Electrochemical Detection of Dopamine Based on Functionalized Electrodes
Abstract
1. Introduction
2. Materials and Methods
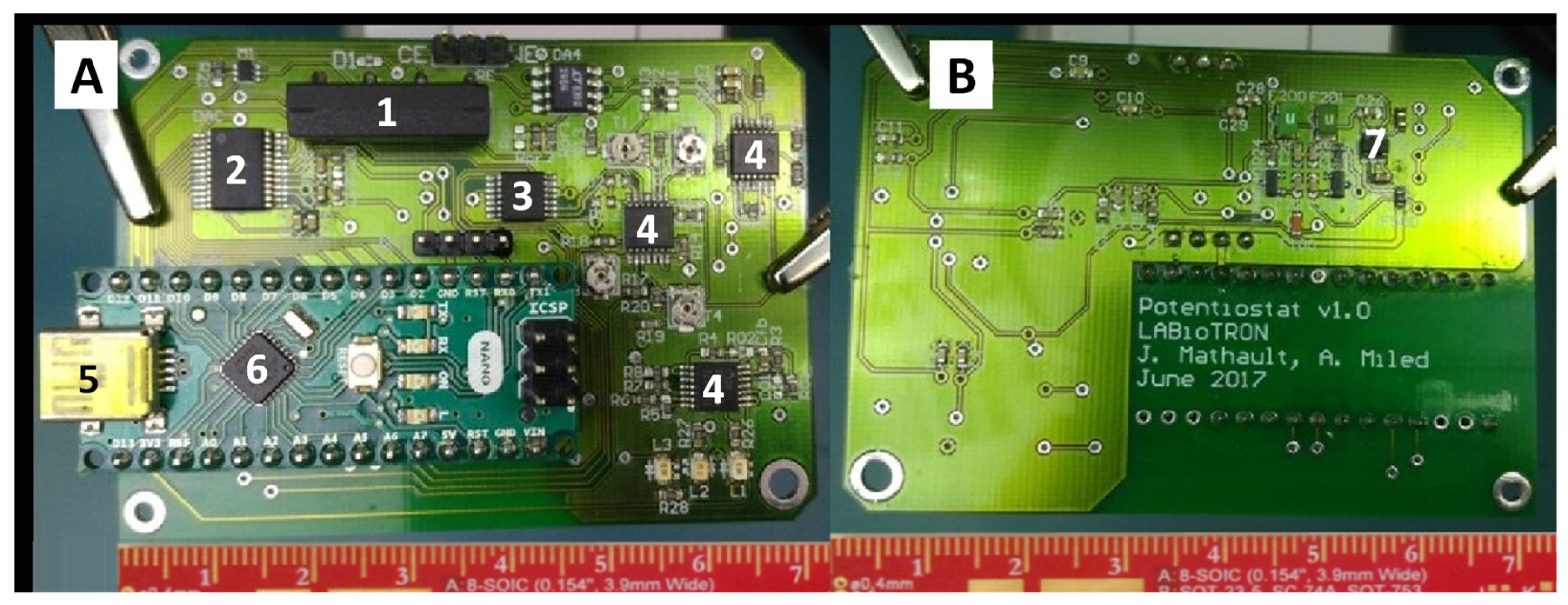
2.1. Potentiostats
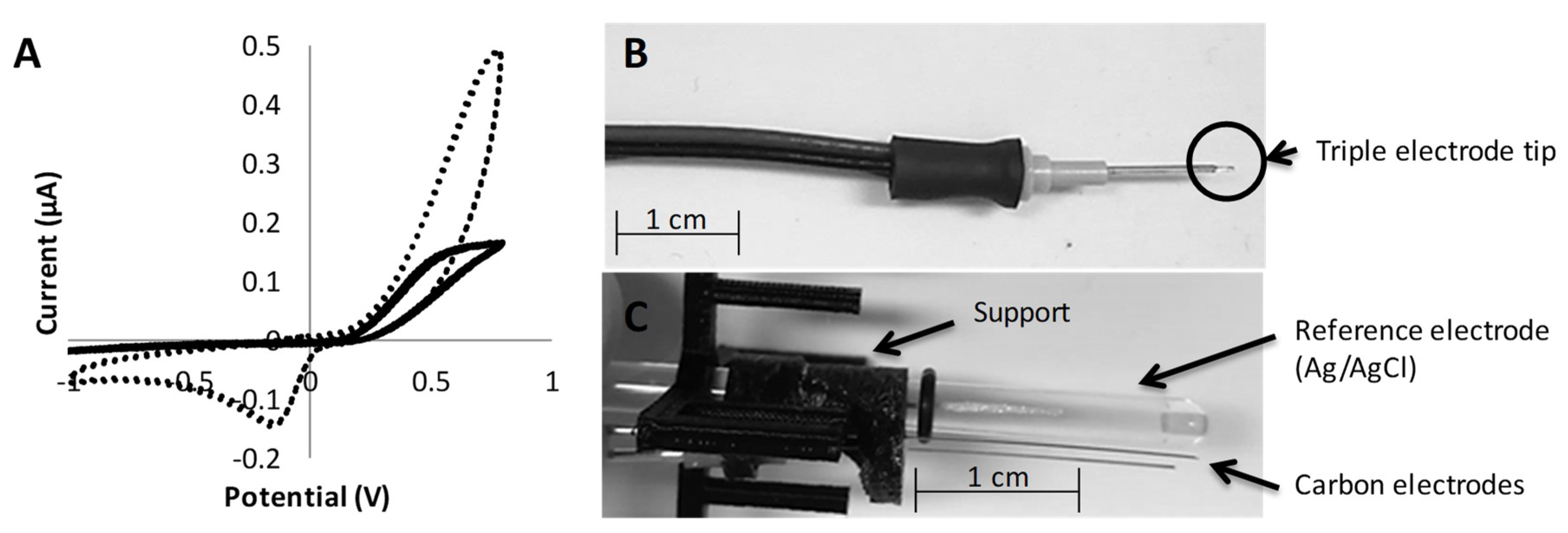
2.2. Custom-Made Electrodes
2.3. Synthesis of Gold Nanoparticles
2.4. Functionalization of the Electrodes
2.4.1. Stock Solutions
2.4.2. Pretreatment Conditions
2.4.3. Functionalization Solution
2.4.4. Functionalization Conditions
2.4.5. Washing and Storage
2.5. Analysis of Neurotransmitters
3. Results and Discussion
3.1. Synthesis and Characterization of the Gold Nanoparticles
3.2. Influence of the Software Parameters on the Detection of Dopamine
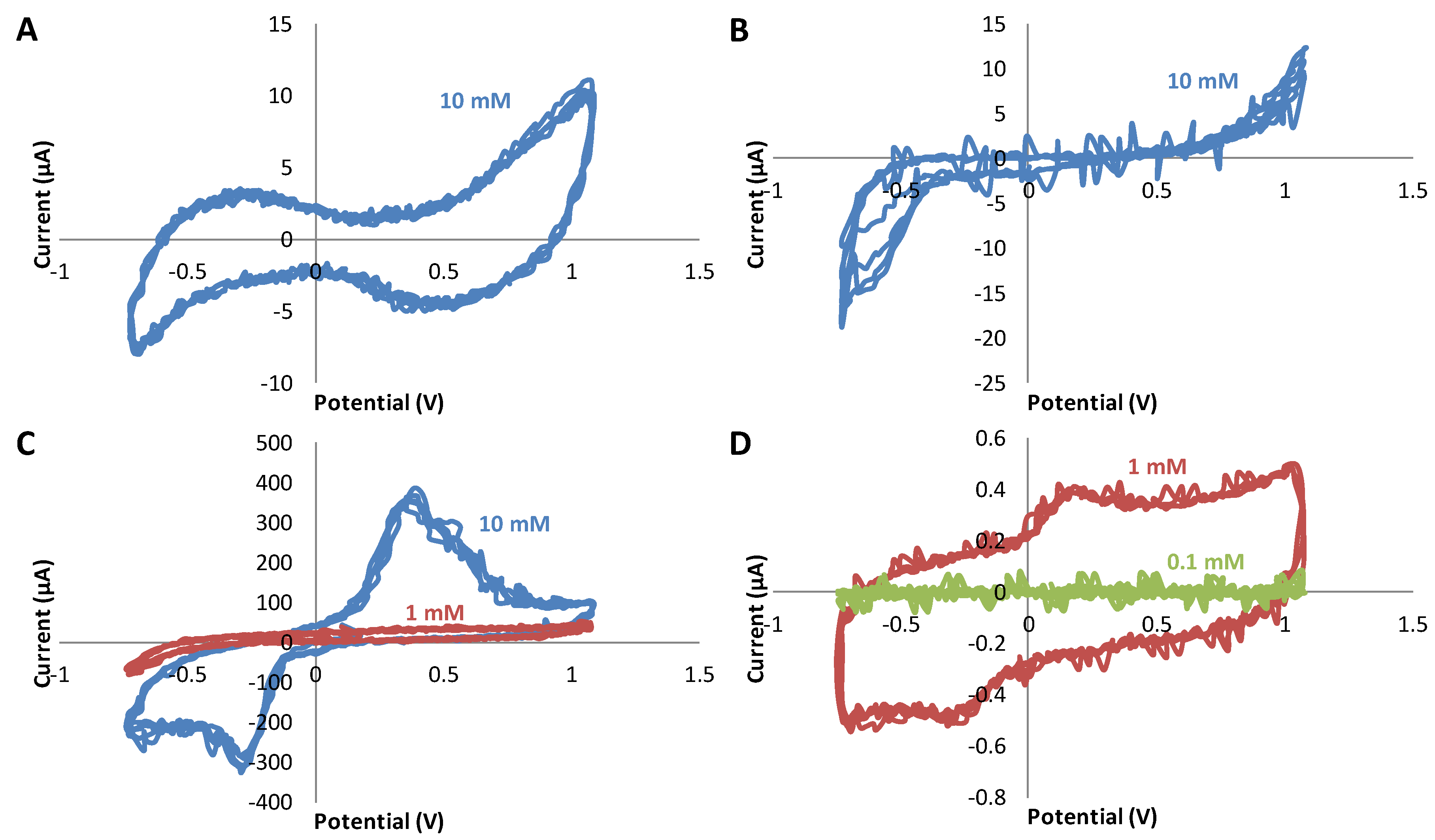
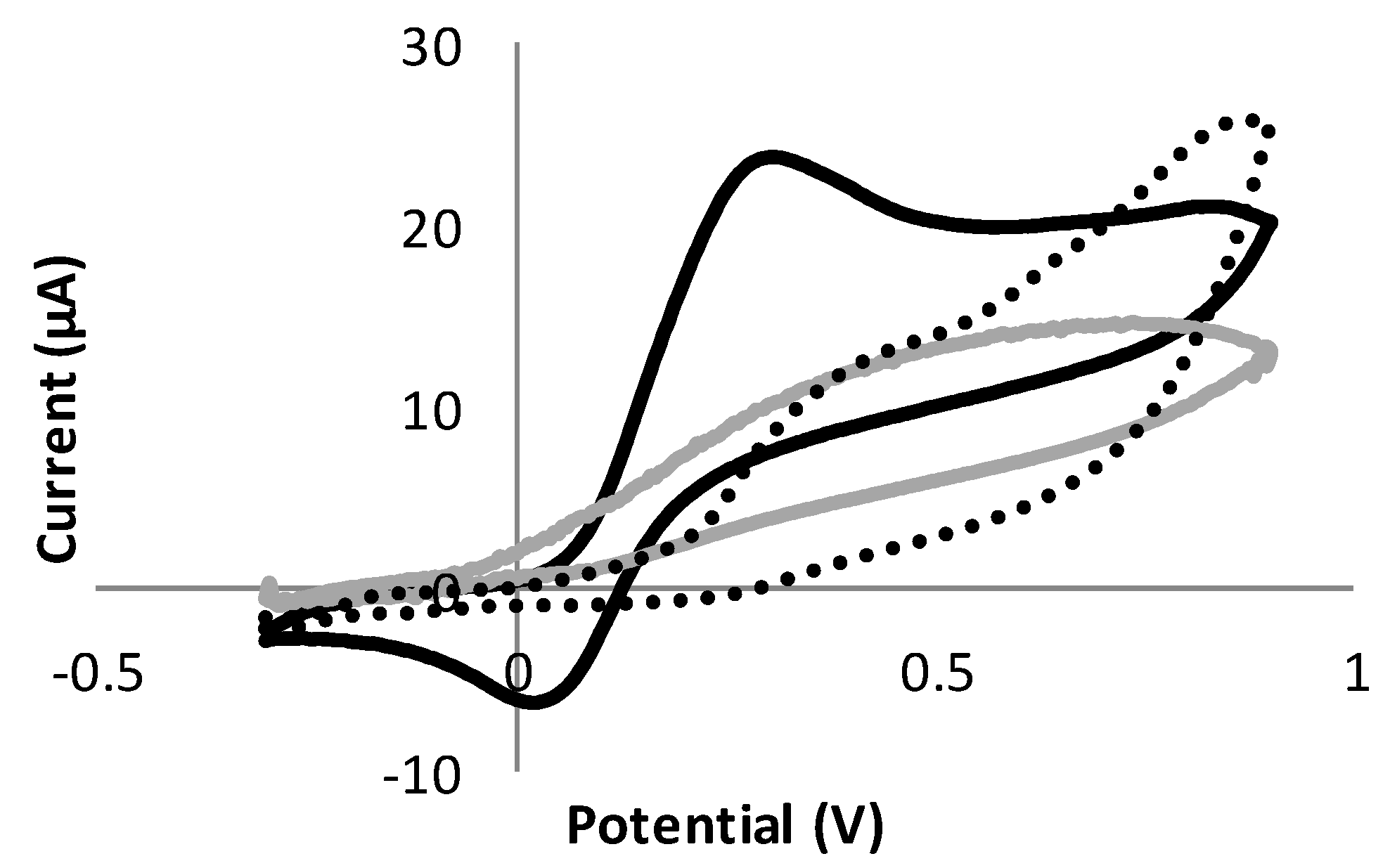
3.3. Study of the Sensitivity of Dopamine Using Triple Electrodes Based on Different Materials
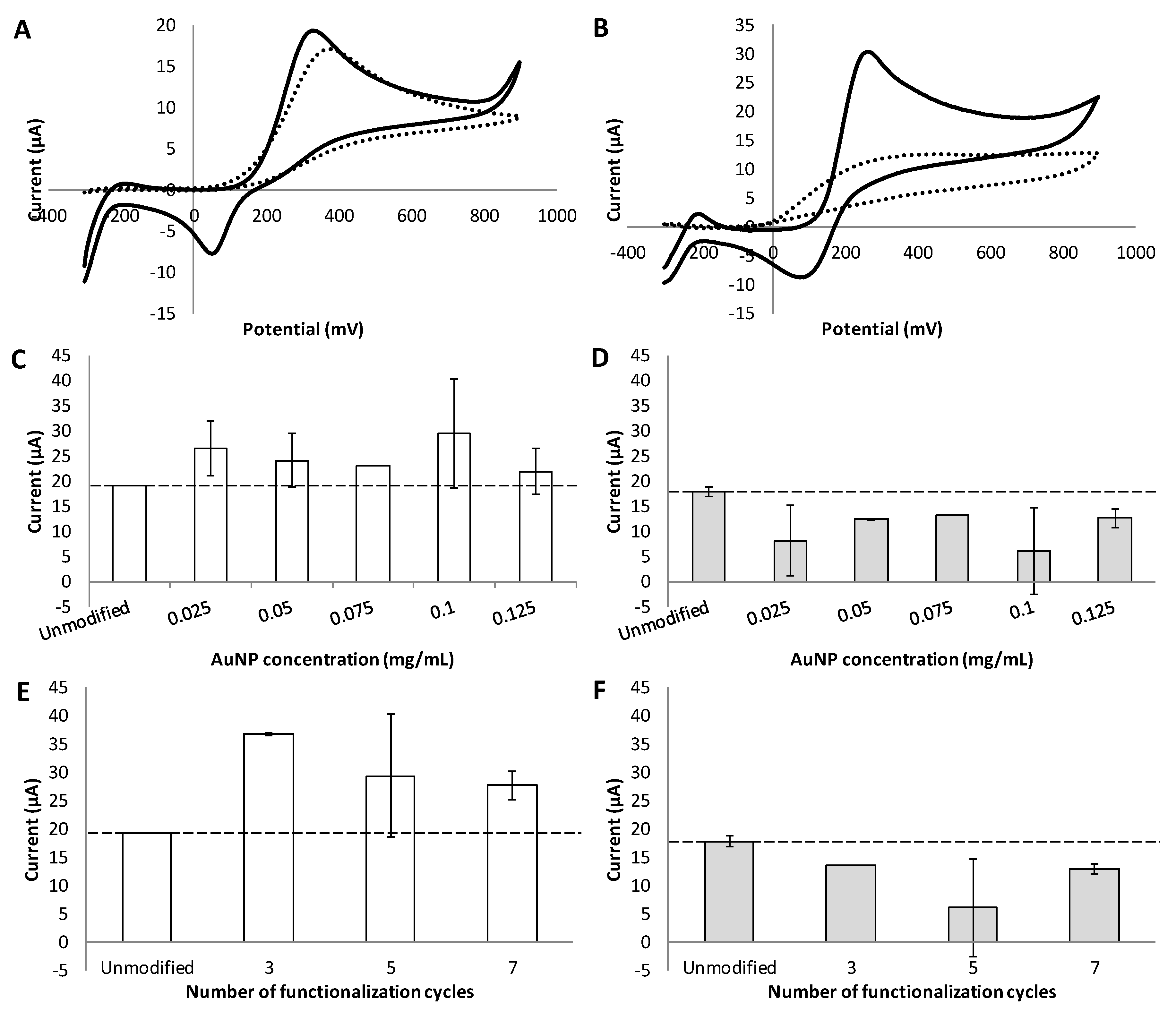
3.4. Effect of Different Functionalization Conditions on the Sensitivity and Selectivity of Dopamine Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Three-electrode setup | 2 carbon (C) fiber electrodes with a diameter of 30 µm and a tip length of 200 µm, and 1 commercial silver/silver-chloride (Ag/AgCl) reference electrode |
| Carbon triple electrode | 2 carbon (C) electrodes with a diameter of 30 µm and a tip length of 200 µm, and 1 platinum–iridium (Pt/Ir) alloy electrode with a diameter of 100 µm and a tip length of 200 µm |
| Commercial Ag/AgCl reference electrode | RRPEAGCL silver/silver-chloride (Ag/AgCl) reference electrode |
| Commercial electrode | RRPE1002C screen-printed carbon electrodes (4 mm × 5 mm carbon working active area electrode) |
| Commercial potentiostat | WaveNow™ potentiostat |
| CTAB | Cetyl trimethylammonium bromide |
| CV | Cyclic voltammetry |
| DNA | Deoxyribonucleic acid |
| DLS | Dynamic light scattering |
| ECL | Electroluminescence |
| FSCV | Fast scan cyclic voltammetry |
| LOD | Limit of detection |
| NaOH | Sodium hydroxide |
| o-PD | o-Phenylenediamine |
| PEG800 | Polyethylene glycol methyl ether thiol with a molecular weight of 800 g·mol−1 |
| RNA | Ribonucleic acid |
| SELEX | Systematic evolution of ligands by exponential enrichment |
| SEM | Scanning electron microscopy |
| SERS | Surface-enhanced Raman spectroscopy |
| Simple electrode | 1 carbon (C) fiber electrode with a diameter of 30 µm |
| Stainless triple electrode | 2 stainless steel electrodes with a diameter of 50 µm, and 1 platinum–iridium (Pt/Ir) alloy electrode with a diameter of 100 µm |
| TEM | Transmission electron microscopy |
| Tungsten 75 triple electrode | 2 tungsten (W) electrodes with a diameter of 75 µm, and 1 platinum–iridium (Pt/Ir) alloy electrode with a diameter of 100 µm |
| Tungsten 125 triple electrode | 2 tungsten (W) electrodes with a diameter of 125 µm diameter, and 1 platinum–iridium (Pt/Ir) alloy electrode with a diameter of 100 µm |
References
- World Health Organization and Alzheimer’s Disease International. Dementia: A Public Health Priority. Available online: http://www.who.int/mental_health/publications/dementia_report_2012/en/ (accessed on 3 August 2019).
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Suzuki, I.; Fukuda, M.; Shirakawa, K.; Jiko, H.; Gotoh, M. Carbon nanotube multi-electrode array chips for noninvasive real-time measurement of dopamine, action potentials, and postsynaptic potentials. Biosens. Bioelectron. 2013, 49, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Kimble, C.J.; Johnson, D.M.; Winter, B.A.; Whitlock, S.V.; Kressin, K.R.; Horne, A.E.; Robinson, J.C.; Bledsoe, J.M.; Tye, S.J.; Chang, S.Y.; et al. Wireless instantaneous neurotransmitter concentration sensing system (WINCS) for intraoperative neurochemical monitoring. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 2009, 4856–4859. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Zhou, Y.; Xu, J.-J.; Chen, H.-Y. Signal-on electrochemiluminescence biosensors based on CdS-carbon nanotube nanocomposite for the sensitive detection of choline and acetylcholine. Adv. Funct. Mater. 2009, 19, 1444–1450. [Google Scholar] [CrossRef]
- Medina-Sanchez, M.; Miserere, S.; Merkoci, A. Nanomaterials and lab-on-a-chip technologies. Lab Chip 2012, 12, 1932–1943. [Google Scholar] [CrossRef] [PubMed]
- Murari, K.; Thakor, N.; Stanacevic, M.; Cauwenberghs, G. Wide-range, picoampere-sensitivity multichannel VLSI potentiostat for neurotransmitter sensing. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2004, 6, 4063–4066. [Google Scholar] [PubMed]
- Jackson, B.P.; Dietz, S.M.; Wightman, R.M. Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Anal. Chem. 1995, 67, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Ghodsevali, E.; Morneau-Gamache, S.; Mathault, J.; Landari, H.; Boisselier, E.; Boukadoum, M.; Gosselin, B.; Miled, A. Miniaturized FDDA and CMOS based potentiostat for bio-applications. Sensors 2017, 17, 810. [Google Scholar] [CrossRef] [PubMed]
- Zamani, H.; Bahrami, H.R.; Chalwadi, P.; Garris, P.A.; Mohseni, P. C-FSCV: Compressive fast-scan cyclic voltammetry for brain dopamine recording. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Pyakurel, P.; Privman Champaloux, E.; Venton, B.J. Fast-scan cyclic voltammetry (FSCV) detection of endogenous octopamine in drosophila melanogaster ventral nerve cord. ACS Chem. Neurosci. 2016, 7, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Jaquins-Gerstl, A.; Michael, A.C. A review of the effects of FSCV and microdialysis measurements on dopamine release in the surrounding tissue. Analyst 2015, 140, 3696–3708. [Google Scholar] [CrossRef] [PubMed]
- Hermans, A.; Keithley, R.B.; Kita, J.M.; Sombers, L.A.; Wightman, R.M. Dopamine detection with fast-scan cyclic voltammetry used with analog background subtraction. Anal. Chem. 2008, 80, 4040–4048. [Google Scholar] [CrossRef] [PubMed]
- Keithley, R.B.; Takmakov, P.; Bucher, E.S.; Belle, A.M.; Owesson-White, C.A.; Park, J.; Wightman, R.M. Higher sensitivity dopamine measurements with faster-scan cyclic voltammetry. Anal. Chem. 2011, 83, 3563–3571. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Chuang, C.; Cao, J.; Ball, V.; Ruch, D.; Buehler, M.J. Excitonic effects from geometric order and disorder explain broadband optical absorption in eumelanin. Nat. Commun. 2014, 5, 3859. [Google Scholar] [CrossRef] [PubMed]
- Bucher, E.S.; Wightman, R.M. Electrochemical analysis of neurotransmitters. Annu. Rev. Anal. Chem. (Palo Alto Calif) 2015, 8, 239–261. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.L.; Venton, B.J.; Heien, M.L.; Wightman, R.M. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin. Chem. 2003, 49, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Rodeberg, N.T.; Sandberg, S.G.; Johnson, J.A.; Phillips, P.E.; Wightman, R.M. Hitchhiker’s guide to voltammetry: Acute and chronic electrodes for in vivo fast-scan cyclic voltammetry. ACS Chem. Neurosci. 2017, 8, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Cheer, J.F.; Heien, M.L.; Garris, P.A.; Carelli, R.M.; Wightman, R.M. Simultaneous dopamine and single-unit recordings reveal accumbens GABAergic responses: Implications for intracranial self-stimulation. Proc. Natl. Acad. Sci. USA 2005, 102, 19150–19155. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xia, M.; Liang, O.; Sun, K.; Cipriano, A.F.; Schroeder, T.; Liu, H.; Xie, Y.-H. Label-Free SERS selective detection of dopamine and serotonin using graphene-au nanopyramid heterostructure. Anal. Chem. 2015, 87, 10255–10261. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, C.; Zhou, T.; Ma, H. Gold nanoparticle based colorimetric probe for dopamine detection based on the interaction between dopamine and melamine. Microchim. Acta 2015, 182, 1003–1008. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, L.; Lan, C.; Zhao, S. Graphene quantum dots as effective probes for label-free fluorescence detection of dopamine. Sens. Actuators B Chem. 2016, 223, 246–251. [Google Scholar] [CrossRef]
- Cao, Q.; Puthongkham, P.; Venton, B.J. Review: New insights into optimizing chemical and 3D surface structures of carbon electrodes for neurotransmitter detection. Anal. Methods 2019, 11, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Zachek, M.K.; Hermans, A.; Wightman, R.M.; McCarty, G.S. Electrochemical dopamine detection: Comparing gold and carbon fiber microelectrodes using background subtracted fast scan cyclic voltammetry. J. Electroanal. Chem. (Lausanne) 2008, 614, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Feng, L.; Ren, J.; Qu, X. Electrochemical detection of dopamine using porphyrin-functionalized graphene. Biosens. Bioelectron. 2012, 34, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Li, W. CTAB functionalized graphene oxide/multiwalled carbon nanotube composite modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens. Bioelectron. 2014, 56, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Zablocka, I.; Wysocka-Zolopa, M.; Winkler, K. Electrochemical detection of dopamine at a gold electrode modified with a polypyrrole-mesoporous silica molecular sieves (MCM-48) film. Int. J. Mol. Sci. 2018, 20, 111. [Google Scholar] [CrossRef] [PubMed]
- Stozhko, N.; Bukharinova, M.; Galperin, L.; Brainina, K. A nanostructured sensor based on gold nanoparticles and nafion for determination of uric acid. Biosensors 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Farghali, R.A.; Ahmed, R.A. Gold nanoparticles-modified screen-printed carbon electrode for voltammetric determination of sildenafil citrate (Viagra) in pure form, biological and pharmaceutical formulations. Int. J. Electrochem. Sci. 2015, 10, 1494–1505. [Google Scholar]
- Masse, F.; Ouellette, M.; Lamoureux, G.; Boisselier, E. Gold nanoparticles in ophthalmology. Med. Res. Rev. 2019, 39, 302–327. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Rezaei, B.; Boroujeni, M.K.; Ensafi, A.A. Fabrication of DNA, o-phenylenediamine, and gold nanoparticle bioimprinted polymer electrochemical sensor for the determination of dopamine. Biosens. Bioelectron. 2015, 66, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, S.; Han, F.; Liu, L.; Xu, L.; Ma, W.; Kuang, H.; Li, A.; Wang, L.; Xu, C. SERS-active Au@Ag nanorod dimers for ultrasensitive dopamine detection. Biosens. Bioelectron. 2015, 71, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Peat, M.A.; Gibb, J.W. High-performance liquid chromatographic determination of indoleamines, dopamine, and norepinephrine in rat brain with fluorometric detection. Anal. Biochem. 1983, 128, 275–280. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Li, X.; Guo, X.; Zhang, B.; Jia, X.; Dai, B. A simple, fast and low-cost turn-on fluorescence method for dopamine detection using in situ reaction. Anal. Chim. Acta 2016, 944, 51–56. [Google Scholar] [CrossRef] [PubMed]
- John, C.E.; Jones, S.R. Fast scan cyclic voltammetry of dopamine and serotonin in mouse brain slices. In Electrochemical Methods for Neuroscience; Michael, A.C., Borland, L.M., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Kara, A.; Rouillard, C.; Mathault, J.; Boisvert, M.; Tessier, F.; Landari, H.; Melki, I.; Laprise-Pelletier, M.; Boisselier, E.; Fortin, M.A.; et al. Towards a multifunctional electrochemical sensing and niosome generation lab-on-chip platform based on a plug-and-play concept. Sensors 2016, 16, 778. [Google Scholar] [CrossRef] [PubMed]
- Mathault, J.; Grenier, D.; Miled, A. Counter/reference-based potentiostat architecture analysis and comparison. In Proceedings of the 2017 15th IEEE International New Circuits and Systems Conference (NEWCAS), Strasbourg, France, 25–28 June 2017; pp. 201–204. [Google Scholar]
- Kozai, T.D.Y.; Jaquins-Gerstl, A.S.; Vazquez, A.L.; Michael, A.C.; Cui, X.T. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem. Neurosci. 2015, 6, 48–67. [Google Scholar] [CrossRef]
- Jaquins-Gerstl, A.; Michael, A.C. Comparison of the brain penetration injury associated with microdialysis and voltammetry. J. Neurosci. Methods 2009, 183, 127–135. [Google Scholar] [CrossRef]
- Peters, J.L.; Miner, L.H.; Michael, A.C.; Sesack, S.R. Ultrastructure at carbon fiber microelectrode implantation sites after acute voltammetric measurements in the striatum of anesthetized rats. J. Neurosci. Methods 2004, 137, 9–23. [Google Scholar] [CrossRef]
- de Oliveira, R.; Zhao, P.; Li, N.; de Santa Maria, L.C.; Vergnaud, J.; Ruiz, J.; Astruc, D.; Barratt, G. Synthesis and in vitro studies of gold nanoparticles loaded with docetaxel. Int. J. Pharm. 2013, 454, 703–711. [Google Scholar] [CrossRef]
- Oh, E.; Susumu, K.; Goswami, R.; Mattoussi, H. One-phase synthesis of water-soluble gold nanoparticles with control over size and surface functionalities. Langmuir 2010, 26, 7604–7613. [Google Scholar] [CrossRef] [PubMed]
- Pine Research Instrumentatio Inc. Screen-Printed Electrode Information-Carbon and Ceramic Electrode Information. Available online: https://www.pineresearch.com/shop/wp-content/uploads/sites/2/2016/10/DRP10036-Screen-Printed-Electrodes-Overview-REV001.pdf (accessed on 3 August 2019).
- Liang, H.-J.; Ling, T.-R.; Rick, J.F.; Chou, T.-C. Molecularly imprinted electrochemical sensor able to enantroselectivly recognize d and l-tyrosine. Anal. Chim. Acta 2005, 542, 83–89. [Google Scholar] [CrossRef]
- McConnell, E.M.; Ventura, K.; Dwyer, Z.; Hunt, V.; Koudrina, A.; Holahan, M.R.; DeRosa, M.C. In vivo use of a multi-DNA aptamer-based payload/targeting system to study dopamine dysregulation in the central nervous system. ACS Chem. Neurosci. 2019, 10, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Shou, M.; Ferrario, C.R.; Schultz, K.N.; Robinson, T.E.; Kennedy, R.T. Monitoring dopamine in vivo by microdialysis sampling and on-line CE-laser-induced fluorescence. Anal. Chem. 2006, 78, 6717–6725. [Google Scholar] [CrossRef] [PubMed]
- Uutela, P.; Karhu, L.; Piepponen, P.; Kaenmaki, M.; Ketola, R.A.; Kostiainen, R. Discovery of dopamine glucuronide in rat and mouse brain microdialysis samples using liquid chromatography tandem mass spectrometry. Anal. Chem. 2009, 81, 427–434. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouellette, M.; Mathault, J.; Niyonambaza, S.D.; Miled, A.; Boisselier, E. Electrochemical Detection of Dopamine Based on Functionalized Electrodes. Coatings 2019, 9, 496. https://doi.org/10.3390/coatings9080496
Ouellette M, Mathault J, Niyonambaza SD, Miled A, Boisselier E. Electrochemical Detection of Dopamine Based on Functionalized Electrodes. Coatings. 2019; 9(8):496. https://doi.org/10.3390/coatings9080496
Chicago/Turabian StyleOuellette, Mathieu, Jessy Mathault, Shimwe Dominique Niyonambaza, Amine Miled, and Elodie Boisselier. 2019. "Electrochemical Detection of Dopamine Based on Functionalized Electrodes" Coatings 9, no. 8: 496. https://doi.org/10.3390/coatings9080496
APA StyleOuellette, M., Mathault, J., Niyonambaza, S. D., Miled, A., & Boisselier, E. (2019). Electrochemical Detection of Dopamine Based on Functionalized Electrodes. Coatings, 9(8), 496. https://doi.org/10.3390/coatings9080496




