Investigation of the Oxidation Mechanism of Dopamine Functionalization in an AZ31 Magnesium Alloy for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate
2.2. Coating Process
2.3. Characterization
2.4. Electrochemical Analysis
2.5. Cell Cultivation
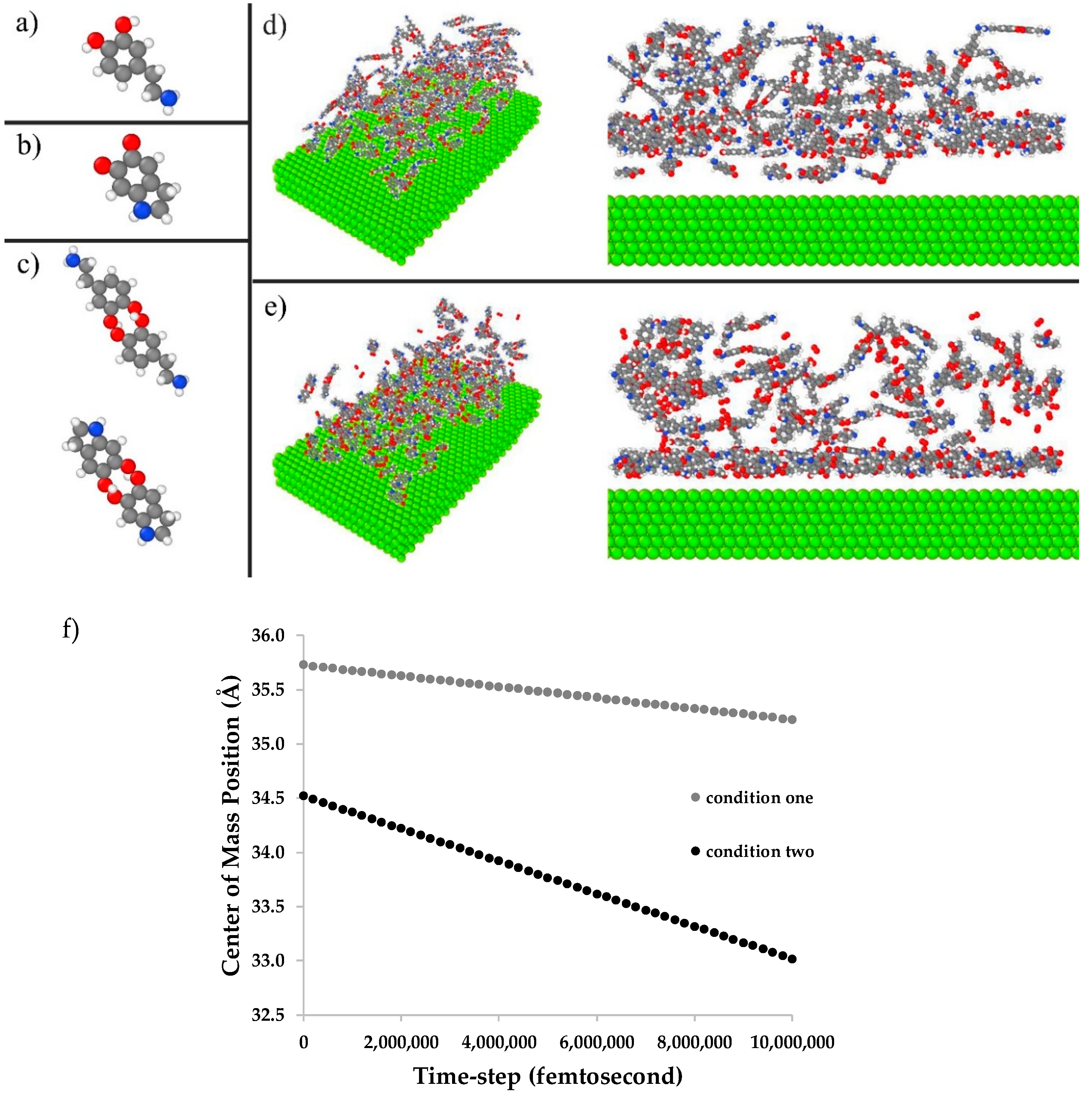
2.6. Molecular Dynamics Simulation
3. Results
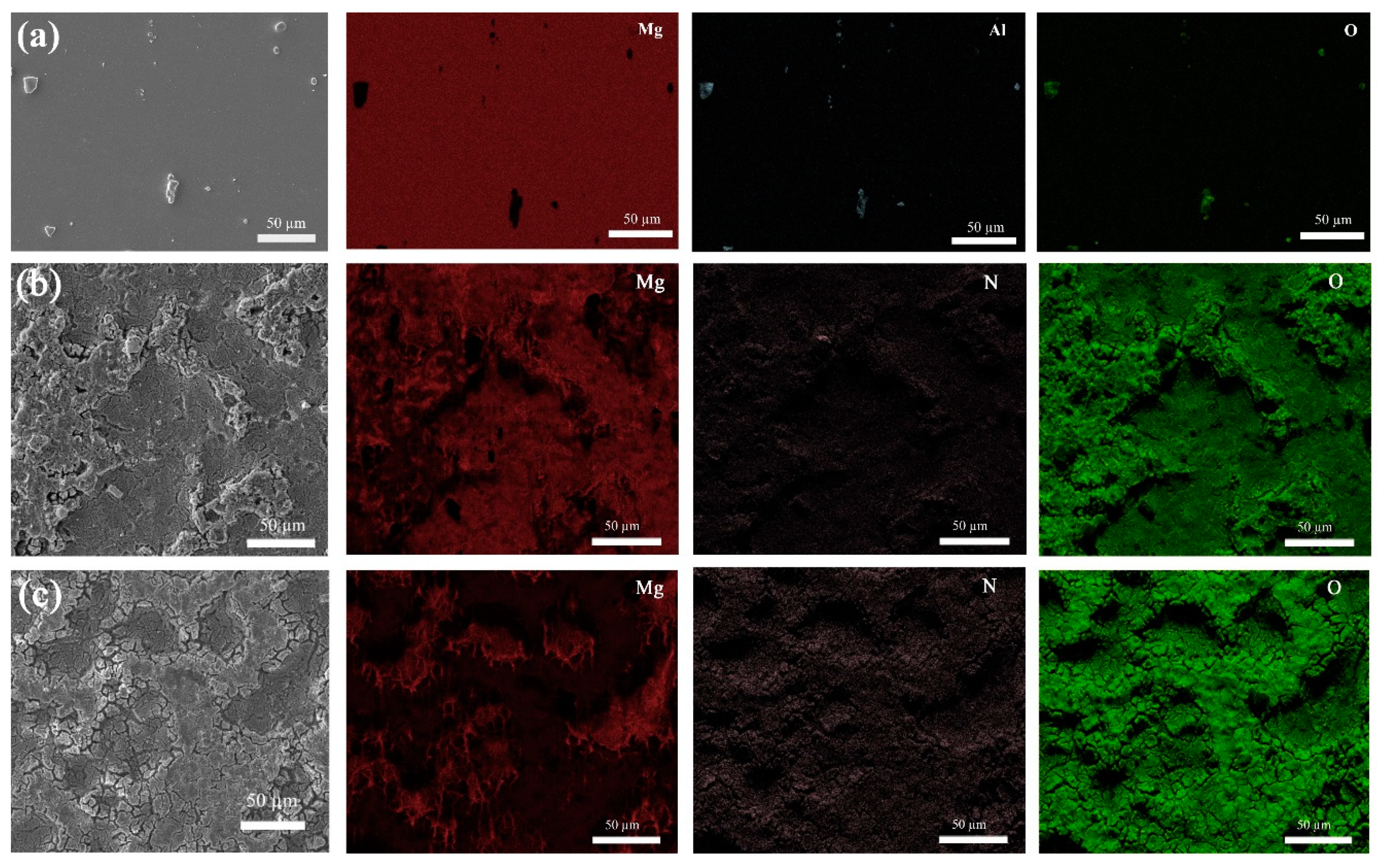
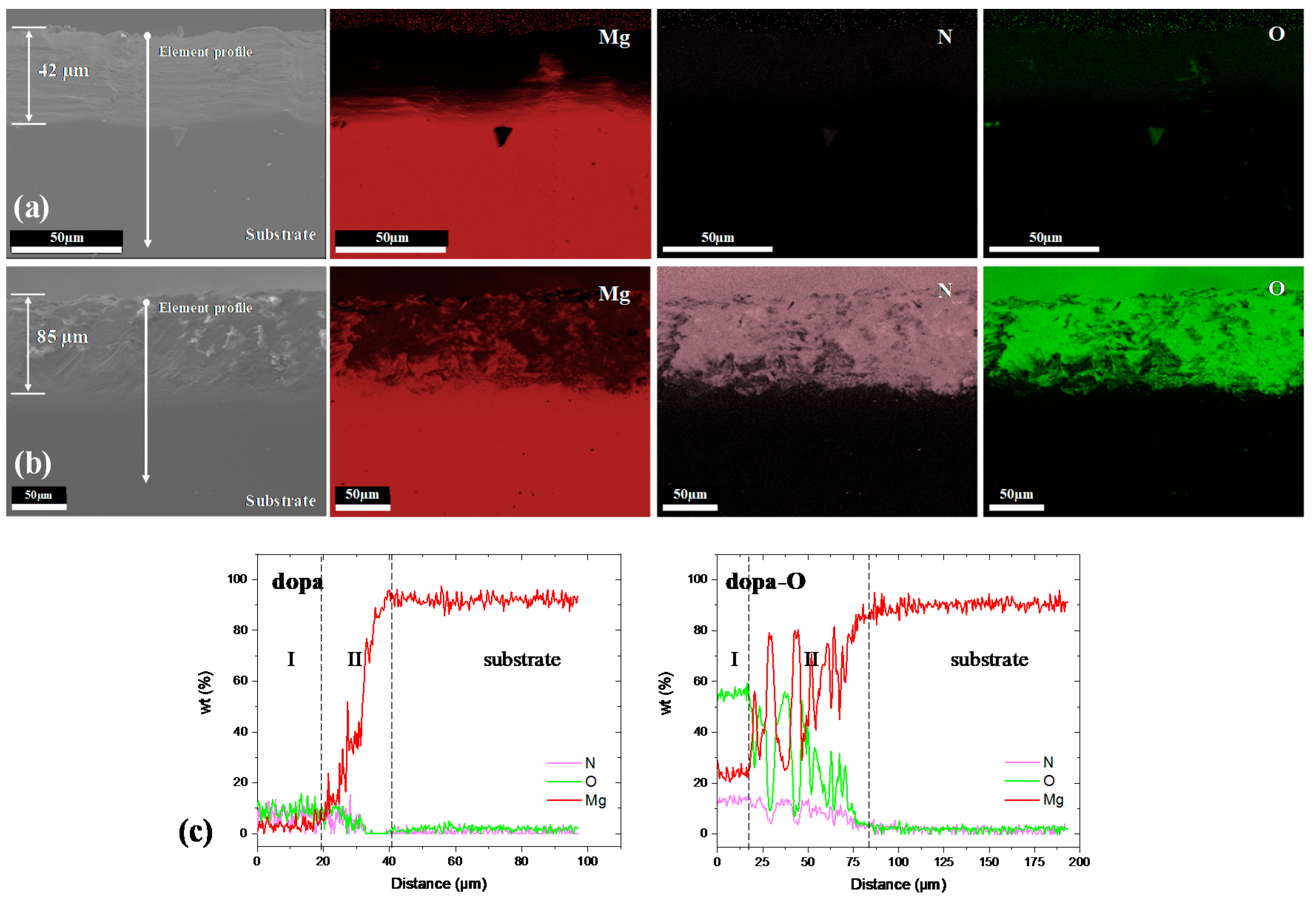
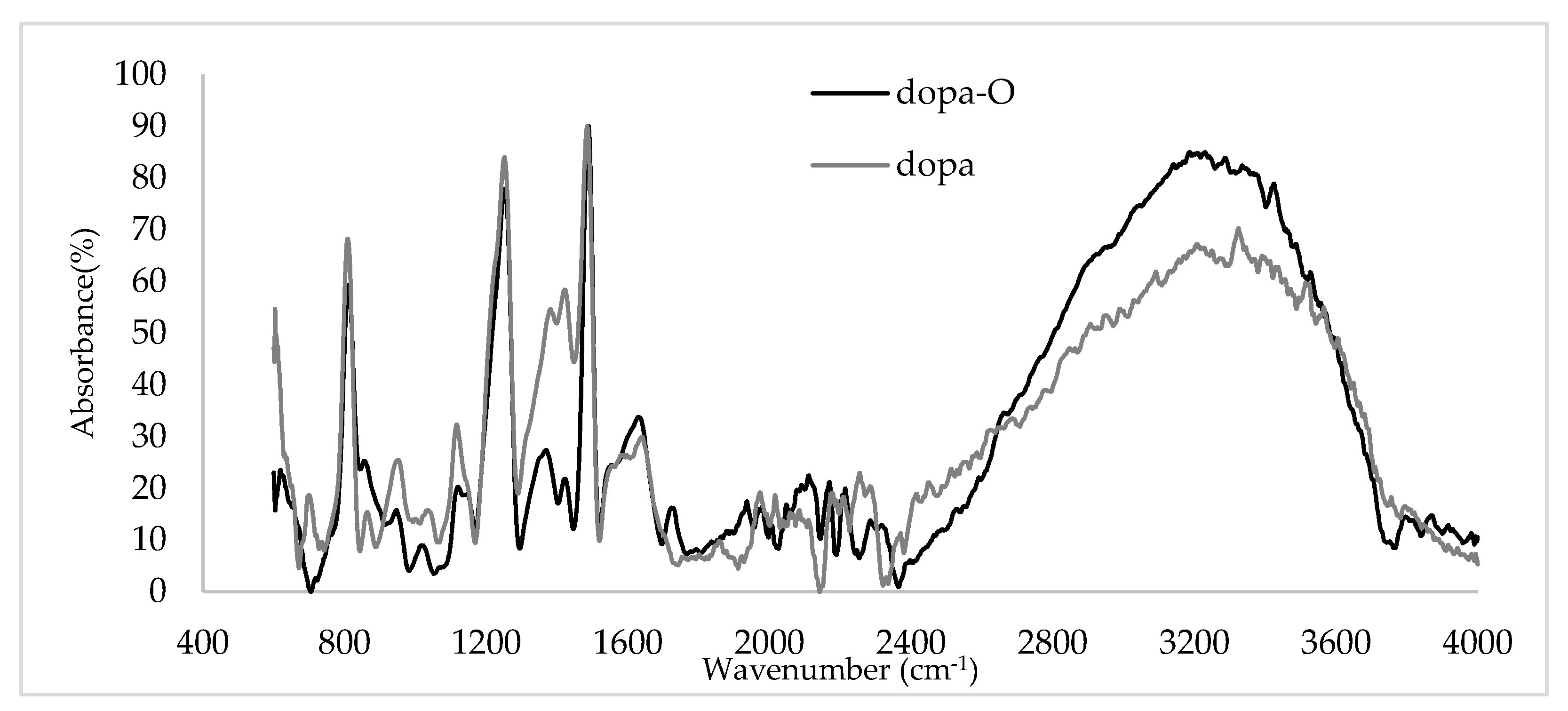
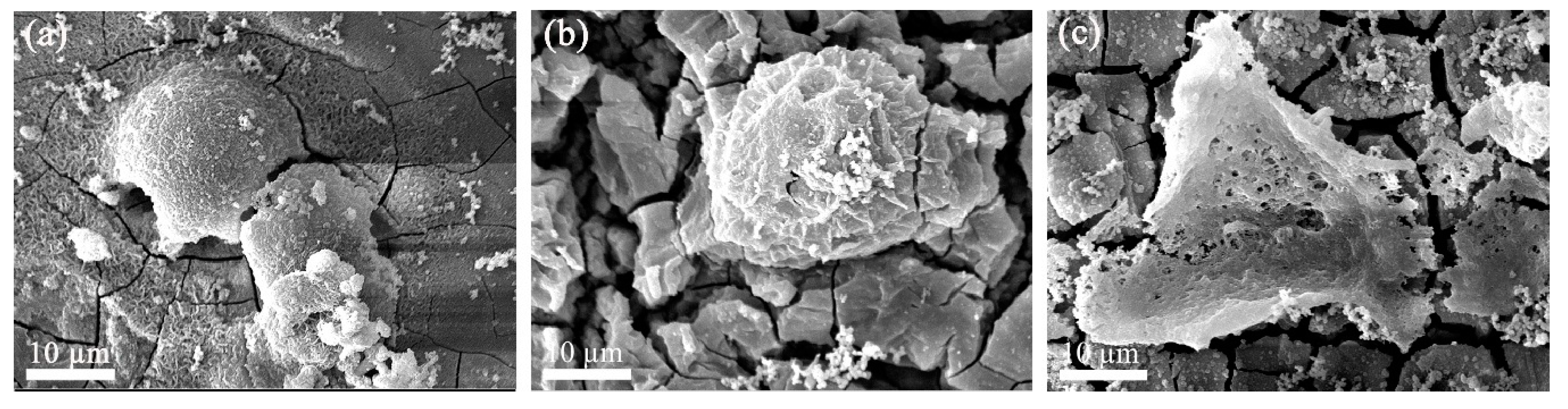
3.1. Characterization of the Coating
3.2. Electrochemical Properties
3.3. Cell Cultivation
3.4. Molecular Dynamics Simulation
4. Discussion
4.1. Characterization of the Coating
4.2. Electrochemical Properties
5. Conclusions
- The molecular dynamic simulation and FTIR analysis show an improvement in the deposition rate and the presence of polydopamine on the substrate.
- The addition of oxygen increases the impedance response of the AZ31 alloy.
- The formation of polydopamine with the presence of amino and hydroxyl functional groups on the surface of the material might be promising for the cell’s response. Further investigations focused on the cell viability and proliferation are necessary to confirm the biocompatibility of the coating.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.N.; Zheng, Y.F. A review on magnesium alloys as biodegradable materials. Front. Mater. Sci. Chin. 2010, 4, 111–115. [Google Scholar] [CrossRef]
- Ghaffarpasand, F.; Shahrezaei, M.; Dehghankhalili, M. Effects of platelet rich plasma on healing rate of long bone non-union fractures: A randomized double-blind placebo controlled clinical trial. Bull. Emerg. Trauma 2016, 4, 134–140. [Google Scholar] [PubMed]
- Shahrezaee, M.; Salehi, M.; Keshtkari, S.; Oryan, A.; Kamali, A.; Shekarchi, B. In vitro and in vivo investigation of pla/pcl scaffold coated with metformin-loaded gelatin nanocarriers in regeneration of critical-sized bone defects. Nanomedicine 2018, 14, 2061–2073. [Google Scholar] [CrossRef] [PubMed]
- Wagener, V.; Schilling, A.; Mainka, A.; Hennig, D.; Gerum, R.; Kelch, M.-L.; Keim, S.; Fabry, B.; Virtanen, S. Cell adhesion on surface-functionalized magnesium. ACS Appl. Mater. Interfaces 2016, 8, 11998–12006. [Google Scholar] [CrossRef] [PubMed]
- Wagener, V.; Killian, M.S.; Turhan, C.M.; Virtanen, S. Albumin coating on magnesium via linker molecules--comparing different coating mechanisms. Colloids Surf. B Biointerfaces 2013, 103, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Beniash, E.; Gawalt, E.; Xu, Z.; Sfeir, C. Biomimetic coating of magnesium alloy for enhanced corrosion resistance and calcium phosphate deposition. Acta Biomater. 2013, 9, 8650–8659. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Xu, Y.; Sun, J.; Yang, L.; Guo, C.; Liang, J.; Cao, B. Preparation and characterization of aminated hydroxyethyl cellulose-induced biomimetic hydroxyapatite coatings on the AZ31 magnesium alloy. Metals 2017, 7, 214. [Google Scholar] [CrossRef]
- Tiyyagura, H.R.; Fuchs-Godec, R.; Gorgieva, S.; Arthanari, S.; Mohan, M.K.; Kokol, V. Biomimetic gelatine coating for less-corrosive and surface bioactive Mg–9Al–1Zn alloys. J. Mater. Res. 2018, 33, 1449–1462. [Google Scholar] [CrossRef]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, L.; Tada, S.; Zhou, D.; Kitajima, T.; Isoshima, T.; Yoshida, Y.; Nakamura, M.; Yan, W.; Ito, Y. Mussel-inspired human gelatin nanocoating for creating biologically adhesive surfaces. Int. J. Nanomed. 2014, 9, 2753–2765. [Google Scholar] [Green Version]
- Zhou, X.; Ouyang, J.; Li, L.; Liu, Q.; Liu, C.; Tang, M.; Deng, Y.; Lei, T. In vitro and in vivo anti-corrosion properties and biocompatibility of 5β-TCP/Mg-3Zn scaffold coated with dopamine-gelatin composite. Surf. Coat. Technol. 2019, 374, 152–163. [Google Scholar] [CrossRef]
- Xu, C.; Xu, K.; Gu, H.; Zheng, R.; Liu, H.; Zhang, X.; Guo, Z.; Xu, B. Dopamine as a robust anchor to immobilize functional molecules on the iron oxide shell of magnetic nanoparticles. J. Am. Chem. Soc. 2004, 126, 9938–9939. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, C.; Gu, Y.; Shen, X.; Zhang, Y.; Lie, B.; Chen, L. Polydopamine-modified poly(l-lactic acid) nanofiber scaffolds immobilized with an osteogenic growth peptide for bone tissue regeneration. R. Soc. Chem. Rsc Adv. 2019, 9, 11722–11736. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Yi, J.; Gao, Q.; Wang, X.; Chen, Y.; Liu, P. Carboxymethyl chitosan functionalization of cped-treated magnesium alloy via polydopamine as intermediate layer. Surf. Coat. Technol. 2014, 258, 664–671. [Google Scholar] [CrossRef]
- Singer, F.; Schlesak, M.; Mebert, C.; Höhn, S.; Virtanen, S. Corrosion properties of polydopamine coatings formed in one-step immersion process on magnesium. Appl. Mater. Interfaces 2015, 7, 26758–26766. [Google Scholar] [CrossRef]
- Lin, B.; Zhong, M.; Zheng, C.; Cao, L.; Wang, D.; Wang, L.; Liang, J.; Cao, B. Preparation and characterization of dopamine-induced biomimetic hydroxyapatite coatings on the AZ31 magnesium alloy. Surf. Coat. Technol. 2015, 281, 82–88. [Google Scholar] [CrossRef]
- Lynge, M.E.; Westen, R.V.D.; Postmab, A.; Stadler, B. Polydopamine—A nature-inspired polymer coating for biomedical science. Nanoscale 2011, 3, 4916–4928. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Yang, H.-C.; Wu, Q.-Y.; Wan, L.-S.; Xu, Z.-K. Polydopamine gradients by oxygen diffusion controlled autoxidation. Chem. Commun. 2013, 49, 10522–10524. [Google Scholar] [CrossRef]
- Sedó, J.; Saiz-Poseu, J.; Busqué, F.; Ruiz-Molina, D. Catechol-based biomimetic functional materials. Adv. Mater. 2013, 25, 653–701. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Dyke, J.C.; Bowman, B.A.; Ko, C.-C.; You, W. Investigation of dopamine analogues: Synthesis, mechanistic understanding and structure-property relationship. Langmuir 2016, 32, 9873–9882. [Google Scholar] [CrossRef] [PubMed]
- Herlinger, E.; Jameson, R.F.; Linert, W. Spontaneous autoxidation of dopamine. J. Chem. Soc. Perkin Trans. 1995, 2, 259–263. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the structure of poly(dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef] [PubMed]
- Bernsmann, F.; Ball, V.; Addiego, F.; Ponche, A.; Michel, M.; Gracio, J.J.D.A.; Toniazzo, V.; Ruch, D. Dopamine-melanin film deposition depends on the used oxidant and buffer solution. Langmuir 2011, 27, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Ponzio, F.; Barthes, J.; Bour, J.; Michel, M.; Bertani, P.; Hemmerle, J.; d’Ischia, M.; Ball, V. Oxidant control of polydopamine surface chemistry in acids: A mechanism-based entry to superhydrophilic-superoleophobic coatings. Chem. Mater. 2016, 28, 4697–4705. [Google Scholar] [CrossRef]
- Zhang, L.; Mohammed, E.A.A.; Adriaens, A. Synthesis and electrochemical behavior of a magnesium fluoride-polydopamine-stearic acid composite coating on AZ31 magnesium alloy. Surf. Coat. Technol. 2016, 307, 56–64. [Google Scholar] [CrossRef]
- Szaraniec, B.; Pielichowsk, K.; Pac, E.; Menaszek, E. Multifunctional polymer coatings for titanium implants. Mater. Sci. Eng. C 2018, 93, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Mayo, S.L.; Olafson, B.D.; Goddard, W.A. Dreiding: A generic force field for molecular simulations. J. Phys. Chem. 1990, 94, 8897–8909. [Google Scholar] [CrossRef]
- Panteva, M.T.; Giambasu, G.M.; York, D.M. Force field for Mg2+, Mn2+, Zn2+, and Cd2+ ions that have balanced interactions with nucleic acids. J. Phys. Chem. 2015, 119, 15460–15470. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, G.H.; Kim, H.; Kim, W.J. Enhanced corrosion resistance of high strength Mg–3Al–1Zn alloy sheets with ultrafine grains in a phosphate-buffered saline solution. Corros. Sci. 2013, 74, 139–148. [Google Scholar] [CrossRef]
- Pawar, S.; Zhou, X.; Thompson, G.E.; Scamans, G.; Fan, Z. The role of intermetallics on the corrosion initiation of twin roll cast AZ31 mg alloy. J. Electrochem. Soc. 2015, 162, C442–C448. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhu, B.; Xu, Y. Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef] [PubMed]
- Simons, W.W. The Sadtler Handbook of Infrared Spectra; Sadtler Research Laboratories: Philadelphia, PA, USA, 1978. [Google Scholar]
- Orazem, M.E.; Orazem, M. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Orishchin, N.; Crane, C.C.; Brownell, M.; Wang, T.; Jenkins, S.; Min Zou, A.N.C. Rapid deposition of uniform polydopamine coatings on nanoparticle surfaces with controllable thickness. Langmuir 2017, 33, 6046–6053. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.H.; Floren, M.; Tan, W. Mussel-inspired polydopamine for bio-surface functionalization. Biosurface Biotribol. 2016, 2, 121–136. [Google Scholar] [CrossRef]
- Kim, H.W.; McCloskey, B.D.; Choi, T.H.; Lee, C.; Kim, M.J.; Freeman, B.D.; Park, H.B. Oxygen concentration control of dopamine-induced high uniformity surface coating chemistry. ACS Appl. Mater. Interfaces 2013, 5, 223–238. [Google Scholar]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: New York, NY, USA, 2014. [Google Scholar]
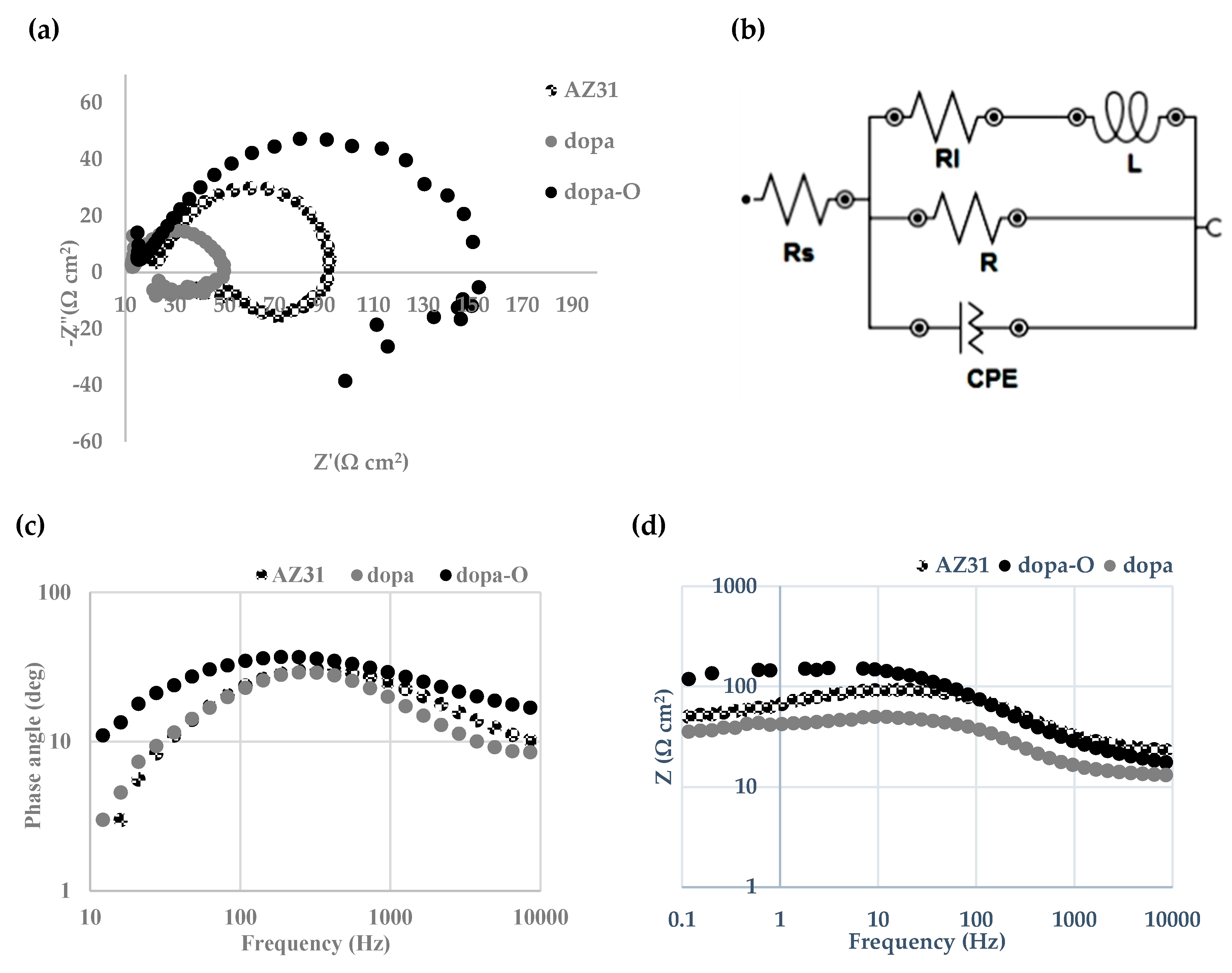

| Sample | Rs (Ω cm2) | R (Ω cm2) | CPE (µMho/cm2) | N | Rl (Ω cm2) | L (H cm2) | X2 (<0.5) |
|---|---|---|---|---|---|---|---|
| AZ31 | 22.1 | 68.7 | 39.6 | 0.841 | 38.3 | 13.9 | 0.385 |
| dopa | 13.152 | 31.44 | 50.83 | 0.652 | 15.408 | 71.52 | 0.427 |
| dopa-O | 15.38 | 142.32 | 105.83 | 0.718 | 185.52 | 264 | 0.206 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanbari, A.; Warchomicka, F.; Sommitsch, C.; Zamanian, A. Investigation of the Oxidation Mechanism of Dopamine Functionalization in an AZ31 Magnesium Alloy for Biomedical Applications. Coatings 2019, 9, 584. https://doi.org/10.3390/coatings9090584
Ghanbari A, Warchomicka F, Sommitsch C, Zamanian A. Investigation of the Oxidation Mechanism of Dopamine Functionalization in an AZ31 Magnesium Alloy for Biomedical Applications. Coatings. 2019; 9(9):584. https://doi.org/10.3390/coatings9090584
Chicago/Turabian StyleGhanbari, Arezoo, Fernando Warchomicka, Christof Sommitsch, and Ali Zamanian. 2019. "Investigation of the Oxidation Mechanism of Dopamine Functionalization in an AZ31 Magnesium Alloy for Biomedical Applications" Coatings 9, no. 9: 584. https://doi.org/10.3390/coatings9090584
APA StyleGhanbari, A., Warchomicka, F., Sommitsch, C., & Zamanian, A. (2019). Investigation of the Oxidation Mechanism of Dopamine Functionalization in an AZ31 Magnesium Alloy for Biomedical Applications. Coatings, 9(9), 584. https://doi.org/10.3390/coatings9090584







