cellulose Nanocrystals
3.2. Thermogravimetric Analysis (TGA)
3.3. Crystallinity of CNC
3.4. Morphological and Dimensional Analysis of CNC
3.5. Fourier Transform Infrared Spectroscopy
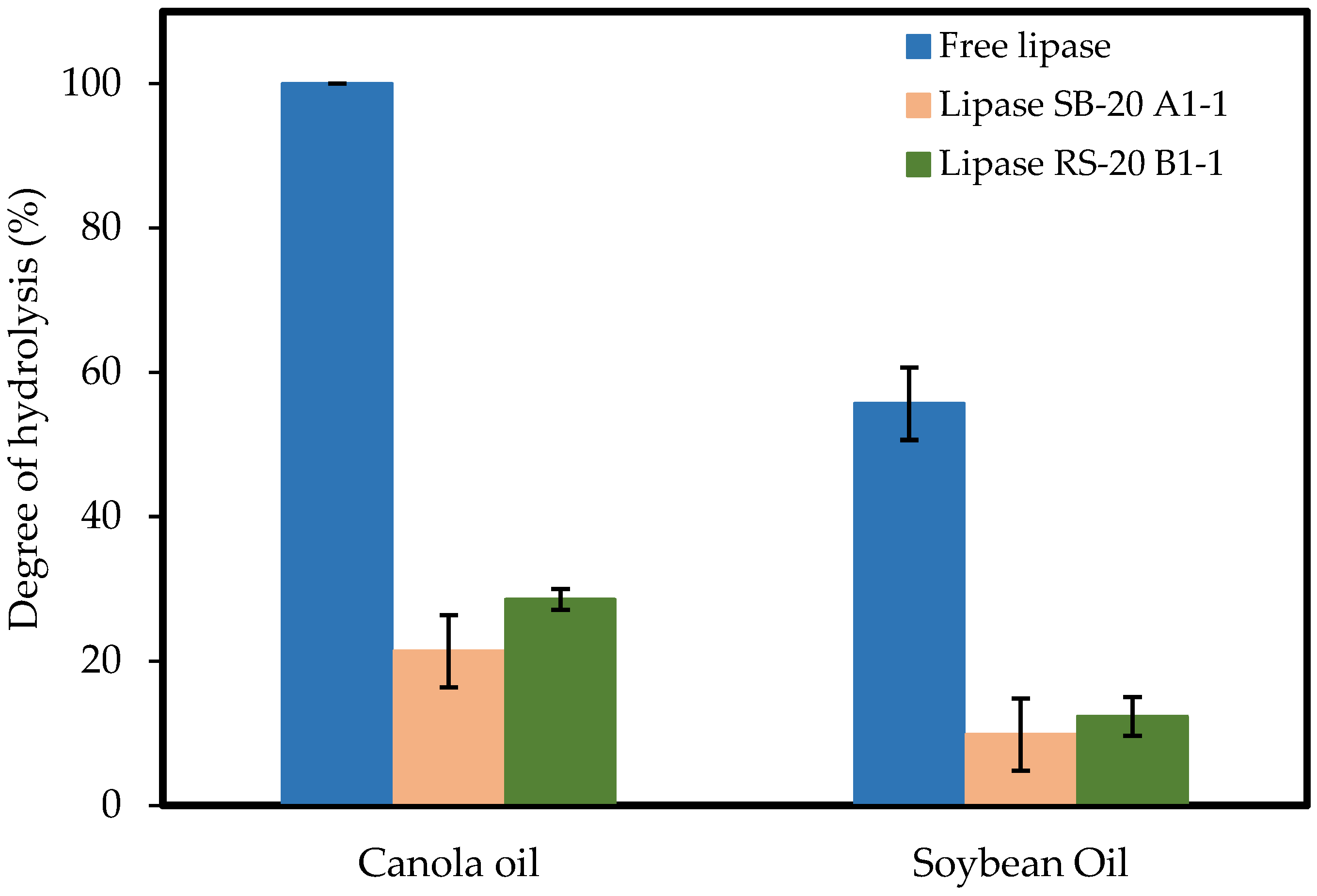
3.6. Degree of Hydrolysis
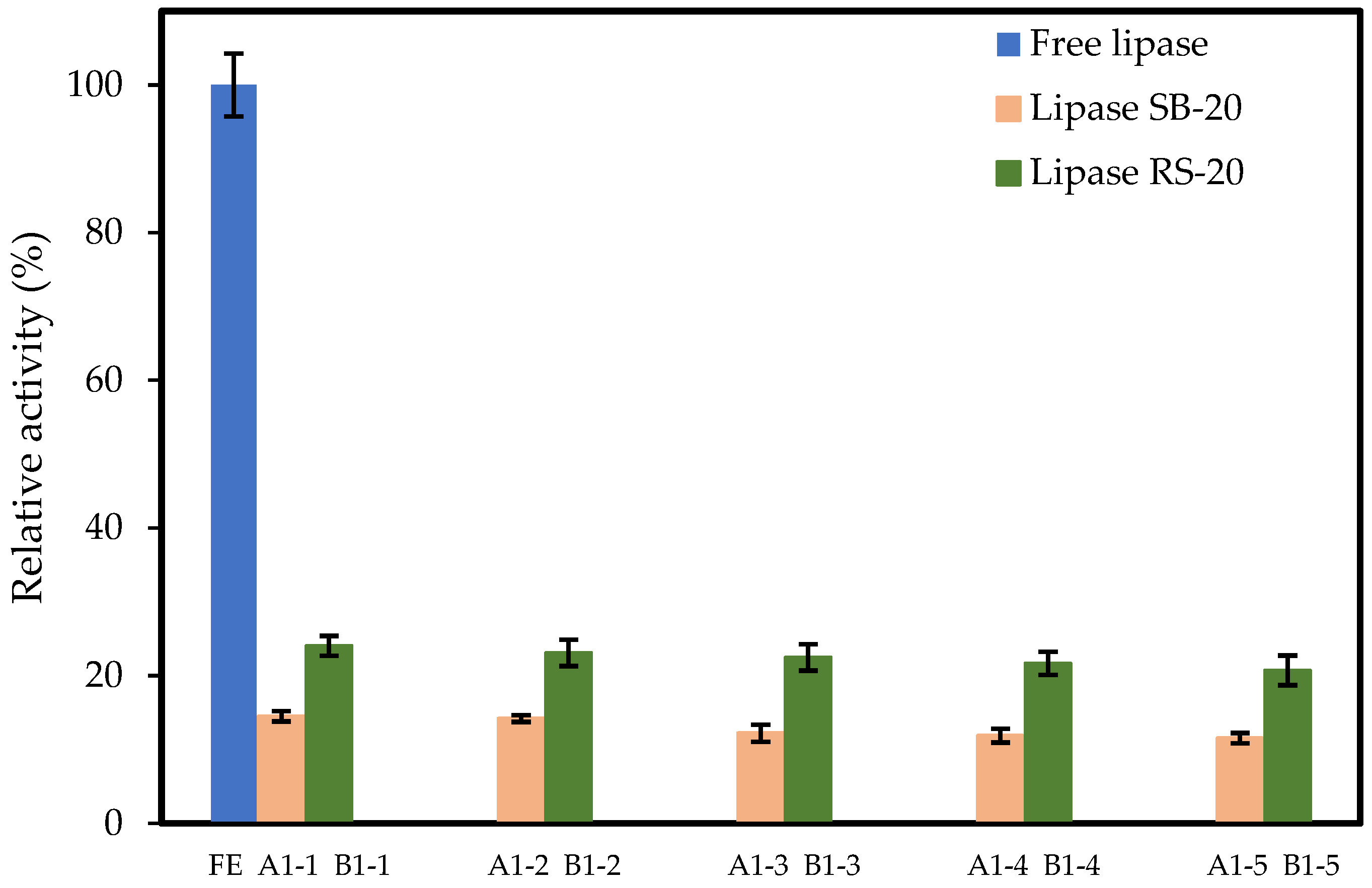
3.7. Reusability of Immobilized Lipase
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez-Ayesta, C.; Carelli, A.A.; Ferreira, M.L. Relation between lipase structures and their catalytic ability to hydrolyse triglycerides and phospholipids. Enzym. Microb. Technol. 2007, 41, 35–43. [Google Scholar] [CrossRef]
- Wang, P. Nanoscale biocatalyst systems. Curr. Opin. Biotechnol. 2006, 17, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Tacias-Pascacio, V.G.; Virgen-Ortíz, J.J.; Jiménez-Pérez, M.; Yates, M.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Evaluation of different lipase biocatalysts in the production of biodiesel from used cooking oil: Critical role of the immobilization support. Fuel 2017, 200, 1–10. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef]
- Sheltami, R.M.; Abdullah, I.; Ahmad, I.; Dufresne, A.; Kargarzadeh, H. Extraction of cellulose nanocrystals from mengkuang leaves (Pandanus tectorius). Carbohydr. Polym. 2012, 88, 772–779. [Google Scholar] [CrossRef]
- Danial, W.H.; Abdul Majid, Z.; Mohd Muhid, M.N.; Triwahyono, S.; Bakar, M.B.; Ramli, Z. The reuse of wastepaper for the extraction of cellulose nanocrystals. Carbohydr. Polym. 2015, 118, 165–169. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Liu, H.; Shang, S.; Song, J.; Wang, D. Extraction and comparison of carboxylated cellulose nanocrystals from bleached sugarcane bagasse pulp using two different oxidation methods. Carbohydr. Polym. 2016, 138, 237–243. [Google Scholar] [CrossRef]
- Budhi, Y.W.; Fakhrudin, M.; Culsum, N.T.U.; Suendo, V.; Iskandar, F. Preparation of cellulose nanocrystals from empty fruit bunch of palm oil by using phosphotungstic acid. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 12063. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Restiawaty, E.; Arabica Yatasya, F.; Ellys; Tresna Umi Culsum, N.; Akhmaloka; Wibisono Budhi, Y. Lipase immobilization onto Cellulose Nanocrystals (CNC) for catalyzing lipolysis of triglycerides. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1143, 12009. [Google Scholar] [CrossRef]
- Culsum, N.T.U.; Melinda, C.; Leman, I.; Wibowo, A.; Budhi, Y.W. Isolation and characterization of cellulose nanocrystals (CNCs) from industrial denim waste using ammonium persulfate. Mater. Today Commun. 2021, 26, 101817. [Google Scholar] [CrossRef]
- Madani, H.; Wibowo, A.; Judawisastra, H.; Nishiyama, N.; Budhi, Y.W. One-step extraction of cellulose nanocrystals from high lignin biomass through ammonium persulfate oxidation method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2022, 13, 15007. [Google Scholar] [CrossRef]
- Hessien, M.M.; Rashad, M.M.; Zaky, R.R.; Abdel-Aal, E.A.; El-Barawy, K.A. Controlling the synthesis conditions for silica nanosphere from semi-burned rice straw. Mater. Sci. Eng. B 2009, 162, 14–21. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T. Biotechnological potential of agro-industrial residues. I: Sugarcane bagasse. Bioresour. Technol. 2000, 74, 69–80. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.; Kim, S.H.; Kim, J.H.; Yu, H.; Kim, H.J.; Yang, Y.-H.; Kan, E.; Kim, Y.H.; Lee, S.H. Biocompatible cellulose nanocrystals as supports to immobilize lipase. J. Mol. Catal. B Enzym. 2015, 122, 170–178. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of Recent Research into Cellulosic Whiskers, Their Properties and Their Application in Nanocomposite Field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef]
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.W.; Luong, J.H.T. Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol. 2012, 30, 283–290. [Google Scholar] [CrossRef]
- Zainuddin, N.; Ahmad, I.; Kargarzadeh, H.; Ramli, S. Hydrophobic kenaf nanocrystalline cellulose for the binding of curcumin. Carbohydr. Polym. 2017, 163, 261–269. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [Green Version]
- Cho, B.-N.; Chino, H.; Tsuji, H.; Kunito, T.; Makishima, H.; Uchida, H.; Matsumoto, S.; Oyaizu, H. Analysis of oil components and hydrocarbon-utilizing microorganisms during laboratory-scale bioremediation of oil-contaminated soil of Kuwait. Chemosphere 1997, 35, 1613–1621. [Google Scholar] [CrossRef]
- Isogai, A.; Kato, Y. Preparation of Polyuronic Acid from Cellulose by TEMPO-mediated Oxidation. Cellulose 1998, 5, 153–164. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; He, Y.; Cui, G.; Abdulrazaq, M.A.; Yan, Y. Enhancing enzyme activity and enantioselectivity of Burkholderia cepacia lipase via immobilization on melamine-glutaraldehyde dendrimer modified magnetic nanoparticles. Chem. Eng. J. 2018, 351, 258–268. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Rooney, D.; Weatherley, L.R. The effect of reaction conditions upon lipase catalysed hydrolysis of high oleate sunflower oil in a stirred liquid–liquid reactor. Process Biochem. 2001, 36, 947–953. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Okita, Y.; Isogai, A. Thermal stabilization of TEMPO-oxidized cellulose. Polym. Degrad. Stab. 2010, 95, 1502–1508. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Tammelin, T.; Schulfter, K.; Kiiskinen, H.; Samela, J.; Bismarck, A. High Performance Cellulose Nanocomposites: Comparing the Reinforcing Ability of Bacterial Cellulose and Nanofibrillated Cellulose. ACS Appl. Mater. Interfaces 2012, 4, 4078–4086. [Google Scholar] [CrossRef] [Green Version]
- Chindaprasirt, P.; Rattanasak, U. Eco-production of silica from sugarcane bagasse ash for use as a photochromic pigment filler. Sci. Rep. 2020, 10, 9890. [Google Scholar] [CrossRef]
- Zhang, P.; Liao, W.; Kumar, A.; Zhang, Q.; Ma, H. Characterization of sugarcane bagasse ash as a potential supplementary cementitious material: Comparison with coal combustion fly ash. J. Clean. Prod. 2020, 277, 123834. [Google Scholar] [CrossRef]
- Avelar, M.H.M.; Cassimiro, D.M.J.; Santos, K.C.; Domingues, R.C.C.; De Castro, H.F.; Mendes, A.A. Hydrolysis of vegetable oils catalyzed by lipase extract powder from dormant castor bean seeds. Ind. Crops Prod. 2013, 44, 452–458. [Google Scholar] [CrossRef]
- Minovska, V.; Winkelhausen, E.; Kuzmanova, S. Lipase immobilized by different techniques on various support materials applied in oil hydrolysis. J. Serbian Chem. Soc. 2005, 70, 609–624. [Google Scholar] [CrossRef]
- Kabbashi, N.A.; Mohammed, N.I.; Alam, M.Z.; Mirghani, M.E.S. Hydrolysis of Jatropha curcas oil for biodiesel synthesis using immobilized Candida cylindracea lipase. J. Mol. Catal. B Enzym. 2015, 116, 95–100. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Elias, N.; Chandren, S.; Razak, F.I.A.; Jamalis, J.; Widodo, N.; Wahab, R.A. Characterization, optimization and stability studies on Candida rugosa lipase supported on nanocellulose reinforced chitosan prepared from oil palm biomass. Int. J. Biol. Macromol. 2018, 114, 306–316. [Google Scholar] [CrossRef]
- Ly, M.; Mekonnen, T.H. Cationic surfactant modified cellulose nanocrystals for corrosion protective nanocomposite surface coatings. J. Ind. Eng. Chem. 2020, 83, 409–420. [Google Scholar] [CrossRef]
- Knill, C.J.; Kennedy, J.F. Degradation of cellulose under alkaline conditions. Carbohydr. Polym. 2003, 51, 281–300. [Google Scholar] [CrossRef]
- Ciftci, D.; Flores, R.A.; Saldaña, M.D.A. Cellulose Fiber Isolation and Characterization from Sweet Blue Lupin Hull and Canola Straw. J. Polym. Environ. 2018, 26, 2773–2781. [Google Scholar] [CrossRef]
- Elenga, R.G.; Djemia, P.; Tingaud, D.; Chauveau, T.; Maniongui, J.G.; Dirras, G. Effects of alkali treatment on the microstructure, composition, and properties of the Raffia textilis fiber. BioResources 2013, 8, 2934–2949. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Ding, B.; Yu, J.; Al-Deyab, S.S. Cellulose nanowhiskers extracted from TEMPO-oxidized jute fibers. Carbohydr. Polym. 2012, 90, 1075–1080. [Google Scholar] [CrossRef]
- Wibowo, A.; Madani, H.; Judawisastra, H.; Restiawaty, E.; Lazarus, C.; Budhi, Y.W. An eco-friendly preparation of cellulose nano crystals from oil palm empty fruit bunches. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 012059. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Xiang, S.; Ma, X.; Liao, S.; Shi, H.; Liu, C.; Shen, Y.; Lv, X.; Yuan, M.; Fan, G.; Huang, J.; et al. Cellulose nanocrystal surface cationization: A New Fungicide with High Activity against Phycomycetes capsici. Molecules 2019, 24, 2467. [Google Scholar] [CrossRef] [Green Version]
- Nagalakshmaiah, M.; El Kissi, N.; Dufresne, A. Ionic Compatibilization of Cellulose Nanocrystals with Quaternary Ammonium Salt and Their Melt Extrusion with Polypropylene. ACS Appl. Mater. Interfaces 2016, 8, 8755–8764. [Google Scholar] [CrossRef]
- Tong, X.; Shen, W.; Chen, X.; Jia, M.; Roux, J.-C. Preparation and mechanism analysis of morphology-controlled cellulose nanocrystals via compound enzymatic hydrolysis of eucalyptus pulp. J. Appl. Polym. Sci. 2020, 137, 48407. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.T.; Ren, X.Y.; Liu, Y.M.; Wei, Y.; Qing, L.; Liao, X. Covalent immobilization of porcine pancreatic lipase on carboxyl-activated magnetic nanoparticles: Characterization and application for enzymatic inhibition assays. Mater. Sci. Eng. C 2014, 38, 278–285. [Google Scholar] [CrossRef]
- Onyianta, A.J.; Dorris, M.; Williams, R.L. Aqueous morpholine pre-treatment in cellulose nanofibril (CNF) production: Comparison with carboxymethylation and TEMPO oxidisation pre-treatment methods. Cellulose 2018, 25, 1047–1064. [Google Scholar] [CrossRef] [Green Version]
- Incani, V.; Danumah, C.; Boluk, Y. Nanocomposites of nanocrystalline cellulose for enzyme immobilization. Cellulose 2013, 20, 191–200. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J. Youngblood, Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Yang, T.; Xu, X.; Li, L.T. Comparison of Linoleic and Conjugated Linoleic Acids in Enzymatic Acidolysis of Tristearin. J. Food Lipids 2001, 8, 149–161. [Google Scholar] [CrossRef]
- Karabulut, I.; Durmaz, G.; Hayaloglu, A.A. C18 Unsaturated fatty acid selectivity of lipases during the acidolysis reaction between tripalmitin and oleic, linoleic, and linolenic acids. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 1301–1307. [Google Scholar] [CrossRef]


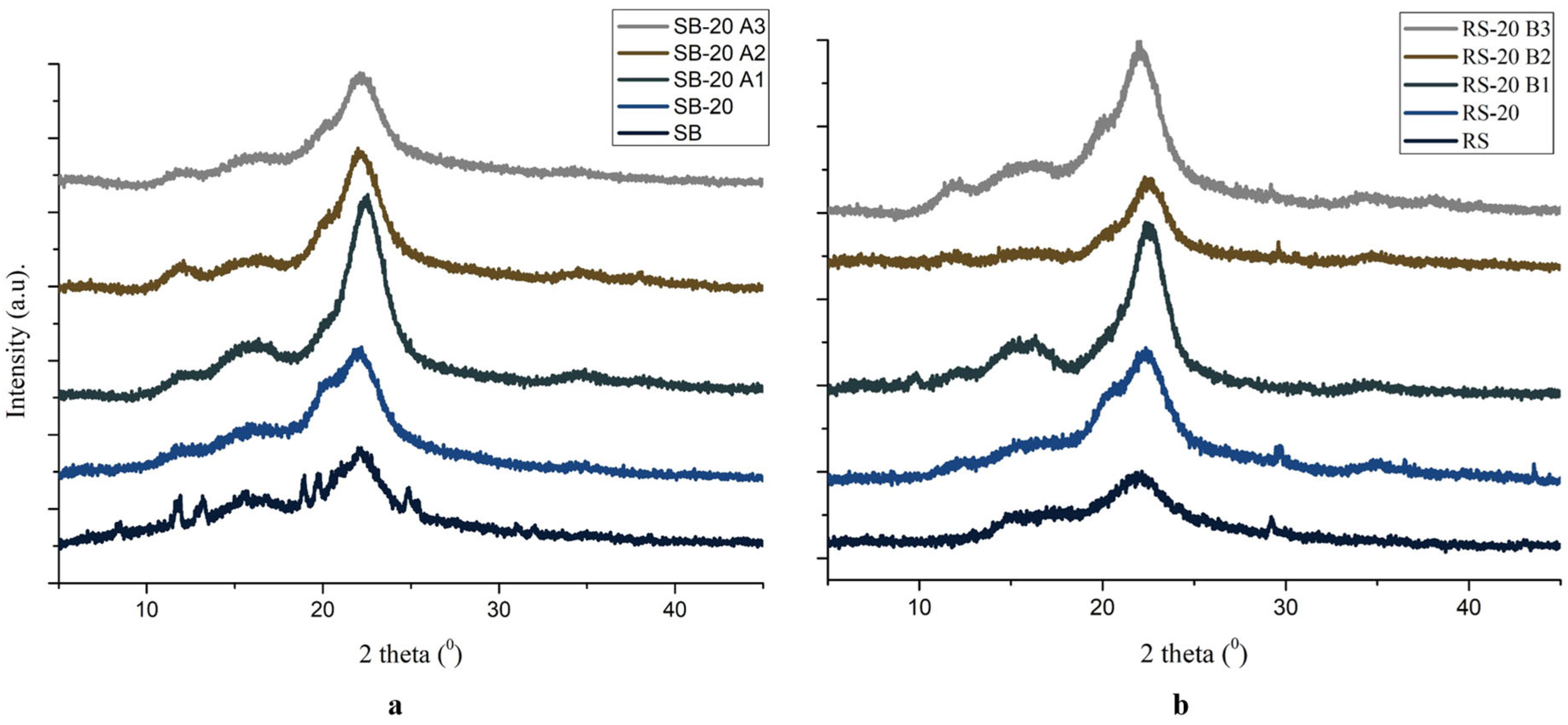
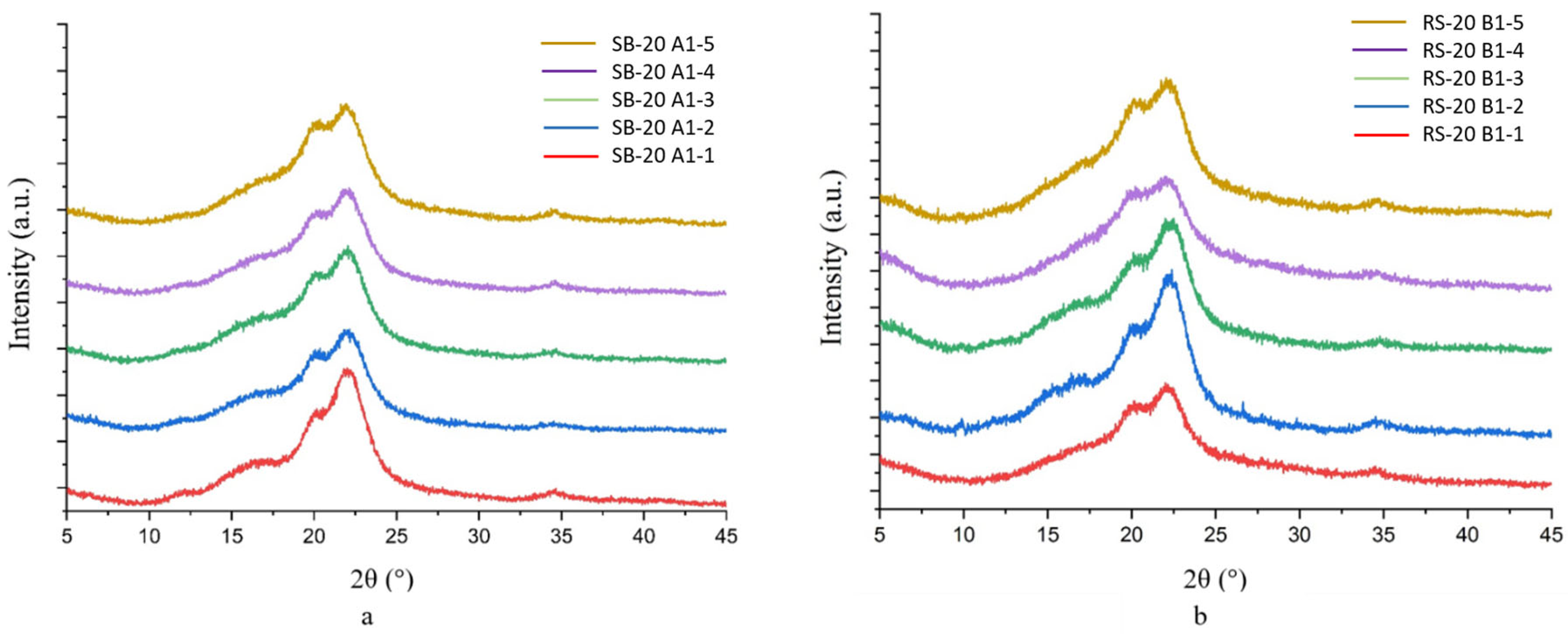
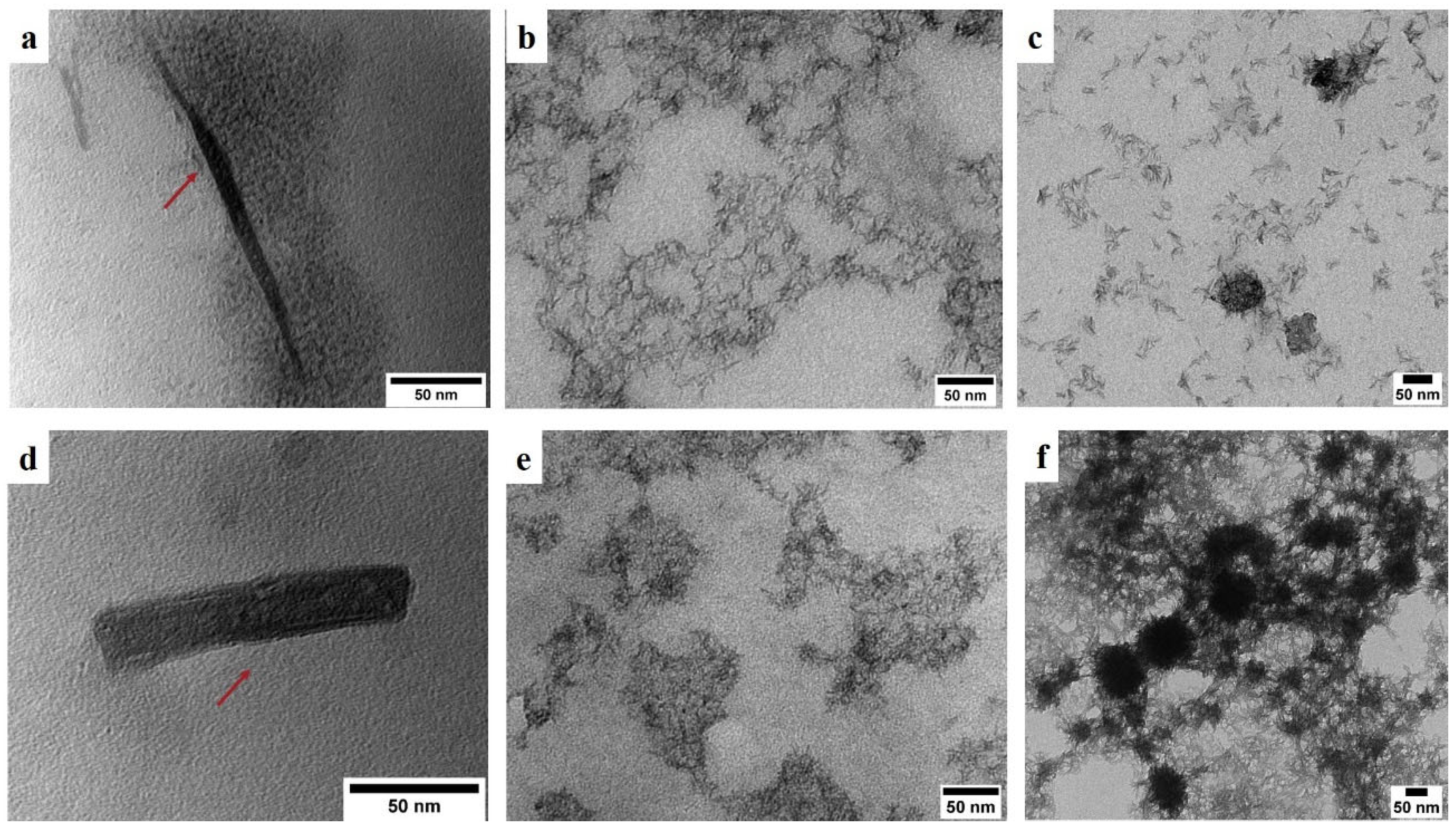


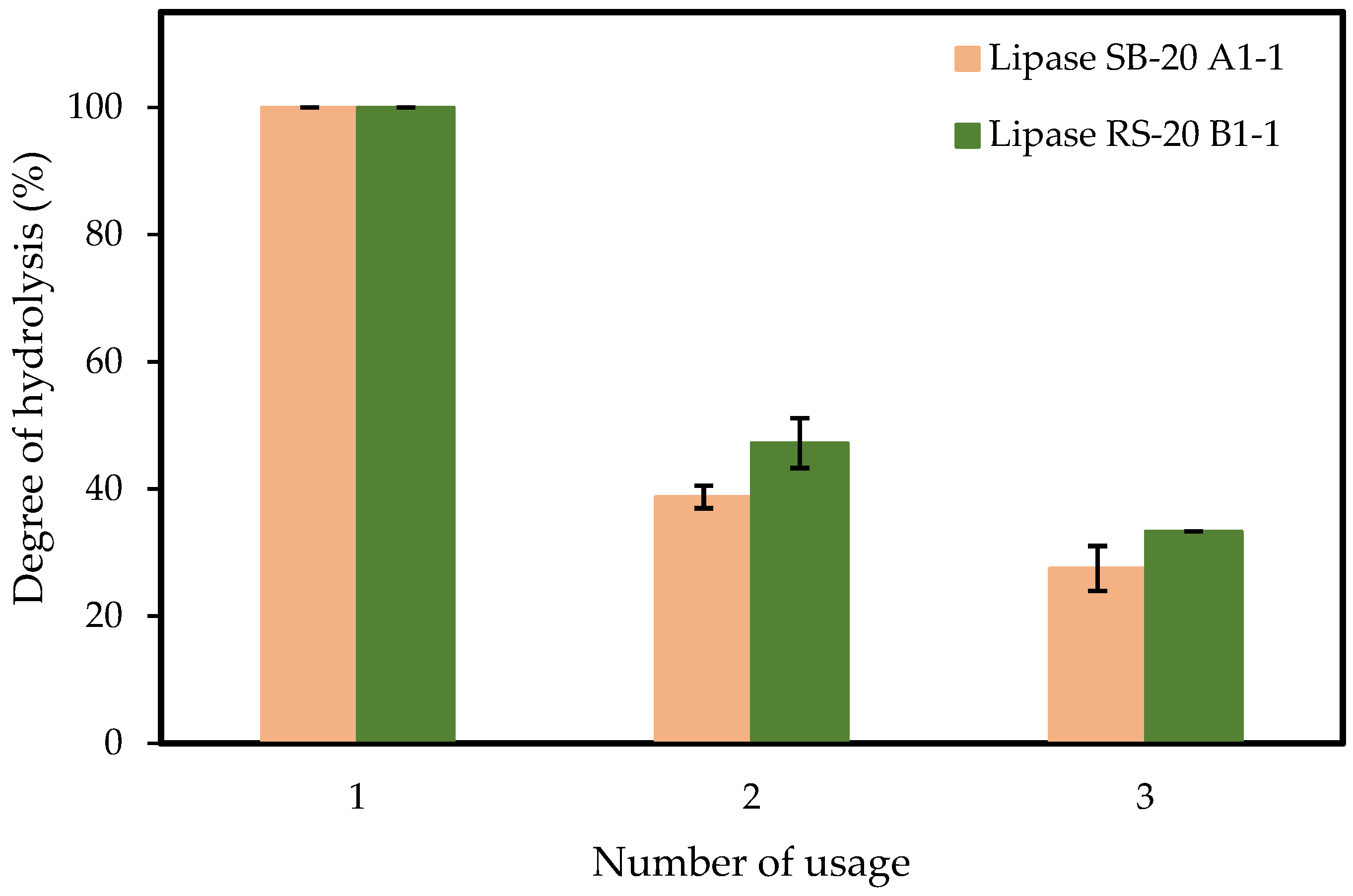

| No | Sample Code | Details | CrI (%) |
|---|---|---|---|
| 1 | SB | Raw materials of sugarcane bagasse | 56 |
| 2 | SB-20 | SB after pretreatment using 20% NaOH | 65 |
| 3 | SB-20 A1 | SB-20 oxidized with TEMPO and 11 mmol NaOCl | 80 |
| 4 | SB-20 A2 | SB-20 oxidized with TEMPO and 9 mmol NaOCl | 77 |
| 5 | SB-20 A3 | SB-20 oxidized with TEMPO and 7 mmol NaOCl | 76 |
| 6 | RS | Raw materials of rice straw | 56 |
| 7 | RS-20 | RS after pretreatment using 20% NaOH | 72 |
| 8 | RS-20 B1 | RS-20 oxidized with TEMPO and 11 mmol NaOCl | 86 |
| 9 | RS-20 B2 | RS-20 oxidized with TEMPO and 9 mmol NaOCl | 85 |
| 10 | RS-20 B3 | RS-20 oxidized with TEMPO and 7 mmol NaOCl | 74 |
| No | Sample Code | Details | CrI (%) |
|---|---|---|---|
| 1 | SB-20 A1-1 | Modification of SB-20 A1 using 2 mM CTAB | 69 |
| 2 | SB-20 A1-2 | Modification of SB-20 A1 using 4 mM CTAB | 66 |
| 3 | SB-20 A1-3 | Modification of SB-20 A1 using 6 mM CTAB | 63 |
| 4 | SB-20 A1-4 | Modification of SB-20 A1 using 8 mM CTAB | 62 |
| 5 | SB-20 A1-5 | Modification of SB-20 A1 using 10 mM CTAB | 62 |
| 6 | RS-20 B1-1 | Modification of RS-20 A1 using 2 mM CTAB | 62 |
| 7 | RS-20 B1-2 | Modification of RS-20 A1 using 4 mM CTAB | 63 |
| 8 | RS-20 B1-3 | Modification of RS-20 A1 using 6 mM CTAB | 66 |
| 9 | RS-20 B1-4 | Modification of RS-20 A1 using 8 mM CTAB | 60 |
| 10 | RS-20 B1-5 | Modification of RS-20 A1 using 10 mM CTAB | 61 |
| No. | Biocatalyst | Activity (U/mg-Enzyme) | Immobilization Efficiency (%) |
|---|---|---|---|
| 1 | Free lipase | 1.04 ± 0.09 | - |
| 2 | Lipase SB-20 A1-1 | 0.26 ± 0.01 | 82.98 ± 7.50 |
| 3 | Lipase RS-20 B1-1 | 0.16 ± 0.01 | 86.63 ± 2.41 |
| Enzyme | Enzyme Source | Support | Substrate | Enzyme Concentration | Time | Degree of Hydrolysis | Ref. |
|---|---|---|---|---|---|---|---|
| Free lipase | Ricinus communis | - | canola oil | 81.3 ± 0.29 U/g-substrate | 3 h | 88% | [29] |
| Free lipase | C. rugosa | - | olive oil | 200 U/g-substrate | 24 h | 98% | [30] |
| Free lipase | C. rugosa | - | canola oil | 3.69 ± 0.29 U/mL-substrate | 6 h | 100% | this work |
| Immobilized lipase RS-20 B1-1 | C. rugosa | cnc | canola oil | 0.75 ± 1.16 U/mL-substrate | 6 h | 30% | this work |
| Immobilized lipase | C. cylindracea | activated carbon | jatropha oil | 4.03 U/mL-substrate | 3 h | 78% | [31] |
| Immobilized lipase | C. rugosa | amberlite IRC-50 | olive oil | 300 U/g-substrate | 24 h | 91% | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restiawaty, E.; Culsum, N.T.U.; Nishiyama, N.; Budhi, Y.W. Preparation, Characterization, and Surface Modification of Cellulose Nanocrystal from Lignocellulosic Biomass for Immobilized Lipase. Fibers 2022, 10, 33. https://doi.org/10.3390/fib10040033
Restiawaty E, Culsum NTU, Nishiyama N, Budhi YW. Preparation, Characterization, and Surface Modification of Cellulose Nanocrystal from Lignocellulosic Biomass for Immobilized Lipase. Fibers. 2022; 10(4):33. https://doi.org/10.3390/fib10040033
Chicago/Turabian StyleRestiawaty, Elvi, Neng Tresna Umi Culsum, Norikazu Nishiyama, and Yogi Wibisono Budhi. 2022. "Preparation, Characterization, and Surface Modification of Cellulose Nanocrystal from Lignocellulosic Biomass for Immobilized Lipase" Fibers 10, no. 4: 33. https://doi.org/10.3390/fib10040033
APA StyleRestiawaty, E., Culsum, N. T. U., Nishiyama, N., & Budhi, Y. W. (2022). Preparation, Characterization, and Surface Modification of Cellulose Nanocrystal from Lignocellulosic Biomass for Immobilized Lipase. Fibers, 10(4), 33. https://doi.org/10.3390/fib10040033







