Compositional and Morphological Changes in Water-Induced Early-Stage Degradation in Lead Halide Perovskites
Abstract
:1. Introduction
2. Materials and Methods
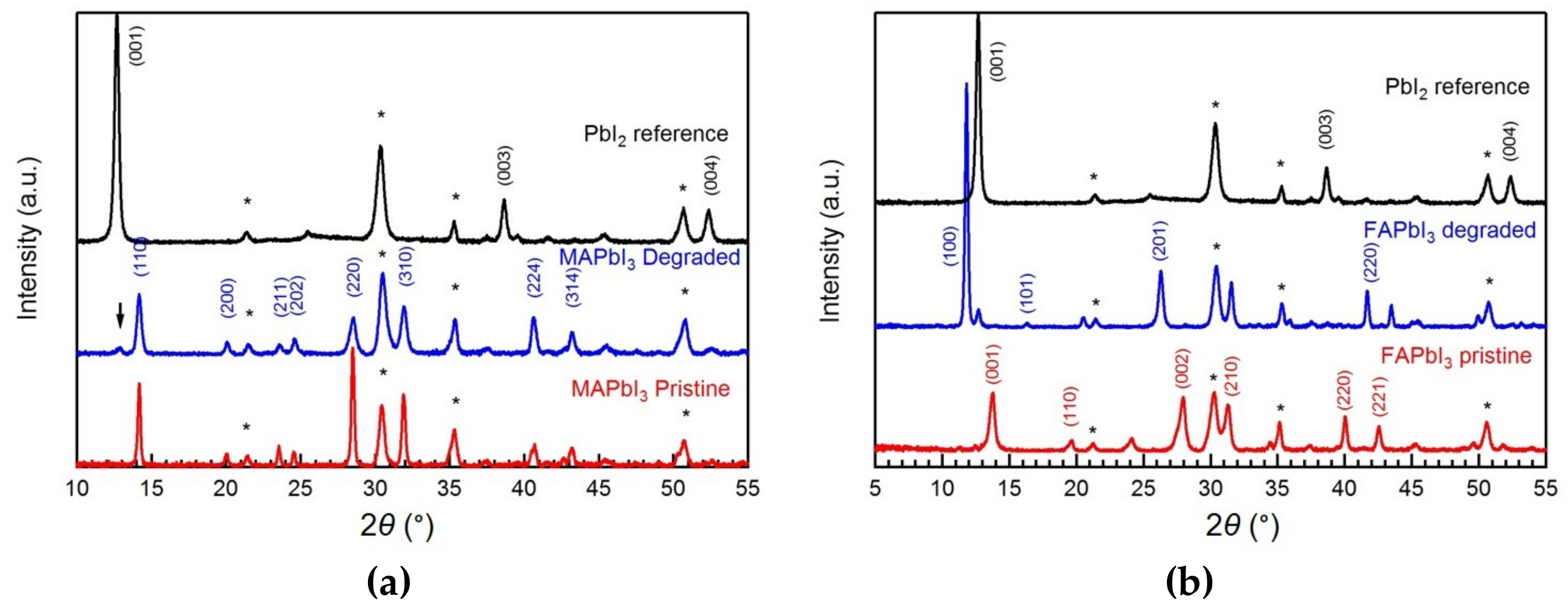
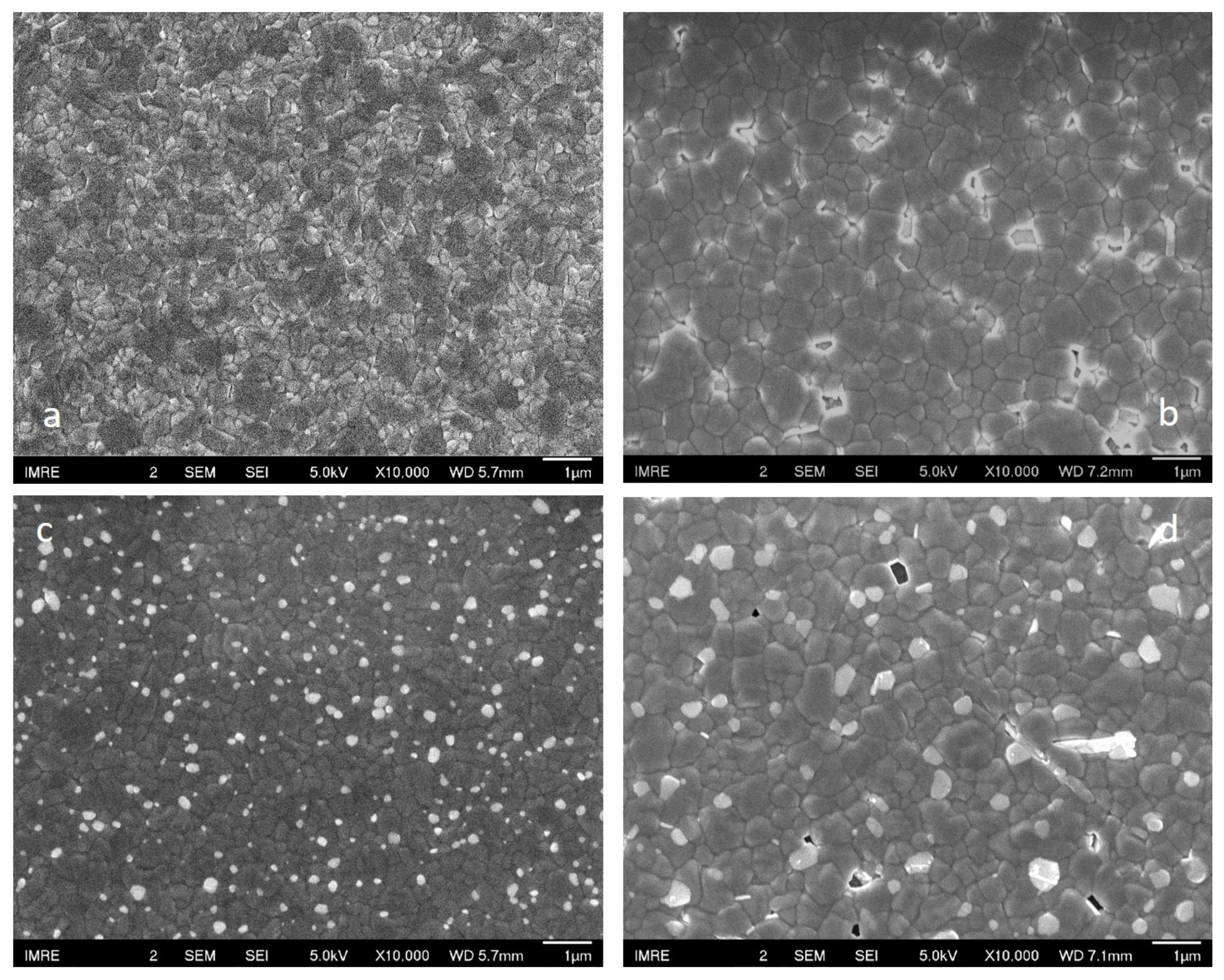
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.T.; Yang, Y.; You, J.B.; Hong, Z.R.; Chang, W.H.; Li, G.; Yang, Y. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Xia, Y.; Wei, Q.; Xing, G.; Chen, Y.; Huang, W. All-inorganic perovskite nanocrystals-based light emitting diodes and solar cells. Chemnanomat 2019, 5, 266–277. [Google Scholar] [CrossRef]
- Leijtens, T.; Eperon, G.E.; Noel, N.K.; Habisreutinger, S.N.; Petrozza, A.; Snaith, H.J. Stability of metal halide perovskite solar cells. Adv. Energy Mater. 2015, 5, 1500963. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Zheng, C.; Gao, D.; Huang, W. Advancements in the stability of perovskite solar cells: Degradation mechanisms and improvement approaches. RSC Adv. 2016, 6, 38079–38091. [Google Scholar] [CrossRef]
- Aristidou, N.; Eames, C.; Islam, M.S.; Haque, S.A. Insights into the increased degradation rate of CH3NH3PbI3 solar cells in combined water and O2 environment. J. Mater. Chem. A 2017, 5, 25469–25475. [Google Scholar] [CrossRef]
- Norbert, H.N.; Felix, L.; Brus, V.V.; Shargaieva, O.; Rappich, J. Unraveling the light-induced degradation mechanisms of CH3NH3PbI3 perovskite film. Adv. Electron. Mater. 2017, 3, 1700158. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Leijtens, T.; Eperon, G.E.; Stranks, S.D.; Nicholas, R.J.; Snaith, H.J. Carbon nanotube/polymer composites as a highly stable hole collection layer in perovskite solar cells. Nano Lett. 2014, 14, 5561–5568. [Google Scholar] [CrossRef]
- Wei, Z.; Zheng, X.; Chen, H.; Long, X.; Wang, Z.; Yang, S. A multifunctional C + epoxy/Ag-paint cathode enables efficient and stable operation of perovskite solar cells in watery environments. J. Mater. Chem. A 2015, 3, 16430–16434. [Google Scholar] [CrossRef]
- Bella, F.; Griffini, G.; Correa-Baena, J.-P.; Saracco, G.; Gratzel, M.; Hagfeldt, A.; Turri, S.; Gerbaldi, C. Improving efficiency and stability of perovskite solar cells with photocurable fluoropolymers. Science 2016, 354, 203–206. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Liu, P.; Cheng, Y.; Zhao, H.; Yang, H. Functionalization of perovskite thin films with moisture-tolerant molecules. Nat. Energy 2016, 1, 15016. [Google Scholar] [CrossRef]
- Hu, Y.; Qiu, T.; Bai, F.; Miao, X.; Zhang, S. Enhancing moisture-tolerance and photovoltaic performances of FAPbI3 by bismuth incorporation. J. Mater. Chem. A 2017, 5, 25258–25265. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Q.; Chmiel, F.P.; Sakai, N.; Herz, L.M.; Snaith, H.J. Efficient ambient-air-stable solar cells with 2D-3D heterostructured butylammonium-caesium-formamidinium lead halide perovskites. Nat. Energy 2017, 2, 17135. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Kim, J.; Jain, A.; Quintero-Bermudez, R.; Tan, H.; Long, G.; Tan, F.; Johnston, A.; Zhao, Y.; Voznyy, O.; et al. Suppression of atomic vacancies via incorporation of isovalent small ions to increase the stability of halide perovskite solar cells in ambient air. Nat. Energy 2018, 3, 648–654. [Google Scholar] [CrossRef]
- Kim, J.H.; Williams, S.T.; Cho, N.; Chueh, C.-C.; Jen, A.K.-Y. Enhanced environmental stability of planar heterojunction perovskite solar cells based on blade-coating. Adv. Energy Mater. 2015, 5, 1401229. [Google Scholar] [CrossRef]
- Song, Z.; Abate, A.; Watthage, S.C.; Liyanage, G.K.; Phillips, A.B.; Steiner, U.; Graetzel, M.; Heben, M.J. Perovskite solar cell stability in humid air: Partially reversible phase transitions in the PbI2–CH3NH3I–H2O system. Adv. Energy Mater. 2016, 6, 1600846. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Hu, Y.; Campoy-Quiles, M.; Alonso, M.I.; Weber, O.J.; Azarhoosh, P.; van Schilfgaarde, M.; Weller, M.T.; Bein, T.; Nelson, J.; et al. Reversible hydration of CH3NH3PbI3 in films, single crystals, and solar cells. Chem. Mater. 2015, 27, 3397–3407. [Google Scholar] [CrossRef]
- Schlipf, J.; Hu, Y.H.; Pratap, S.; Bießmann, L.; Hohn, N.; Porcar, L.; Bein, T.; Docampo, P.; Muller-Buschbaum, P. Shedding light on the moisture stability of 3D/2D hybrid perovskite heterojunction thin films. ACS Appl. Energy Mater. 2019, 2, 1011–1018. [Google Scholar] [CrossRef]
- Ke, J.C.-R.; Walton, A.S.; Lewis, D.J.; Tedstone, A.; O’Brien, P.; Thomas, A.G.; Flavell, W.R. In situ investigation of degradation at organometal halide perovskite surfaces by X-ray photoelectron spectroscopy at realistic water vapour pressure. Chem. Commun. 2017, 53, 5231–5234. [Google Scholar] [CrossRef] [Green Version]
- Bi, D.; Tress, W.; Dar, M.I.; Gao, P.; Luo, J.; Renevier, C.; Schenk, K.; Abate, A.; Giordano, F.; Baena, J.-P.C.; et al. Efficient luminescent solar cells based on tailored mixed-cation perovskites. Sci. Adv. 2016, 2, e1501170. [Google Scholar] [CrossRef]
- Jesper, J.; Juan-Pablo, C.B.; Elham, H.A.; Bertrand, P.; Samuel, D.S.; Marine, E.F.B.; Wolfgang, T.; Kurt, S.; Joel, T.; Jacques, E.M.; et al. Unreacted PbI2 as a double-edge sword for enhancing the performance of perovskite solar cells. J. Am. Chem. Soc. 2016, 138, 10331–10343. [Google Scholar] [CrossRef]
- Wang, C.C.; Gao, Y.L. Stability of perovskites at the surface analytic level. J. Phys. Chem. Lett. 2018, 9, 4657–4666. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Hu, Y.H. Catalytic role of H2O in degradation of inorganic-organic perovskite (CH3NH3PbI3) in air. Int. J. Energy Res. 2017, 41, 1063–1069. [Google Scholar] [CrossRef]
- Philippe, B.; Park, B.-W.; Lindblad, R.; Oscarsson, J.; Ahmadi, S.; Johansson, E.M.J.; Rensmo, H. Chemical and electronic structure characterization of lead halide perovskites and stability behavior under different exposures—A photoelectron spectroscopy investigation. Chem. Mater. 2015, 27, 1720–1731. [Google Scholar] [CrossRef]
- You, J.; Yang, Y.; Hong, Z.; Song, T.B.; Meng, L.; Liu, Y.; Jiang, C.; Zhou, H.; Chang, W.H.; Li, G.; et al. Moisture assisted perovskite film growth for high performance solar cells. Appl. Phys. Lett. 2014, 105, 183902. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Xiong, J.; Li, J.; Daoud, W.A. Mechanism of water effect on enhancing the photovoltaic performance of triple-cation hybrid perovskite solar cells. ACS Appl. Mater. Interfaces 2019, 11, 12699–12708. [Google Scholar] [CrossRef]
- Solanki, A.; Lim, S.S.; Mhaisalkar, S.; Sum, T.C. Role of water in suppressing recombination pathways in CH3NH3PbI3 perovskite solar cells. ACS Appl. Mater. Interfaces 2019, 11, 25474–25482. [Google Scholar] [CrossRef]
- Yang, J.M.; Yuan, Z.C.; Liu, X.J.; Braun, S.; Li, Y.Q.; Tang, J.X.; Gao, F.; Duan, C.G.; Fahlman, M.; Bao, Q.Y. Oxygen- and water-induced energetics degradation in organometal halide perovskites. ACS Appl. Mater. Interfaces 2018, 10, 16225–16230. [Google Scholar] [CrossRef]
- Christians, J.A.; Miranda Herrera, P.A.; Kamat, P.V. Transformation of the excited state and photovoltaic efficiency of CH3NH3PbI3 perovskite upon controlled exposure to humidified Air. J. Am. Chem. Soc. 2015, 137, 1530–1538. [Google Scholar] [CrossRef]
- Shirayama, M.; Kato, M.; Miyadera, T.; Sugita, T.; Fujiseki, T.; Hara, S.; Kadowaki, H.; Murata, D.; Chikamatsu, M.; Fujiwara, H.; et al. Degradation mechanism of CH3NH3PbI3 perovskite materials upon exposure to humid air. J. Appl. Phys. 2016, 119, 115501. [Google Scholar] [CrossRef]
- Li, D.; Bretschneider, S.A.; Bergmann, V.W.; Hermes, I.M.; Mars, J.; Klasen, A.; Lu, H.; Tremel, W.; Mezger, M.; Butt, H.J.; et al. Humidity-induced grain boundaries in MAPbI3 perovskite films. J. Phys. Chem. C 2016, 120, 6363–6368. [Google Scholar] [CrossRef]
- Ralaiarisoa, M.; Salzmann, I.; Zu, F.S.; Koch, N. Effect of water, oxygen, and air exposure on CH3NH3PbI3−xClx perovskite surface electronic properties. Adv. Electron. Mater. 2018, 4, 1800307. [Google Scholar] [CrossRef]
- Chen, S.; Goh, T.W.; Sabba, D.; Chua, J.; Mathews, N.; Huan, C.H.A.; Sum, T.C. Energy level alignment at the methylammonium lead iodide/copper phthalocyanine interface. APL Mater. 2014, 2, 081512. [Google Scholar] [CrossRef] [Green Version]
- Hawash, Z.; Raga, S.R.; Son, D.Y.; Ono, L.K.; Park, N.G.; Qi, Y.B. Interfacial modification of perovskite solar cells using an ultrathin MAI layer leads to enhanced energy level alignment, efficiencies, and reproducibility. J. Phys. Chem. Lett. 2017, 8, 3947–3953. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S.; Hayakawa, K.; Cojocaru, L.; Tsuruta, R.; Sato, T.; Mase, K.; Uchida, S.; Nakayama, Y. Electronic structures and chemical states of methylammonium lead triiodide thin films and the impact of annealing and moisture exposure. J. Appl. Phys. 2018, 123, 165501. [Google Scholar] [CrossRef]
- Smecca, E.; Numata, Y.; Deretzis, I.; Pellegrino, G.; Boninelli, S.; Miyasaka, T.; La Magna, A.; Alberti, A. Stability of solution-processed MAPbI3 and FAPbI3 layers. Phys. Chem. Chem. Phys. 2016, 18, 13413–13422. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.P.; Chi, W.J.; Li, Z.S. Effects of water molecules on the chemical stability of MAGeI3 perovskite explored from a theoretical viewpoint. Phys. Chem. Chem. Phys. 2016, 18, 24526–24536. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Geng, W.; Tang, Z.; Yam, C.; Fan, X.; Liu, J.; Lau, W.; Liu, L. Uncovering the veil of the degradation in perovskite CH3NH3PbI3 upon humidity exposure: A first-principles study. J. Phys. Chem. Lett. 2015, 6, 3289–3295. [Google Scholar] [CrossRef]
- Liu, T.; Zong, Y.; Zhou, Y.; Yang, M.; Li, Z.; Game, O.S.; Zhu, K.; Zhu, R.; Gong, Q.; Padture, N.P. High-performance formamidinium-based perovskite solar cells via microstructure-mediated δ-to-α phase transformation. Chem. Mater. 2017, 29, 3246–3250. [Google Scholar] [CrossRef]
- Tseng, W.S.; Jao, M.H.; Hsu, C.C.; Huang, J.S.; Wu, C.I.; Yeh, N.C. Stabilization of hybrid perovskite CH3NH3PbI3 thin films by graphene passivation. Nanoscale 2017, 9, 19227–19235. [Google Scholar] [CrossRef]
- Yang, J.L.; Fransishyn, K.M.; Kelly, T.L. Comparing the effect of mesoporous and planar metal oxides on the stability of methylammonium lead iodide thin films. Chem. Mater. 2016, 28, 7344–7352. [Google Scholar] [CrossRef]
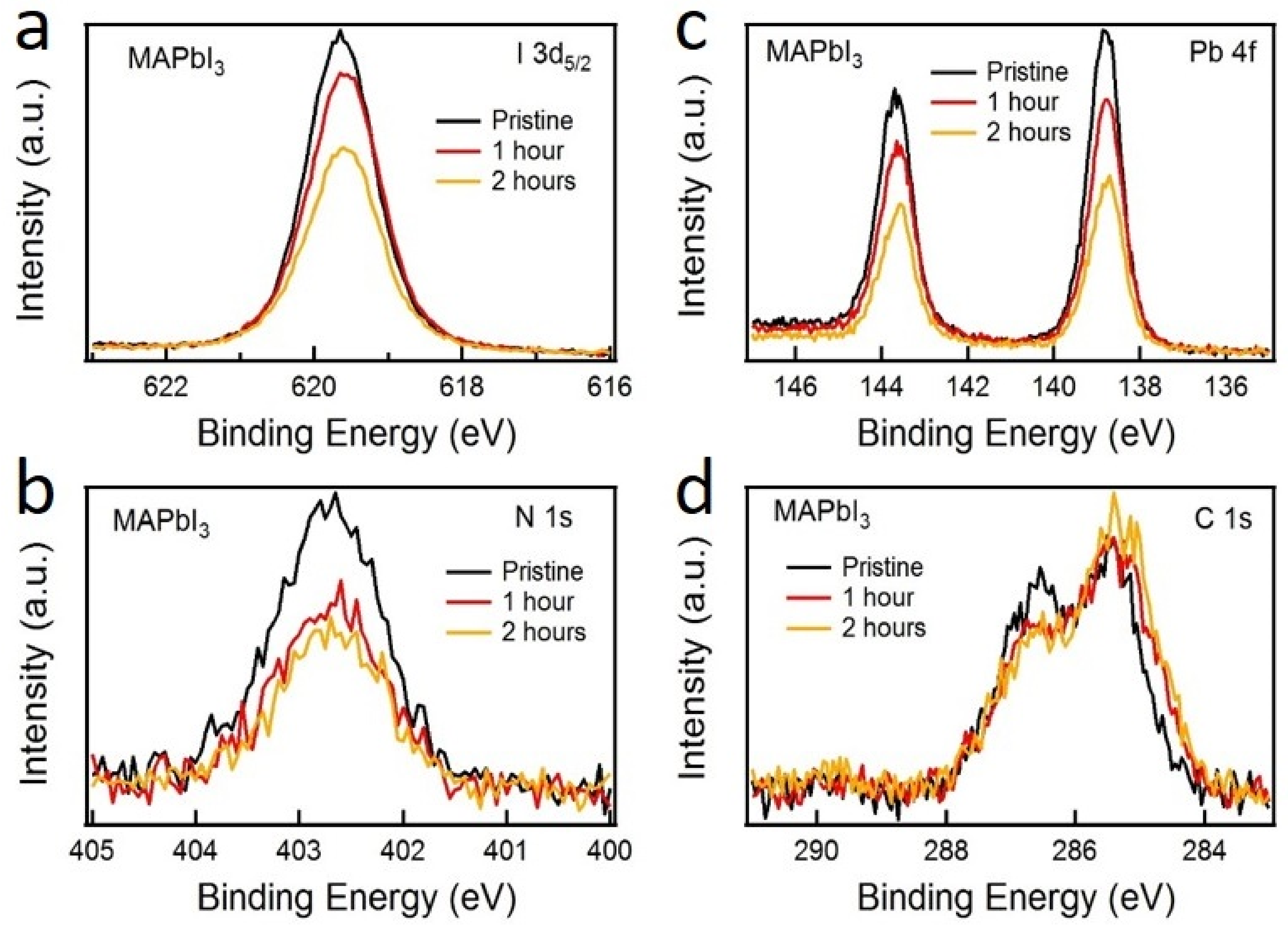
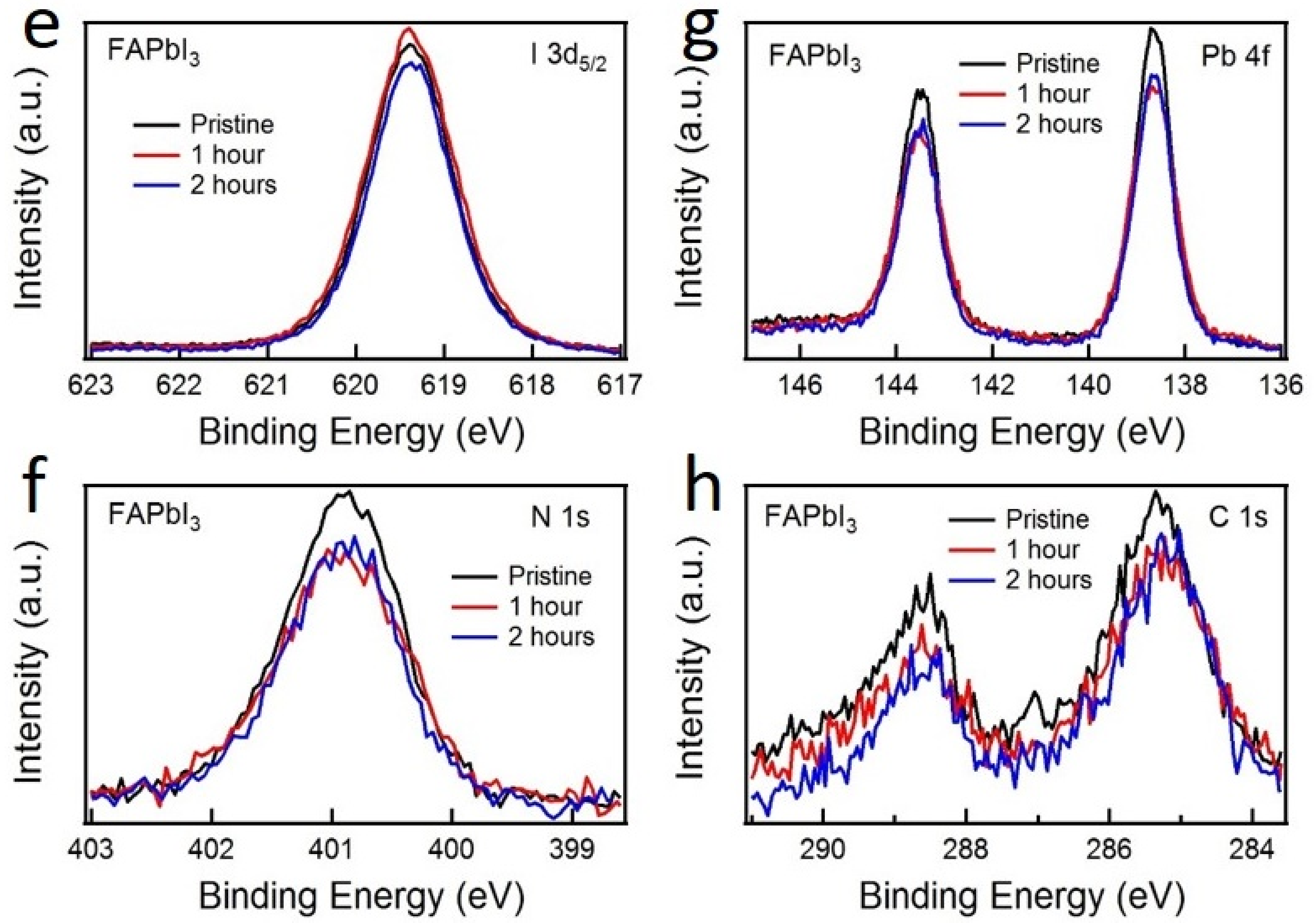
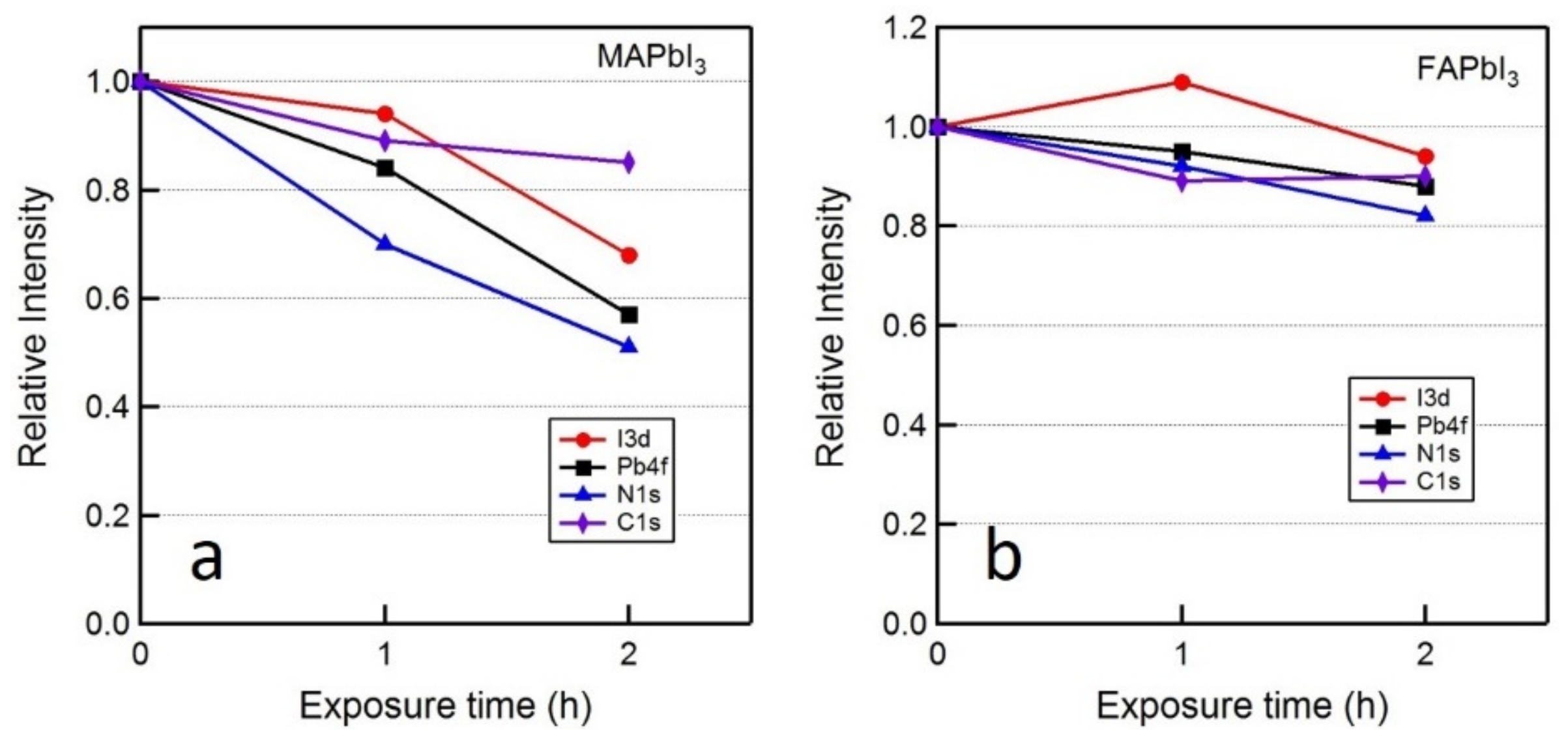
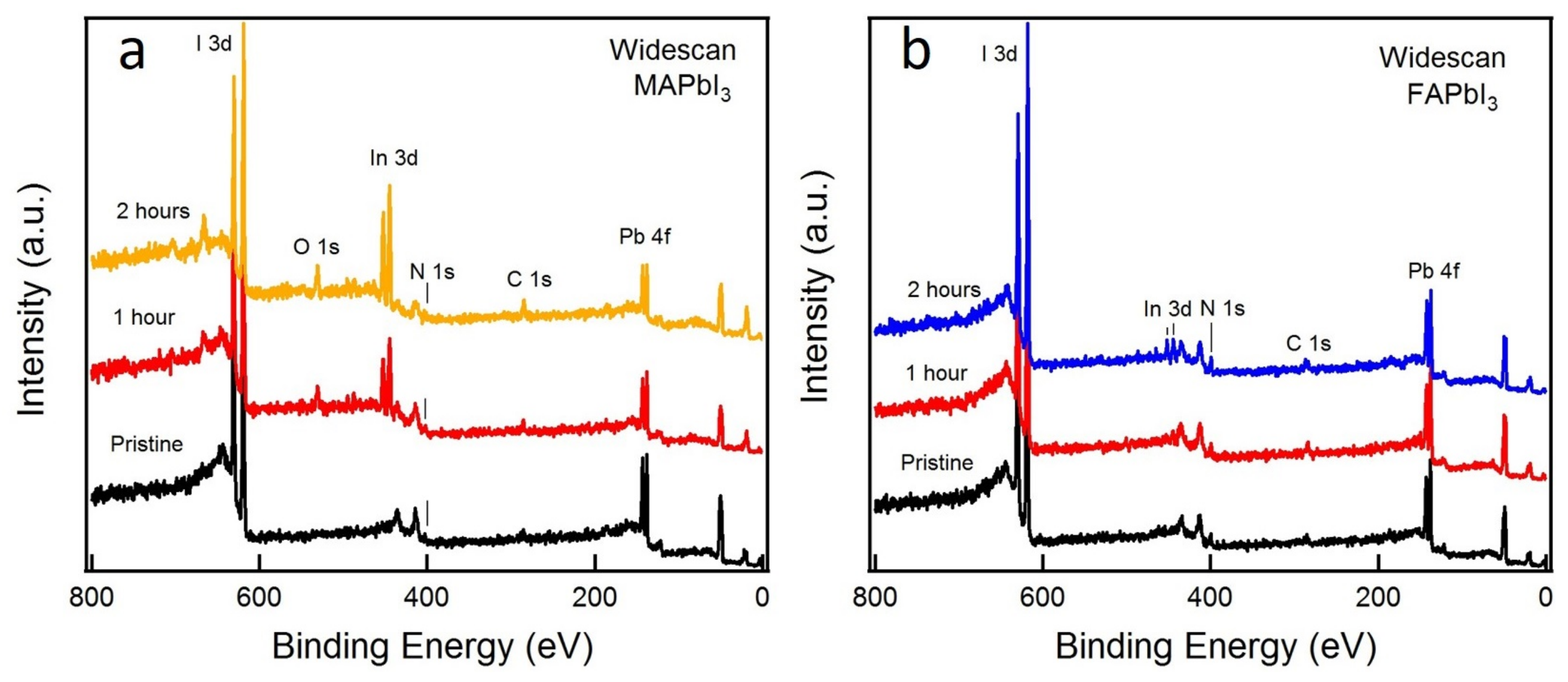

| Exposure Time | MAPbI3 | FAPbI3 | ||||
|---|---|---|---|---|---|---|
| I/Pb | N/Pb | C/Pb | I/Pb | N/Pb | C/Pb | |
| 0 h | 3.1 | 1.6 | 1.5 | 3.1 | 2.2 | 0.8 |
| 1 h | 3.5 | 1.0 | 1.5 | 3.6 | 2.1 | 0.7 |
| 2 h | 3.7 | 1.5 | 2.4 | 3.3 | 2.1 | 0.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Solanki, A.; Pan, J.; Sum, T.C. Compositional and Morphological Changes in Water-Induced Early-Stage Degradation in Lead Halide Perovskites. Coatings 2019, 9, 535. https://doi.org/10.3390/coatings9090535
Chen S, Solanki A, Pan J, Sum TC. Compositional and Morphological Changes in Water-Induced Early-Stage Degradation in Lead Halide Perovskites. Coatings. 2019; 9(9):535. https://doi.org/10.3390/coatings9090535
Chicago/Turabian StyleChen, Shi, Ankur Solanki, Jisheng Pan, and Tze Chein Sum. 2019. "Compositional and Morphological Changes in Water-Induced Early-Stage Degradation in Lead Halide Perovskites" Coatings 9, no. 9: 535. https://doi.org/10.3390/coatings9090535
APA StyleChen, S., Solanki, A., Pan, J., & Sum, T. C. (2019). Compositional and Morphological Changes in Water-Induced Early-Stage Degradation in Lead Halide Perovskites. Coatings, 9(9), 535. https://doi.org/10.3390/coatings9090535





