Surface Stoichiometry and Optical Properties of Cux–TiyCz Thin Films Deposited by Magnetron Sputtering
Abstract
1. Introduction
2. Materials and Methods
2.1. Cux–TiyCz Film Synthesis (Combination of DC-MS and HiPIMS)
2.2. Ar Etching and Thickness Measurement
2.3. Film Characterization
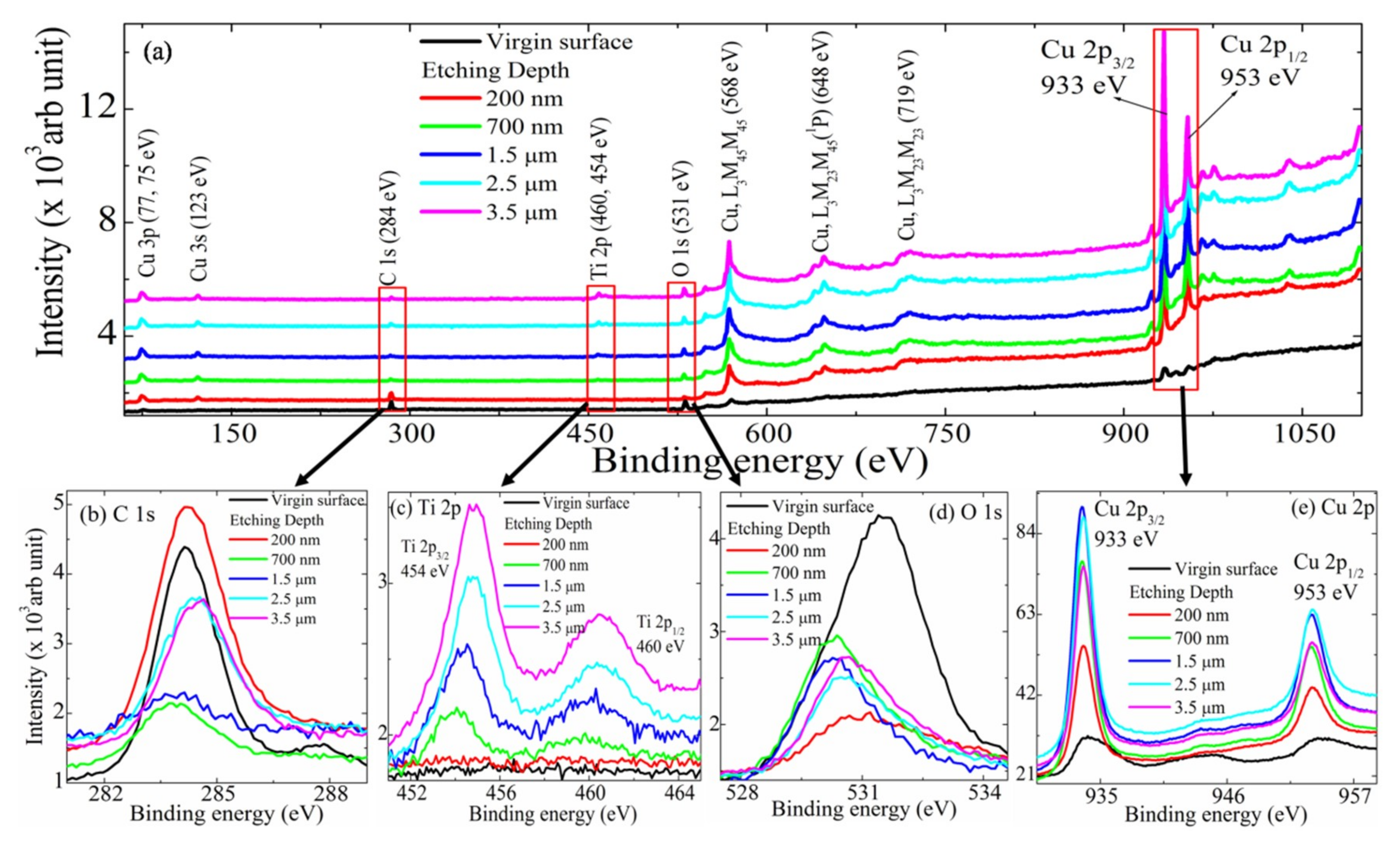
3. Results and Discussion
3.1. XPS of Cux–TiyCz Film
3.2. TEM
3.3. UV-VIS Spectra of Cux-TiyCz Film
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Callister, W.D. Materials Science and Engineering: An Introduction; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Xu, X.; Li, W.; Wang, Y.; Dong, G.; Jing, S.; Wang, Q.; Feng, Y.; Fan, X.; Ding, H. Study of the preparation of Cu-TiC composites by reaction of soluble Ti and ball-milled carbon coating TiC. Results Phys. 2018, 9, 486–492. [Google Scholar] [CrossRef]
- Rathod, S.; Modi, O.; Prasad, B.; Chrysanthou, A.; Vallauri, D.; Deshmukh, V.; Shah, A. Cast in situ Cu–TiC composites: Synthesis by SHS route and characterization. Mater. Sci. Eng. A 2009, 502, 91–98. [Google Scholar] [CrossRef]
- Bagheri, G.A. The effect of reinforcement percentages on properties of copper matrix composites reinforced with TiC particles. J. Alloy. Compd. 2016, 676, 120–126. [Google Scholar] [CrossRef]
- Tjong, S.; Lau, K. Abrasive wear behavior of TiB2 particle-reinforced copper matrix composites. Mater. Sci. Eng. A 2000, 282, 183–186. [Google Scholar] [CrossRef]
- Akhtar, F.; Askari, S.J.; Shah, K.A.; Du, X.; Guo, S. Microstructure, mechanical properties, electrical conductivity and wear behavior of high volume TiC reinforced Cu-matrix composites. Mater. Charact. 2009, 60, 327–336. [Google Scholar] [CrossRef]
- Sapate, S.G.; Uttarwar, A.; Rathod, R.C.; Paretkar, R.K. Analyzing dry sliding wear behaviour of copper matrix composites reinforced with pre-coated SiCp particles. Mater. Des. 2009, 30, 376–386. [Google Scholar] [CrossRef]
- Buytoz, S.; Dagdelen, F.; Islak, S.; Kok, M.; Kir, D.; Ercan, E. Effect of the TiC content on microstructure and thermal properties of Cu–TiC composites prepared by powder metallurgy. J. Therm. Anal. Calorim. 2014, 117, 1277–1283. [Google Scholar] [CrossRef]
- Jha, P.; Gautam, R.K.; Tyagi, R.; Kumar, D. Sliding wear behavior of TiC-reinforced Cu-4 wt.% Ni matrix composites. J. Mater. Eng. Perform. 2016, 25, 4210–4218. [Google Scholar] [CrossRef]
- Sabbaghian, M.; Shamanian, M.; Akramifard, H.R.; Esmailzadeh, M. Effect of friction stir processing on the microstructure and mechanical properties of Cu–TiC composite. Ceram. Int. 2014, 40, 12969–12976. [Google Scholar] [CrossRef]
- Zhuang, J.; Liu, Y.; Cao, Z.; Li, Y. The influence of technological process on dry sliding wear behaviour of titanium carbide reinforcement copper matrix composites. Mater. Trans. 2010, 51, 2311–2317. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, K.Y. Effects of process parameters on microstructure and properties of TiC reinforced Cu-matrix composites. Adv. Mater. Res. 2012, 557–559, 318–323. [Google Scholar] [CrossRef]
- Karadag, M.; Acikbas, G. Investigation of electrical and mechanical properties of Cu matrix TiC reinforced composites. Sch. J. Eng. Tech. 2018, 6, 58–63. [Google Scholar]
- Stranak, V.; Cada, M.; Hubicka, Z.; Tichy, M.; Hippler, R. Time-resolved investigation of dual high power impulse magnetron sputtering with closed magnetic field during deposition of Ti–Cu thin films. J. Appl. Phys. 2010, 108, 043305. [Google Scholar] [CrossRef]
- Helmersson, U.; Lattemann, M.; Bohlmark, J.; Ehiasarian, A.P.; Gudmundsson, J.T. Ionized physical vapor deposition (IPVD): A review of technology and applications. Thin Solid Film. 2006, 513, 1–24. [Google Scholar] [CrossRef]
- Bohlmark, J.; Lattemann, M.; Gudmundsson, J.; Ehiasarian, A.; Gonzalvo, Y.A.; Brenning, N.; Helmersson, U. The ion energy distributions and ion flux composition from a high power impulse magnetron sputtering discharge. Thin Solid Film. 2006, 515, 1522–1526. [Google Scholar] [CrossRef]
- Sarakinos, K.; Braun, A.; Zilkens, C.; Mráz, S.; Schneider, J.; Zoubos, H.; Patsalas, P. Exploring the potential of high power impulse magnetron sputtering for growth of diamond-like carbon films. Surf. Coat. Technol. 2012, 206, 2706–2710. [Google Scholar] [CrossRef]
- Aijaz, A.; Sarakinos, K.; Lundin, D.; Brenning, N.; Helmersson, U. A strategy for increased carbon ionization in magnetron sputtering discharges. Diam. Relat. Mater. 2012, 23, 1. [Google Scholar] [CrossRef]
- Stranak, V.; Wulff, H.; Rebl, H.; Zietz, C.; Arndt, K.; Bogdanowicz, R.; Nebe, B.; Bader, R.; Podbielski, A.; Hubicka, Z.; et al. Deposition of thin titanium–copper films with antimicrobial effect by advanced magnetron sputtering methods. Mater. Sci. Eng. 2011, C31, 1512–1519. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Fiermans, L.; Vennik, J.; Dekeyser, W. Electron and Ion Spectroscopy of Solids; Plenum: New York, NY, USA, 1978. [Google Scholar]
- Wang, P.H.; Yap, P.S.; Lim, T.T. C–N–S tridoped TiO2 for photocatalytic degradation of tetracycline under visible-light irradiation. Appl. Catal. A 2011, 399, 252–261. [Google Scholar] [CrossRef]
- Majumdar, A.; Das, G.; Basvani, K.R.; Heinicke, J.; Hippler, R. Role of nitrogen in the formation of HC-N films by CH4/N2 barrier discharge plasma: Aliphatic tendency. J. Phys. Chem. B 2009, 113, 15734–15741. [Google Scholar] [CrossRef]
- Majumdar, A.; Drache, S.; Wulff, H.; Mukhopadhyay, A.K.; Bhattacharyya, S.; Helm, C.A.; Hippler, R. Strain effects by surface oxidation of Cu3N thin films deposited by DC magnetron sputtering. Coatings 2017, 7, 64. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database. Available online: https://srdata.nist.gov/xps/ (accessed on 26 April 2019).
- Fu, Y.; Dua, H.; Zhang, S.; Huang, W. XPS characterization of surface and interfacial structure of sputtered TiNi films on Si substrate. Mater. Sci. Eng. 2005, A403, 25–31. [Google Scholar] [CrossRef]
- Chrysanthou, A.; Erbaccio, G. Production of copper-matrix composites by in situ processing. J. Mater. Sci. 1995, 30, 6339–6344. [Google Scholar] [CrossRef]
- Stranak, V.; Quaas, M.; Bogdanowicz, R.; Steffen, H.; Wulff, H.; Hubicka, Z.; Tichy, M.; Hippler, R. Effect of nitrogen doping on TiOxNy thin film formation at reactive high-power pulsed magnetron sputtering. J. Phys. D Appl. Phys. 2010, 43, 285203. [Google Scholar] [CrossRef]
- Stranak, V.; Cada, M.; Quaas, M.; Block, S.; Bogdanowicz, R.; Kment, S.; Wulff, H.; Hubicka, Z.; Helm, C.A.; Tichy, M.; Hippler, R. Physical properties of homogeneous TiO2 films prepared by high power impulse magnetron sputtering as a function of crystallographic phase and nanostructure. J. Phys. D: Appl. Phys. 2009, 42, 105204. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, A.; Mukhopadhyay, A.K.; Das, S.C.; Bhattacharjee, G.; Majumdar, A.; Hippler, R. Surface Stoichiometry and Optical Properties of Cux–TiyCz Thin Films Deposited by Magnetron Sputtering. Coatings 2019, 9, 551. https://doi.org/10.3390/coatings9090551
Roy A, Mukhopadhyay AK, Das SC, Bhattacharjee G, Majumdar A, Hippler R. Surface Stoichiometry and Optical Properties of Cux–TiyCz Thin Films Deposited by Magnetron Sputtering. Coatings. 2019; 9(9):551. https://doi.org/10.3390/coatings9090551
Chicago/Turabian StyleRoy, Avishek, Arun Kumar Mukhopadhyay, Sadhan Chandra Das, Gourab Bhattacharjee, Abhijit Majumdar, and Rainer Hippler. 2019. "Surface Stoichiometry and Optical Properties of Cux–TiyCz Thin Films Deposited by Magnetron Sputtering" Coatings 9, no. 9: 551. https://doi.org/10.3390/coatings9090551
APA StyleRoy, A., Mukhopadhyay, A. K., Das, S. C., Bhattacharjee, G., Majumdar, A., & Hippler, R. (2019). Surface Stoichiometry and Optical Properties of Cux–TiyCz Thin Films Deposited by Magnetron Sputtering. Coatings, 9(9), 551. https://doi.org/10.3390/coatings9090551





