Ultra-Long-Term Reliable Encapsulation Using an Atomic Layer Deposited HfO2/Al2O3/HfO2 Triple-Interlayer for Biomedical Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Setup
3. Results and Discussion
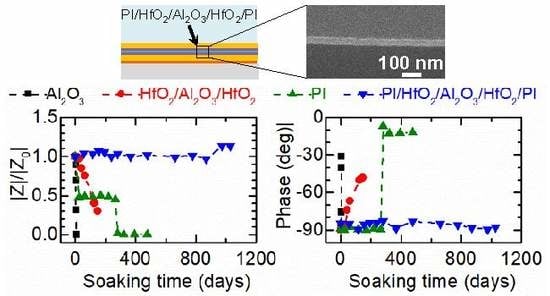
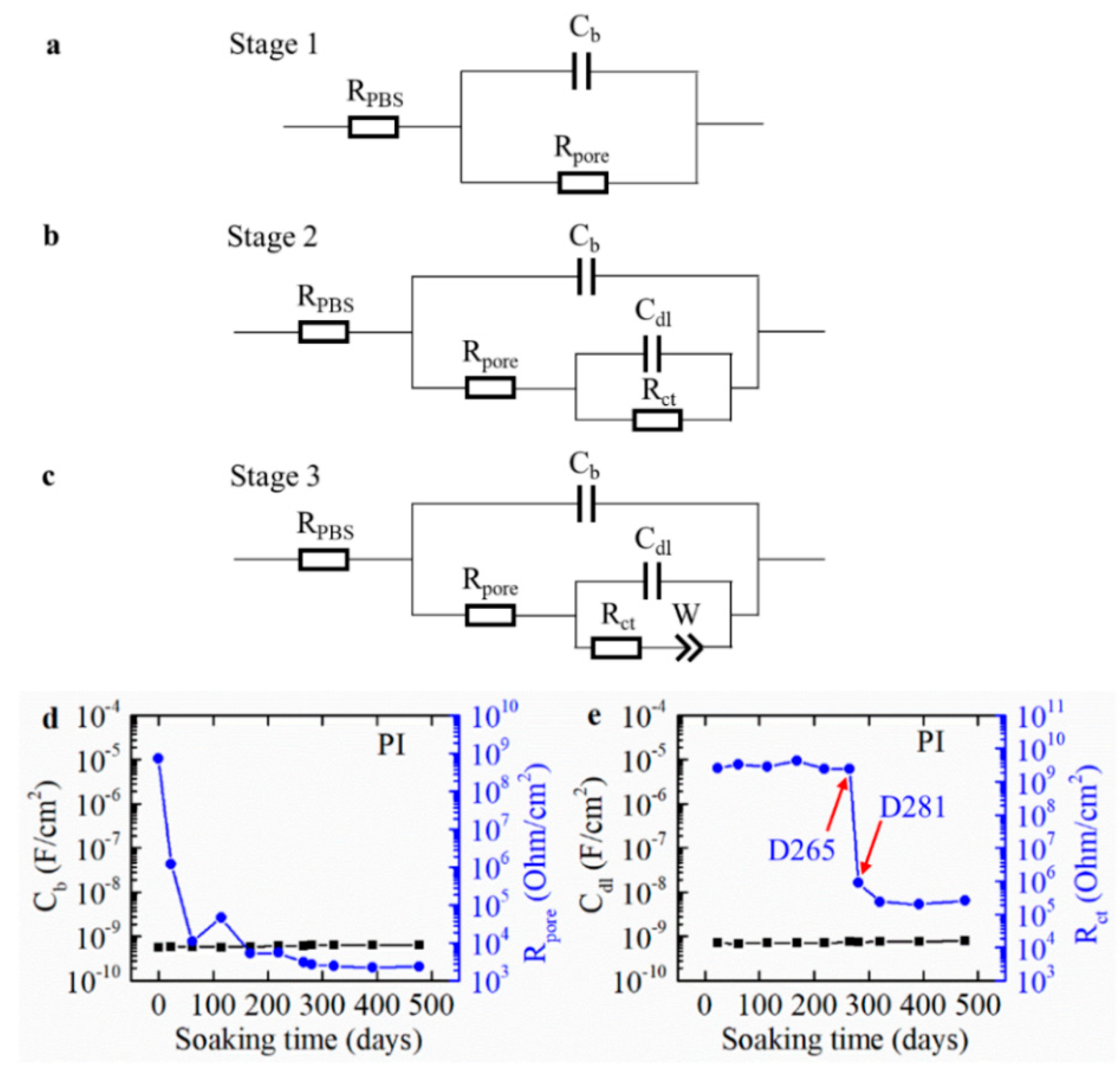
3.1. EIS
3.2. Optical Microscope Observation
3.3. Equivalent Circuit Modeling of the Measured EIS Curve
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lacour, S.P.; Courtine, G.; Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 2016, 1, 1–14. [Google Scholar] [CrossRef]
- Xue, Y.; Shiuan, Y.; McIlvried, L.A.; Copits, B.A.; Noh, K.N.; Zhang, J.; Samineni, V.K.; Payne, M.A.; Won, S.M.; Kim, B.H.; et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 2018, 565, 361–365. [Google Scholar]
- Jiang, G.; Zhou, D.D. Implantable Neural Prostheses 2; Springer: New York, NY, USA, 2010; ISBN 978-0-387-98119-2. [Google Scholar]
- Op De Beeck, M.; Verplancke, R.; Schaubroeck, D.; Cuypers, D.; Cauwe, M. Ultra-thin biocompatible implantable chip for bidirectional communication with peripheral nerves. In Proceedings of the 2017 IEEE Biomedical Circuits and Systems Conference (BioCAS), Turin, Italy, 19–21 October 2017; pp. 1–4. [Google Scholar]
- Yang, Y.; Deng, Z.D. Stretchable sensors for environmental monitoring. Appl. Phys. Rev. 2019, 6, 011309. [Google Scholar] [CrossRef] [Green Version]
- Song, E.; Fang, H.; Jin, X.; Zhao, J.; Jiang, C.; Yu, K.J.; Zhong, Y.; Xu, D.; Li, J.; Fang, G.; et al. Thin, transferred layers of silicon dioxide and silicon nitride as water and ion barriers for implantable flexible electronic systems. Adv. Electron. Mater. 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric biomaterials for medical implants and devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Kim, S.H.; Moon, J.H.; Kim, J.H.; Jeong, S.M.; Lee, S.H. Flexible, stretchable and implantable PDMS encapsulated cable for implantable medical device. Biomed. Eng. Lett. 2011, 1, 199–203. [Google Scholar] [CrossRef]
- de Beeck, M.O.; Jarboui, A.; Cauwe, M.; Declercq, H.; Uytterhoeven, G.; Cornelissen, M.; Vanfleteren, J.; Van Hoof, C. Improved chip & component encapsulation by dedicated diffusion barriers to reduce corrosion sensitivity in biological and humid environments. In Proceedings of the 2013 Eurpoean Microelectronics Packaging Conference (EMPC), Grenoble, France, 9–12 September 2013; pp. 1–6. [Google Scholar]
- Lecomte, A.; Degache, A.; Descamps, E.; Dahan, L.; Bergaud, C. In vitro and in vivo biostability assessment of chronically-implanted Parylene C neural sensors. Sens. Actuators B Chem. 2017, 251, 1001–1008. [Google Scholar] [CrossRef]
- Xie, X.; Rieth, L.; Merugu, S.; Tathireddy, P.; Solzbacher, F. Plasma-assisted atomic layer deposition of Al2O3 and parylene C bi-layer encapsulation for chronic implantable electronics. Appl. Phys. Lett. 2012, 101, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Minnikanti, S.; Diao, G.; Pancrazio, J.J.; Xie, X.; Rieth, L.; Solzbacher, F.; Peixoto, N. Lifetime assessment of atomic-layer-deposited Al2O3-Parylene C bilayer coating for neural interfaces using accelerated age testing and electrochemical characterization. Acta Biomater. 2014, 10, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J. Material challenge for flexible organic devices. Mater. Today 2006, 9, 38–45. [Google Scholar] [CrossRef]
- Yoon, K.H.; Kim, H.S.; Han, K.S.; Kim, S.H.; Lee, Y.E.K.; Shrestha, N.K.; Song, S.Y.; Sung, M.M. Extremely high barrier performance of organic-inorganic nanolaminated thin films for organic light-emitting diodes. ACS Appl. Mater. Interfaces 2017, 9, 5399–5408. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.W.; Jung, E.; Chae, H.; Cho, S.M. Optimization of Al2O3/ZrO2 nanolaminate structure for thin-film encapsulation of OLEDs. Org. Electron. Phys. Mater. Appl. 2012, 13, 2436–2441. [Google Scholar] [CrossRef]
- Iacob, N.; Chindriş, I. Low-temperature atomic layer deposition of Al2O3 thin coatings for corrosion protection of steel: Surface and electrochemical analysis. Corros. Sci. 2012, 53, 2168–2175. [Google Scholar]
- Abdulagatov, A.I.; Yan, Y.; Cooper, J.R.; Zhang, Y.; Gibbs, Z.M.; Cavanagh, A.S.; Yang, R.G.; Lee, Y.C.; George, S.M. Al2O3 and TiO2 atomic layer deposition on copper for water corrosion resistance. ACS Appl. Mater. Interfaces 2011, 3, 4593–4601. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.Z.; Rieth, L.; Tathireddy, P.; Solzbacher, F. Long-term in-vivo investigation of parylene-C as encapsulation material for neural interfaces. Procedia Eng. 2011, 25, 483–486. [Google Scholar] [CrossRef]
- Morales, J.M.H. Evaluating Biocompatible Barrier Films as Encapsulants of Medical Micro Devices. Ph.D. Thesis, Université Grenoble Alpes, Grenoble, France, 2016. [Google Scholar]
- Steven, M.G. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]
- Kim, L.H.; Kim, K.; Park, S.; Jeong, Y.J.; Kim, H.; Chung, D.S.; Kim, S.H.; Park, C.E. Al2O3/TiO2 nanolaminate thin film encapsulation for organic thin film transistors via plasma-enhanced atomic layer deposition. ACS Appl. Mater. Interfaces 2014, 6, 6731–6738. [Google Scholar] [CrossRef]
- Hirvikorpi, T.; Laine, R.; Vähä-Nissi, M.; Kilpi, V.; Salo, E.; Li, W.-M.; Lindfors, S.; Vartiainen, J.; Kenttä, E.; Nikkola, J.; et al. Barrier properties of plastic films coated with an Al2O3 layer by roll-to-toll atomic layer deposition. Thin Solid Films 2014, 550, 164–169. [Google Scholar] [CrossRef]
- Vanhaverbeke, C.; Cauwe, M.; Stockman, A.; Op de Beeck, M.; De Smet, H. Comparison of copper electroplating, copper wet etching and linear sweep voltammetry as techniques to investigate the porosity of atomic layer deposited Al2O3. Thin Solid Films 2019, 686, 137424. [Google Scholar] [CrossRef]
- Kim, L.H.; Jang, J.H.; Jeong, Y.J.; Kim, K.; Baek, Y.; Kwon, H.-J.; An, T.K.; Nam, S.; Kim, S.H.; Jang, J.; et al. Highly-impermeable Al2O3/HfO2 moisture barrier films grown by low-temperature plasma-enhanced atomic layer deposition. Org. Electron. Phys. Mater. Appl. 2017, 50, 296–303. [Google Scholar] [CrossRef]
- Correa, G.C.; Bao, B.; Strandwitz, N.C. Chemical stability of titania and alumina thin films formed by atomic layer deposition. ACS Appl. Mater. Interfaces 2015, 7, 14816–14821. [Google Scholar] [CrossRef]
- Daubert, J.S.; Hill, G.T.; Gotsch, H.N.; Gremaud, A.P.; Ovental, J.S.; Williams, P.S.; Oldham, C.J.; Parsons, G.N. Corrosion protection of copper using Al2O3, TiO2, ZnO, HfO2, and ZrO2 Atomic layer deposition. ACS Appl. Mater. Interfaces 2017, 9, 4192–4201. [Google Scholar] [CrossRef] [PubMed]
- Nehm, F.; Klumbies, H.; Richter, C.; Singh, A.; Schroeder, U.; Mikolajick, T.; Mönch, T.; Hoßbach, C.; Albert, M.; Bartha, J.W.; et al. Breakdown and protection of ALD moisture barrier thin films. ACS Appl. Mater. Interfaces 2015, 7, 22121–22127. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Laiwalla, F.; Lee, J.; Ritasalo, R.; Pudas, M.; Larson, L.; Leung, V.; Nurmikko, A. Conformal hermetic sealing of wireless microelectronic implantable chiplets by multilayered atomic layer deposition (ALD). Adv. Funct. Mater. 2019, 29, 1–10. [Google Scholar] [CrossRef]
- Ahn, S.H.; Jeong, J.; Kim, S.J. Emerging encapsulation technologies for long-term reliability of microfabricated implantable devices. Micromachines 2019, 10, 508. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-R. Comprehensive Handbook of Chemical Bond Energies; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Schaubroeck, D.; Verplancke, R.; Cauwe, M.; Cuypers, D.; Baumans, K.; Op De Beeck, M. Polyimide-ALD-polyimide layers as hermetic encapsulant for implants. In Proceedings of the XXXI International Conference on Surface Modification Technologies (SMT31), Mons, Belgium, 5–7 July 2017; pp. 1–6. [Google Scholar]
- Yang, Y.; Chiesura, G.; Plovie, B.; Vervust, T.; Luyckx, G.; Degrieck, J.; Sekitani, T.; Vanfleteren, J. Design and integration of flexible sensor matrix for in situ monitoring of polymer composites. ACS Sens. 2018, 3, 1698–1705. [Google Scholar] [CrossRef]
- Hassler, C.; Boretius, T.; Stieglitz, T. Polymers for neural implants. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 18–33. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, K.; Vervust, T.; Vanfleteren, J. Multifunctional and miniaturized flexible sensor patch: Design and application for in situ monitoring of epoxy polymerization. Sens. Actuators B Chem. 2018, 261, 144–152. [Google Scholar] [CrossRef]
- Yang, Y.; Martens, T.; Vandecasteele, B.; Degrendele, L.; Mader, L.; De Vriese, L.; Sekitani, T.; Kaufmann, M.; Van Put, S.; Vervust, T.; et al. 3D multifunctional composites based on large-area stretchable circuit with thermoforming technology. Adv. Electron. Mater. 2018, 4, 1800071. [Google Scholar] [CrossRef]
- Meyer, J.-U.; Stieglitz, T.; Scholz, O.; Haberer, W.; Beutel, H. High density interconnects and flexible hybrid assemblies for active biomedical implants. IEEE Trans. Adv. Packag. 2001, 24, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Herth, E.; Guerchouche, K.; Rousseau, L.; Calvet, L.E.; Loyez, C. A biocompatible and flexible polyimide for wireless sensors. Microsyst. Technol. 2017, 23, 5921–5929. [Google Scholar] [CrossRef]
- Loveday, D.; Peterson, P.; Rodgers, B. Evaluation of organic coatings with electrochemical impedance spectroscopy part 2: Application of EIS to coatings. JCT Coat. Tech. 2004, 1, 88–93. [Google Scholar]
- van Westing, E.P.M.; Ferrari, G.M.; De Wit, J.H.W. The determination of coating performance using electrochemical impedance spectroscopy. Electrochim. Acta 1994, 39, 899–910. [Google Scholar] [CrossRef]
- Loveday, D.; Peterson, P.; Rodgers, B. Evaluation of organic coatings with electrochemical impedance spectroscopy, part 3: Protocols for testing coatings with EIS. JCT Coat. Tech. 2005, 2, 22–27. [Google Scholar]
- Vidulich, G.A.; Evans, D.F.; Kay, R.L. The dielectric constant of water and heavy water between 0 and 40 degree. J. Phys. Chem. 1967, 71, 656–662. [Google Scholar] [CrossRef]
- HD MicroSystems “PI-2600 Series—Low Stress Applications” Product Bulletin. Available online: https://www.dupont.com/content/dam/dupont/products-and-services/electronic-and-electrical-materials/semiconductor-fabrication-and-packaging-materials/documents/PI-2600_ProcessGuide.pdf (accessed on 11 September 2019).
- Woods, V.; Trumpis, M.; Bent, B.; Palopoli-Trojani, K.; Chiang, C.H.; Wang, C.; Yu, C.; Insanally, M.N.; Froemke, R.C.; Viventi, J. Long-term recording reliability of liquid crystal polymer μ ECoG arrays. J. Neural Eng. 2018, 15, 066024. [Google Scholar] [CrossRef]
- Forssell, M.; Ong, X.C.; Khilwani, R.; Burak Ozdoganlar, O.; Fedder, G.K. Insulation of thin-film parylene-C/platinum probes in saline solution through encapsulation in multilayer ALD ceramic films. Biomed. Microdevices 2018, 20, 61. [Google Scholar] [CrossRef]
- Zhang, Y.; Seghete, D.; Abdulagatov, A.; Gibbs, Z.; Cavanagh, A.; Yang, R.; George, S.; Lee, Y.-C. Investigation of the defect density in ultra-thin Al2O3 films grown using atomic layer deposition. Surf. Coat. Technol. 2011, 205, 3334–3339. [Google Scholar] [CrossRef]
- Klumbies, H.; Schmidt, P.; Hähnel, M.; Singh, A.; Schroeder, U.; Richter, C.; Mikolajick, T.; Hoßbach, C.; Albert, M.; Bartha, J.W.; et al. Thickness dependent barrier performance of permeation barriers made from atomic layer deposited alumina for organic devices. Org. Electron. Phys. Mater. Appl. 2015, 17, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Corrosion Part 4—Equivalent Circuit Models. Available online: https://www.metrohm-autolab.com/download/Applicationnotes/Autolab_Application_Note_COR04.pdf (accessed on 11 September 2019).
- Chang, K.; Chemical, E.; Hsu, C.; Ji, W.; Chang, C. Advanced anticorrosive coatings prepared from electroactive polyimide/graphene nanocomposites with synergistic effects of redox catalytic capability and gas barrier properties. Express Polym. Lett. 2015, 8, 243–255. [Google Scholar] [CrossRef]
- Yota, J.; Shen, H.; Ramanathan, R. Characterization of atomic layer deposition HfO2, Al2O3, and plasma-enhanced chemical vapor deposition Si3N4 as metal–insulator–metal capacitor dielectric for GaAs HBT technology. J. Vac. Sci. Technol. 2012, 31, 01A134. [Google Scholar] [CrossRef]
- Vyas, R.N.; Li, K.; Wang, B. Modifying randles circuit for analysis of polyoxometalate layer-by-layer films. J. Phys. Chem. B 2010, 114, 15818–15824. [Google Scholar] [CrossRef] [PubMed]
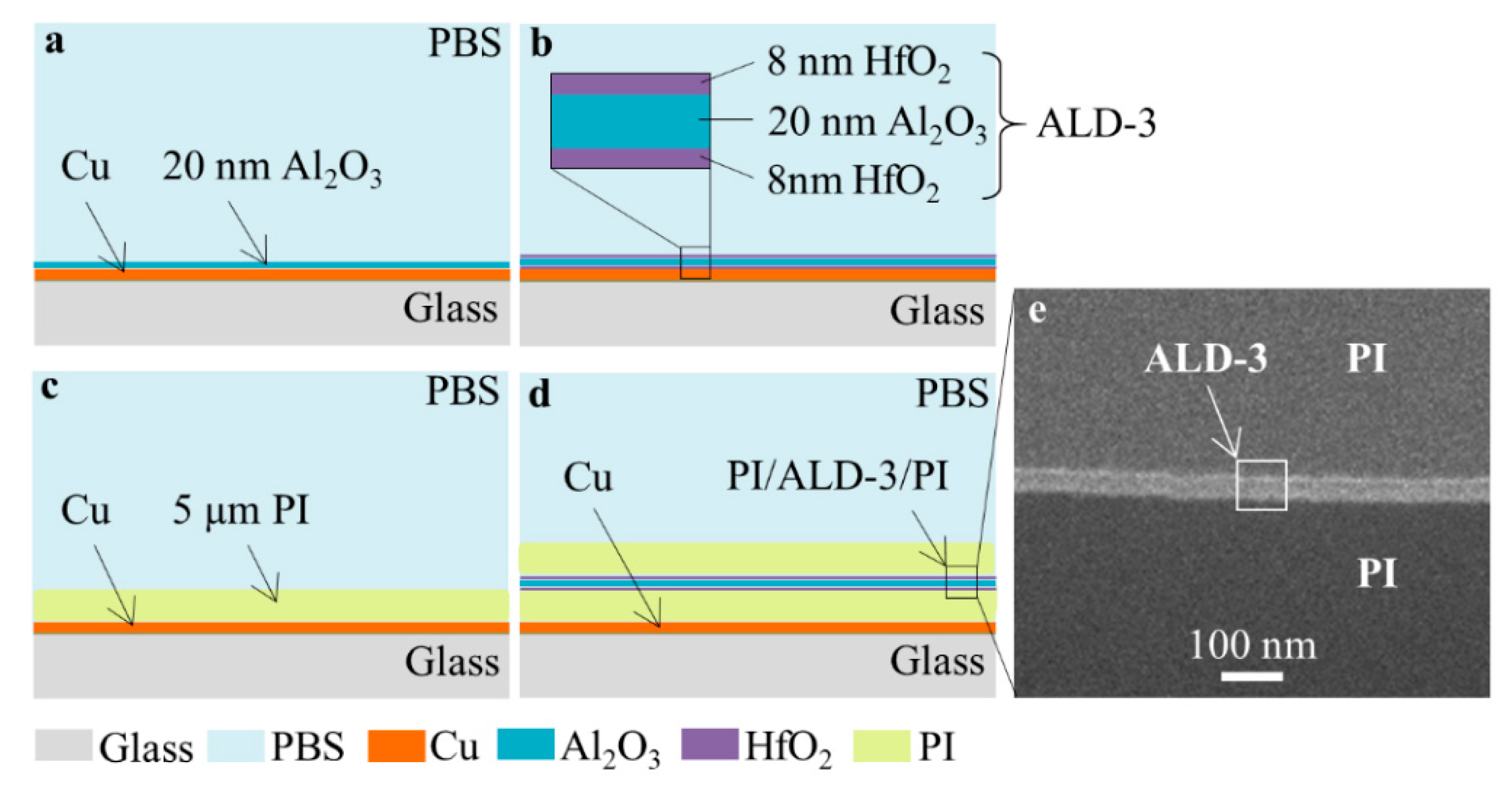
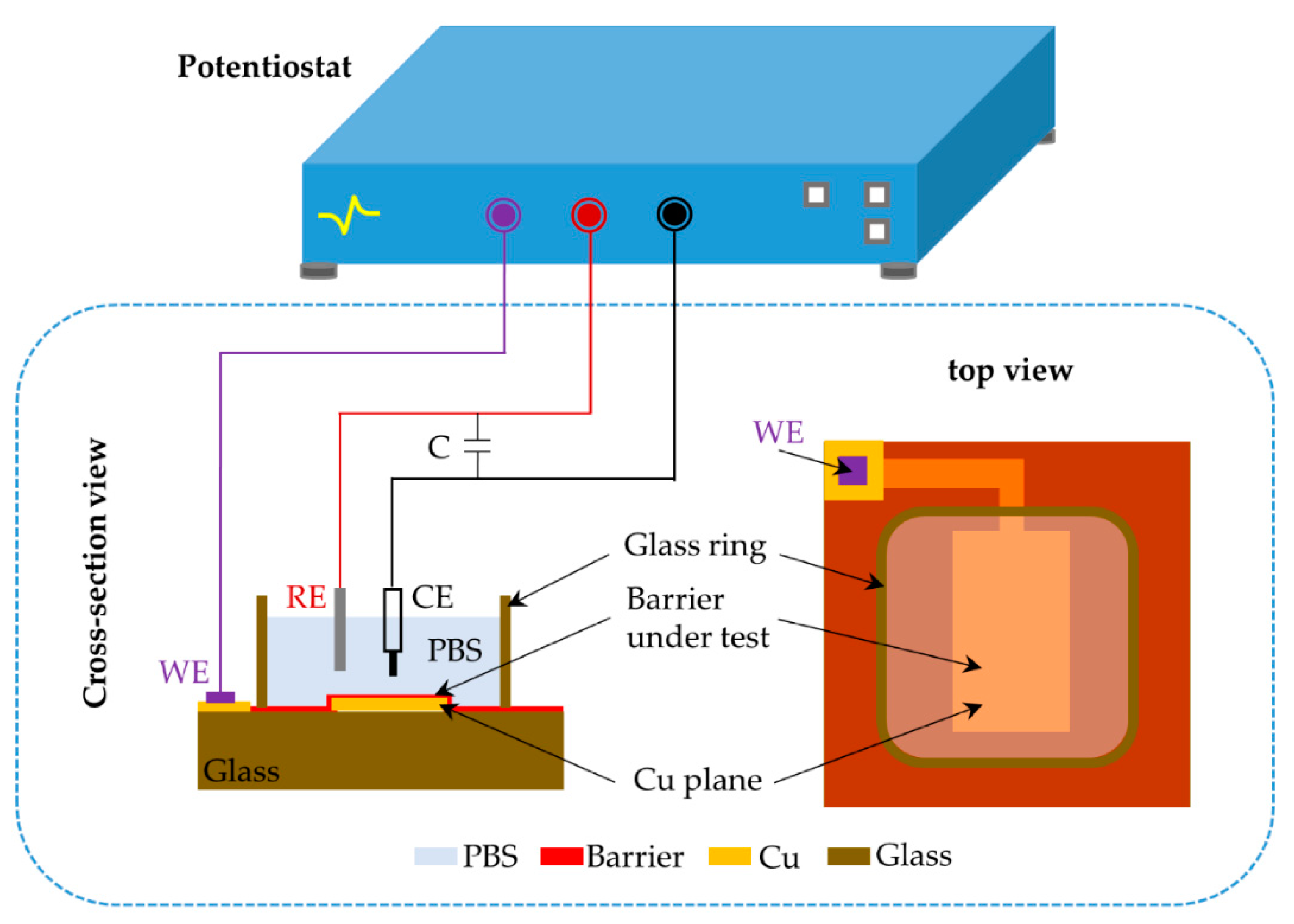
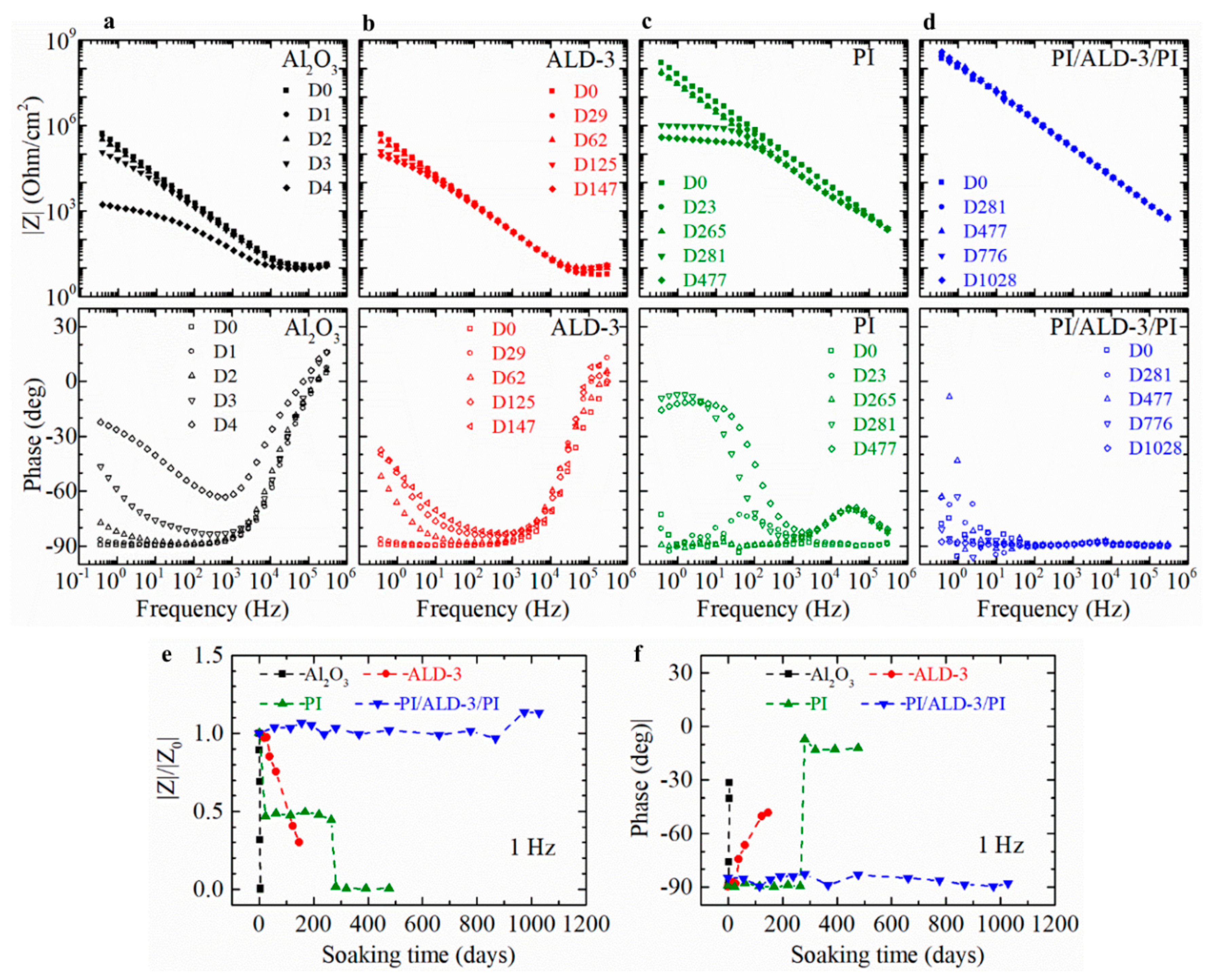
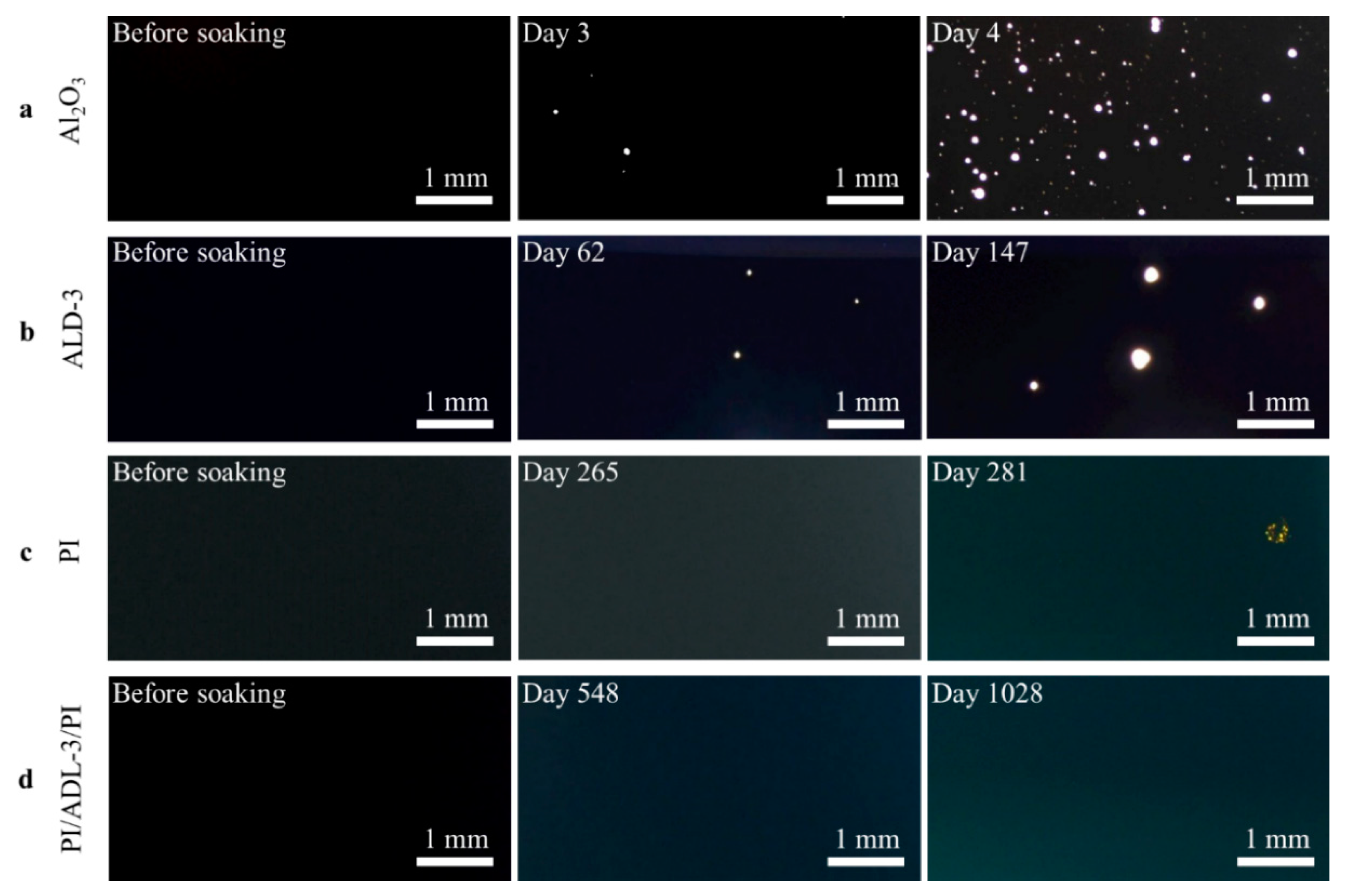
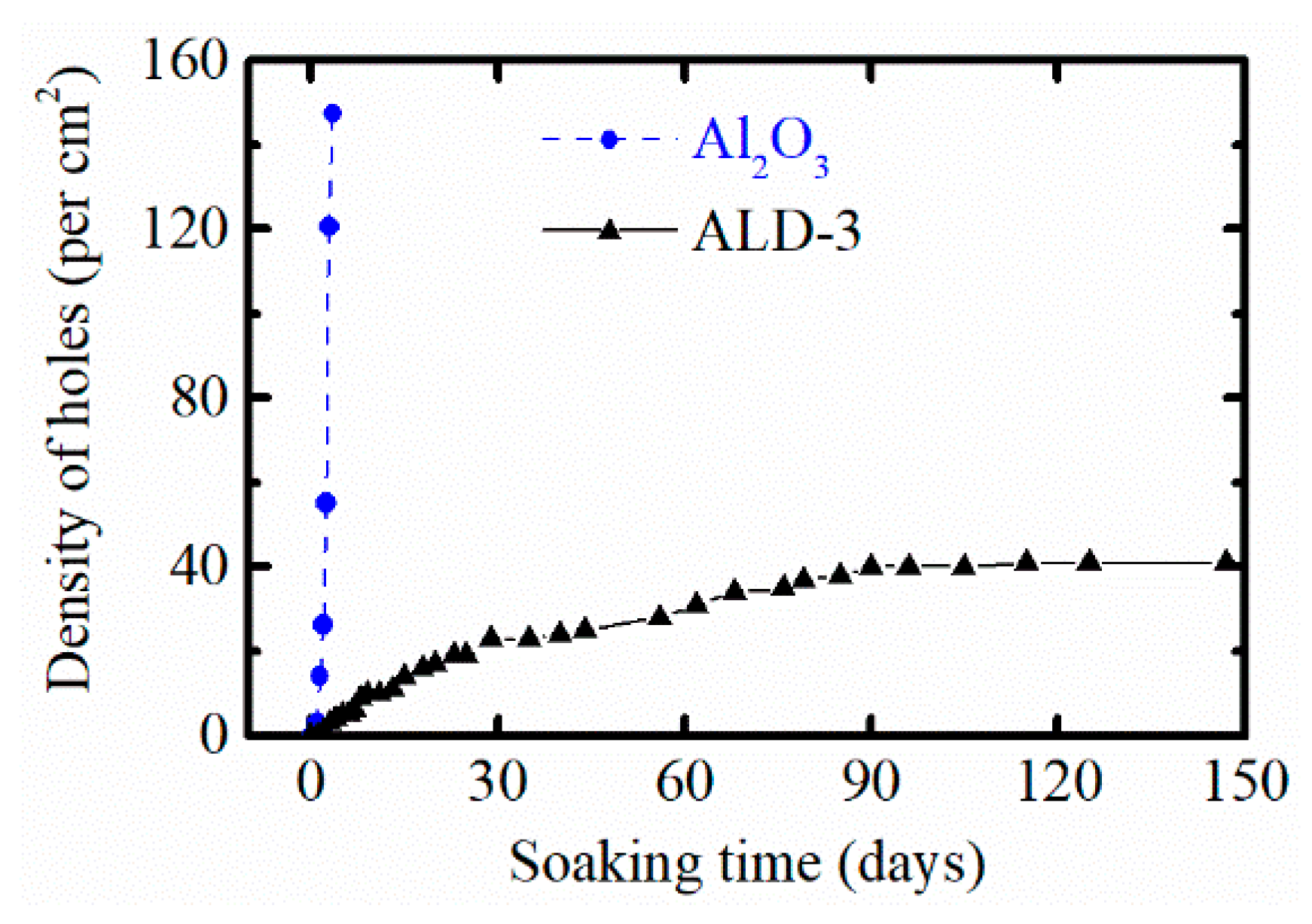
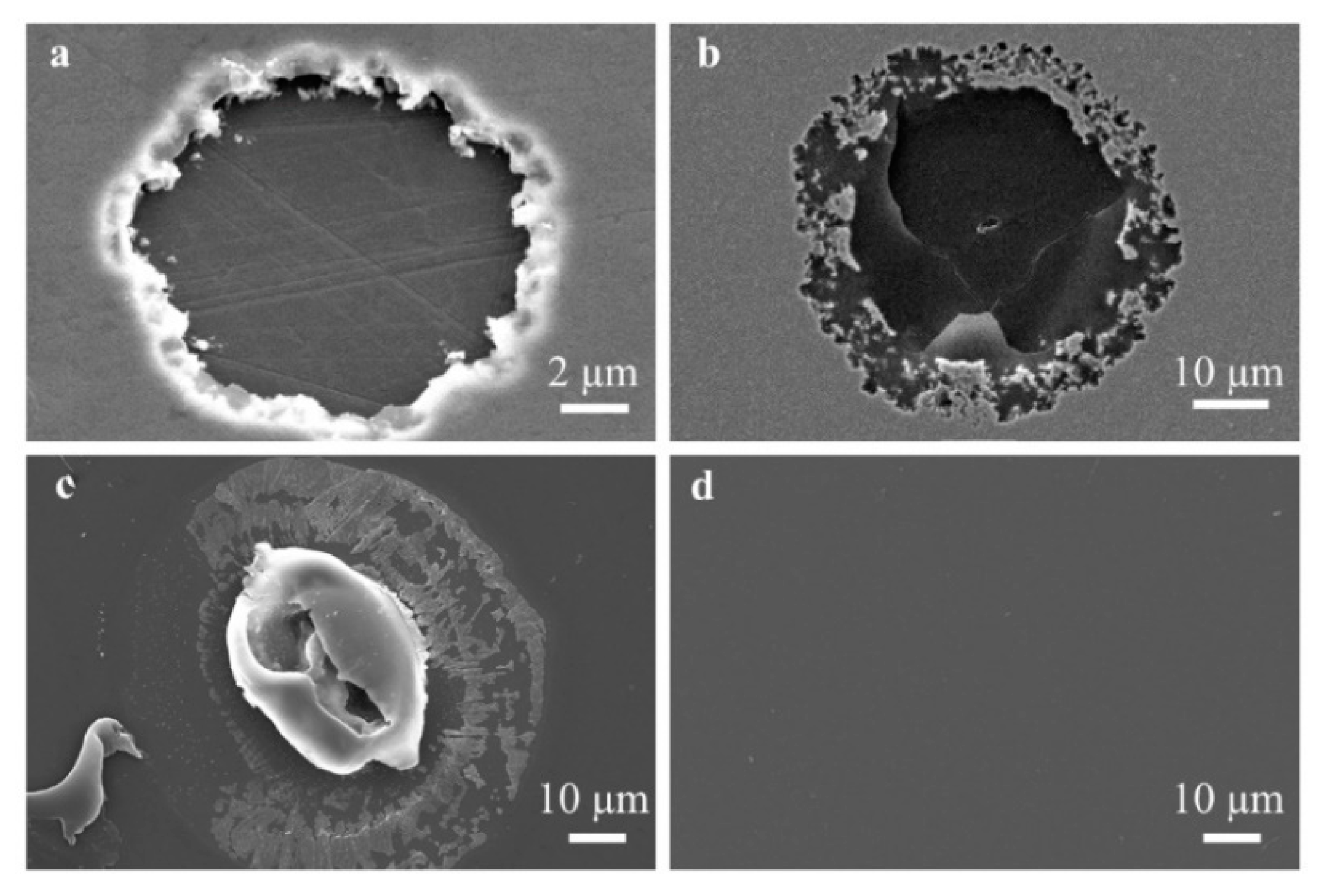
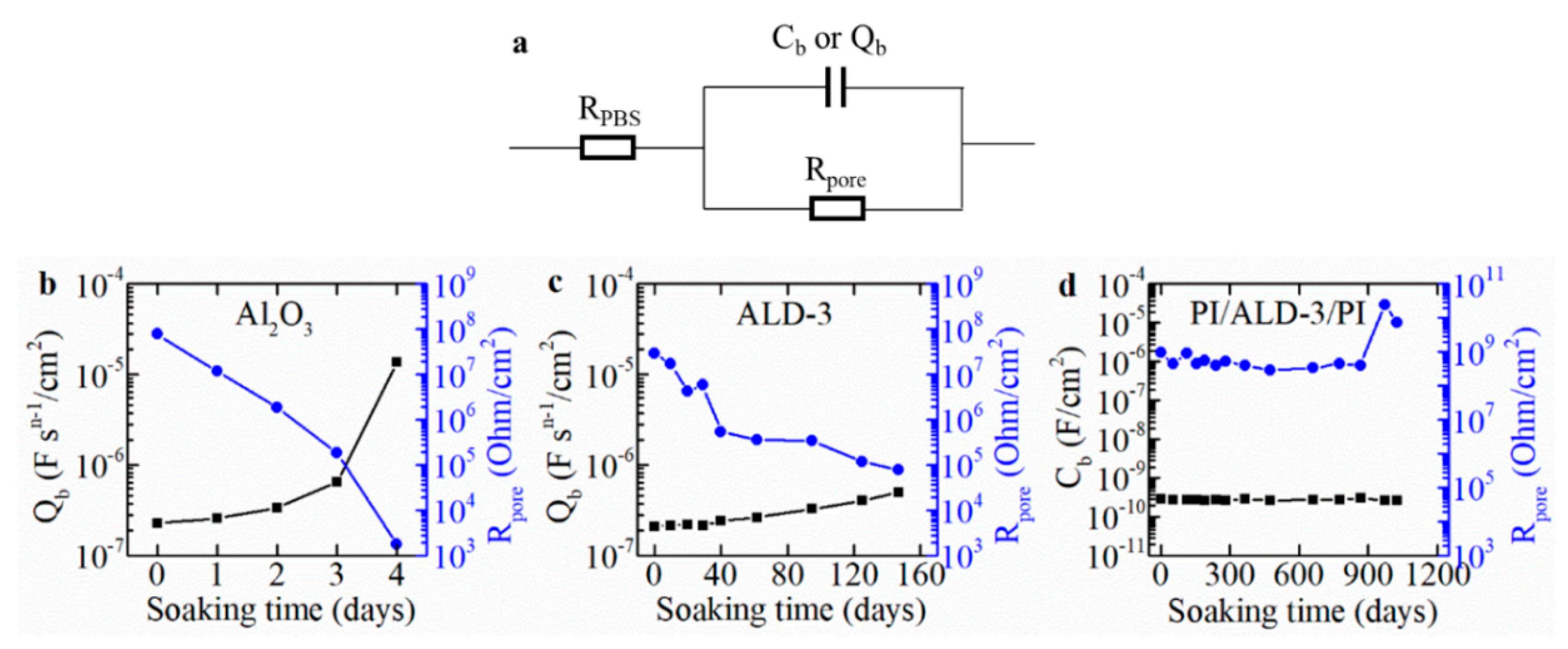
| Barriers | Acceleration Soaking Temperature and Lifetime | Equivalent Lifetime at 37 °C (Q10 = 2) | Ref. |
|---|---|---|---|
| 52 nm Al2O3 + 6 µm Parylene C | 60 °C in PBS, 215 days | 3.0 years | [12] |
| 25 µm LCP | 60 °C in PBS, 221 days | 3.1 years | [44] |
| 10 µm Parylene C + 50 nm TiO2 + 80 nm Al2O3-TiO2 | 60 °C in PBS, 106 days | 1.5 years | [45] |
| 5 µm PI + 8 nm HfO2 + 20 nm Al2O3 + 8 nm HfO2 + 5 µm PI | 60 °C in PBS, 1028 days | >14.3 years | This work |
| Materials | A (m2) | εr | d (m) |
|---|---|---|---|
| Al2O3 | 2 × 10−4 | 10.3 [50] | 2.0 × 10−8 |
| HfO2 | 2 × 10−4 | 18.7 [50] | 8.0 × 10−9 |
| PI | 2 × 10−4 | 2.9 [43] | 5.0 × 10−6 |
| Barriers | C (F/cm2) |
|---|---|
| Al2O3 | 4.56 × 10−7 |
| ALD-3 | 3.66 × 10−7 |
| PI | 5.14 × 10−10 |
| PI/ALD-3/PI | 2.57 × 10−10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Cauwe, M.; Yang, Y.; Schaubroeck, D.; Mader, L.; Op de Beeck, M. Ultra-Long-Term Reliable Encapsulation Using an Atomic Layer Deposited HfO2/Al2O3/HfO2 Triple-Interlayer for Biomedical Implants. Coatings 2019, 9, 579. https://doi.org/10.3390/coatings9090579
Li C, Cauwe M, Yang Y, Schaubroeck D, Mader L, Op de Beeck M. Ultra-Long-Term Reliable Encapsulation Using an Atomic Layer Deposited HfO2/Al2O3/HfO2 Triple-Interlayer for Biomedical Implants. Coatings. 2019; 9(9):579. https://doi.org/10.3390/coatings9090579
Chicago/Turabian StyleLi, Changzheng, Maarten Cauwe, Yang Yang, David Schaubroeck, Lothar Mader, and Maaike Op de Beeck. 2019. "Ultra-Long-Term Reliable Encapsulation Using an Atomic Layer Deposited HfO2/Al2O3/HfO2 Triple-Interlayer for Biomedical Implants" Coatings 9, no. 9: 579. https://doi.org/10.3390/coatings9090579
APA StyleLi, C., Cauwe, M., Yang, Y., Schaubroeck, D., Mader, L., & Op de Beeck, M. (2019). Ultra-Long-Term Reliable Encapsulation Using an Atomic Layer Deposited HfO2/Al2O3/HfO2 Triple-Interlayer for Biomedical Implants. Coatings, 9(9), 579. https://doi.org/10.3390/coatings9090579