Compositional, Optical and Electrical Characteristics of SiOx Thin Films Deposited by Reactive Pulsed DC Magnetron Sputtering
Abstract
1. Introduction
2. Experimental Details
2.1. Deposition of SiOx Thin Films by Pulsed DC Magnetron Sputtering
2.2. Film Characterization
3. Results and Discussion
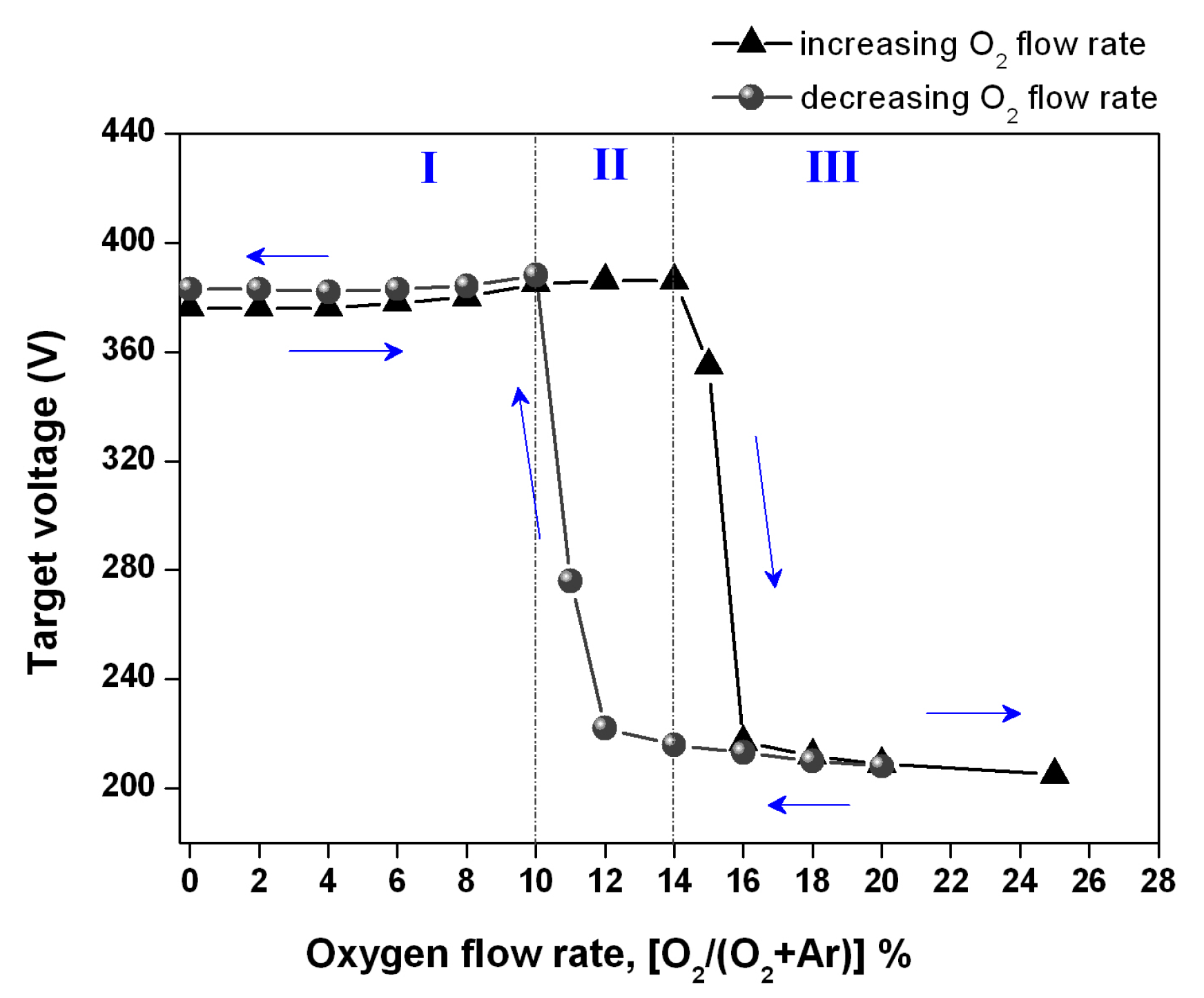
3.1. Target Voltage Control Method and Hysteresis Effect
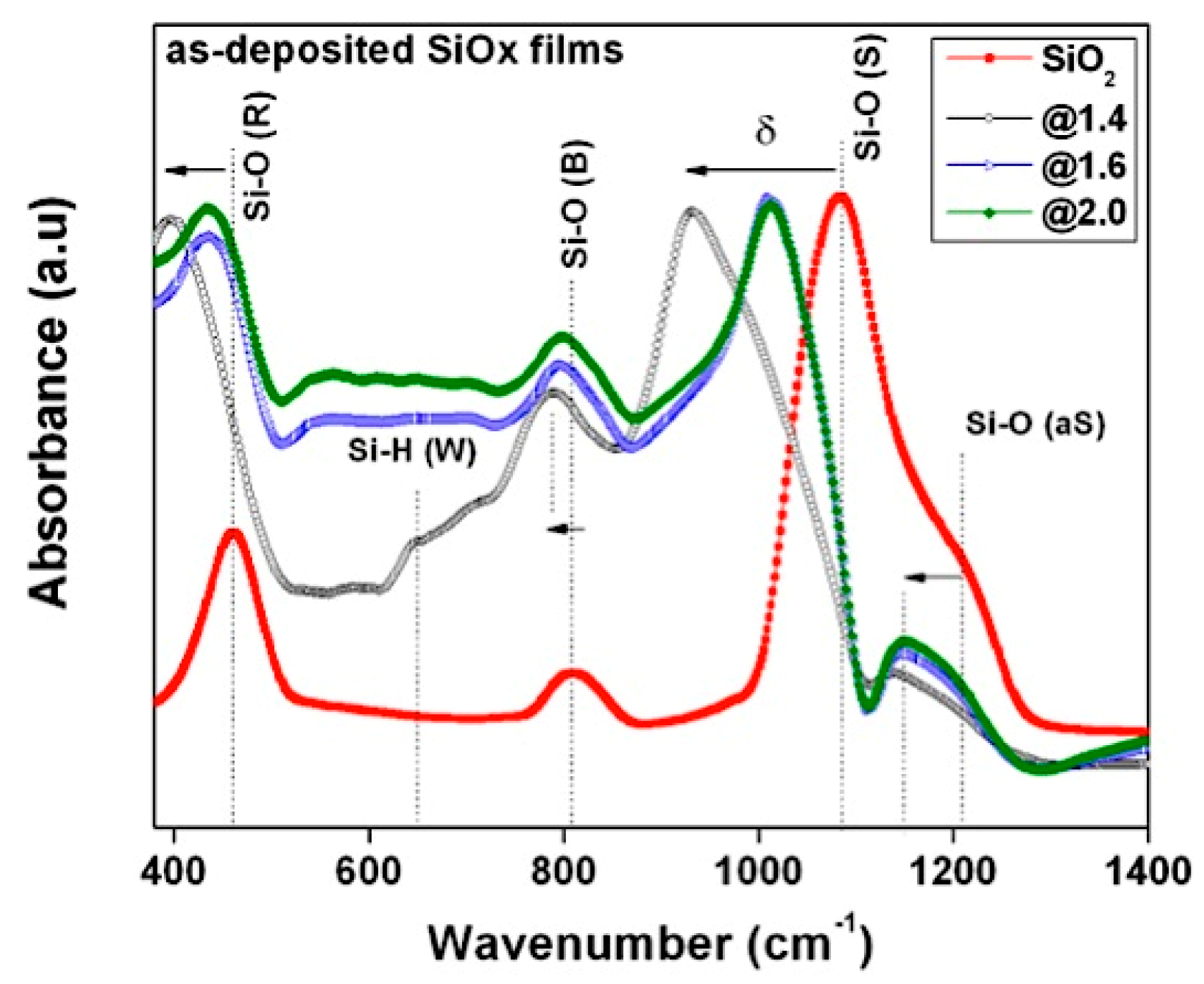
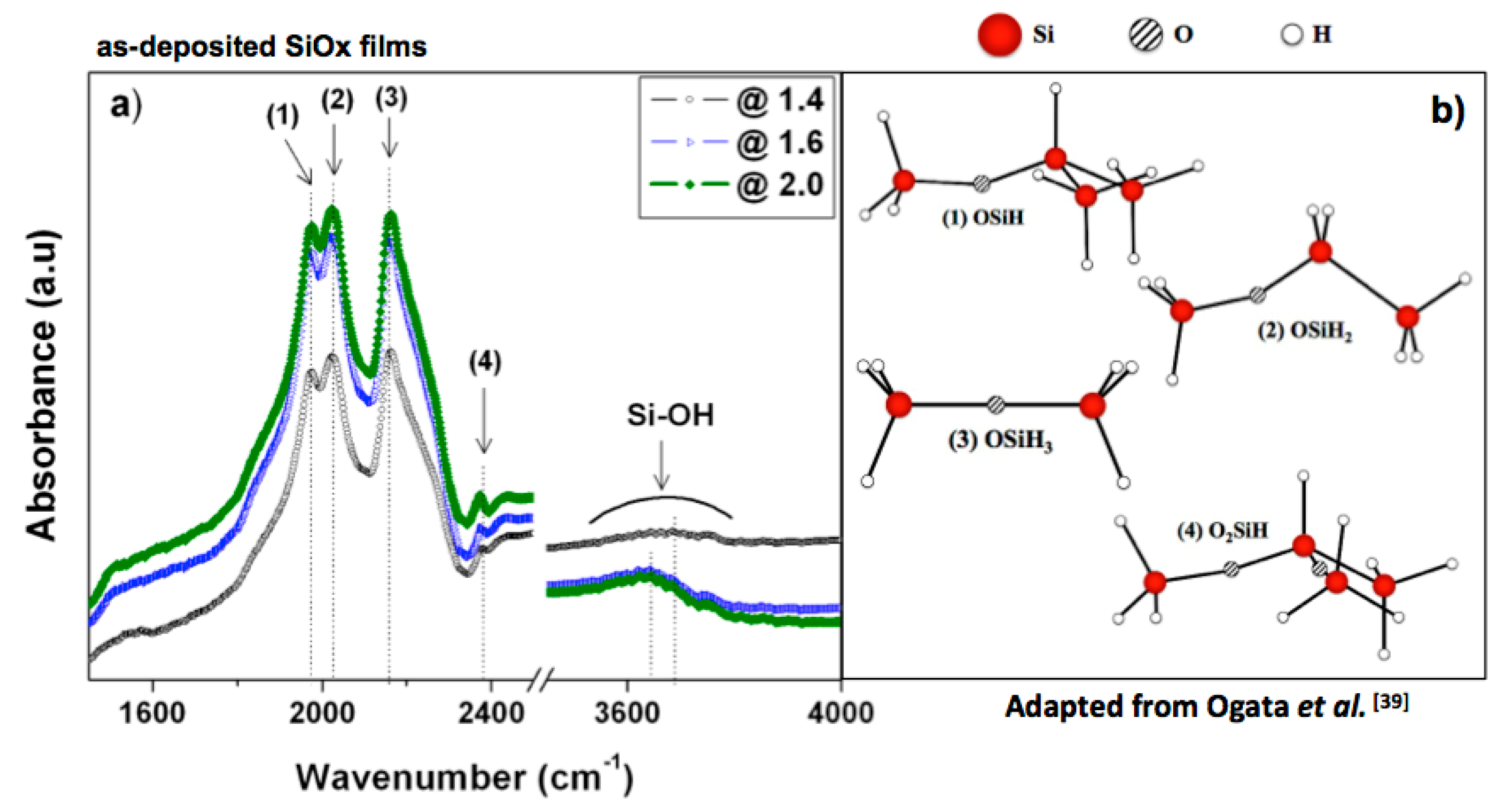
3.2. Film Characteristics
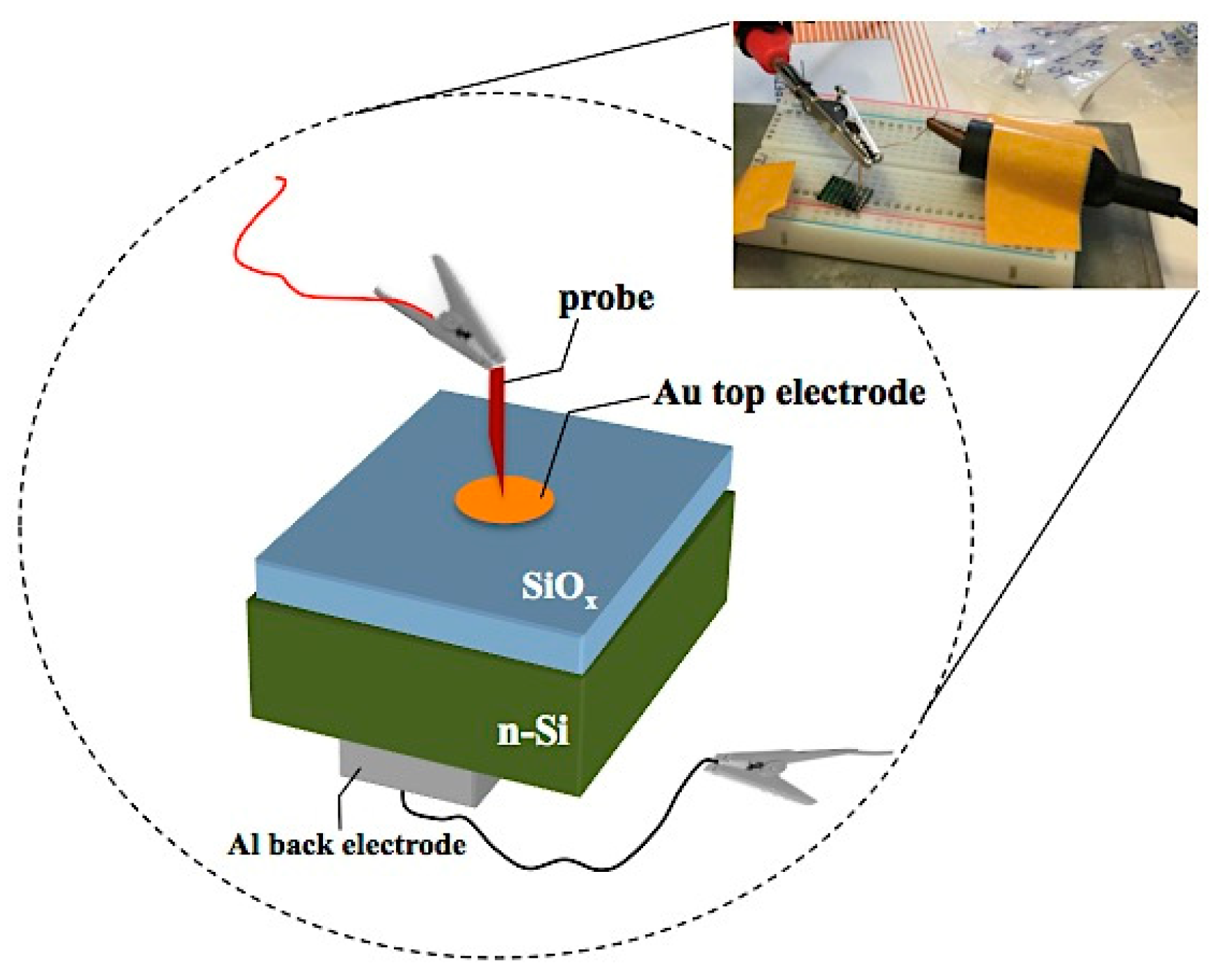
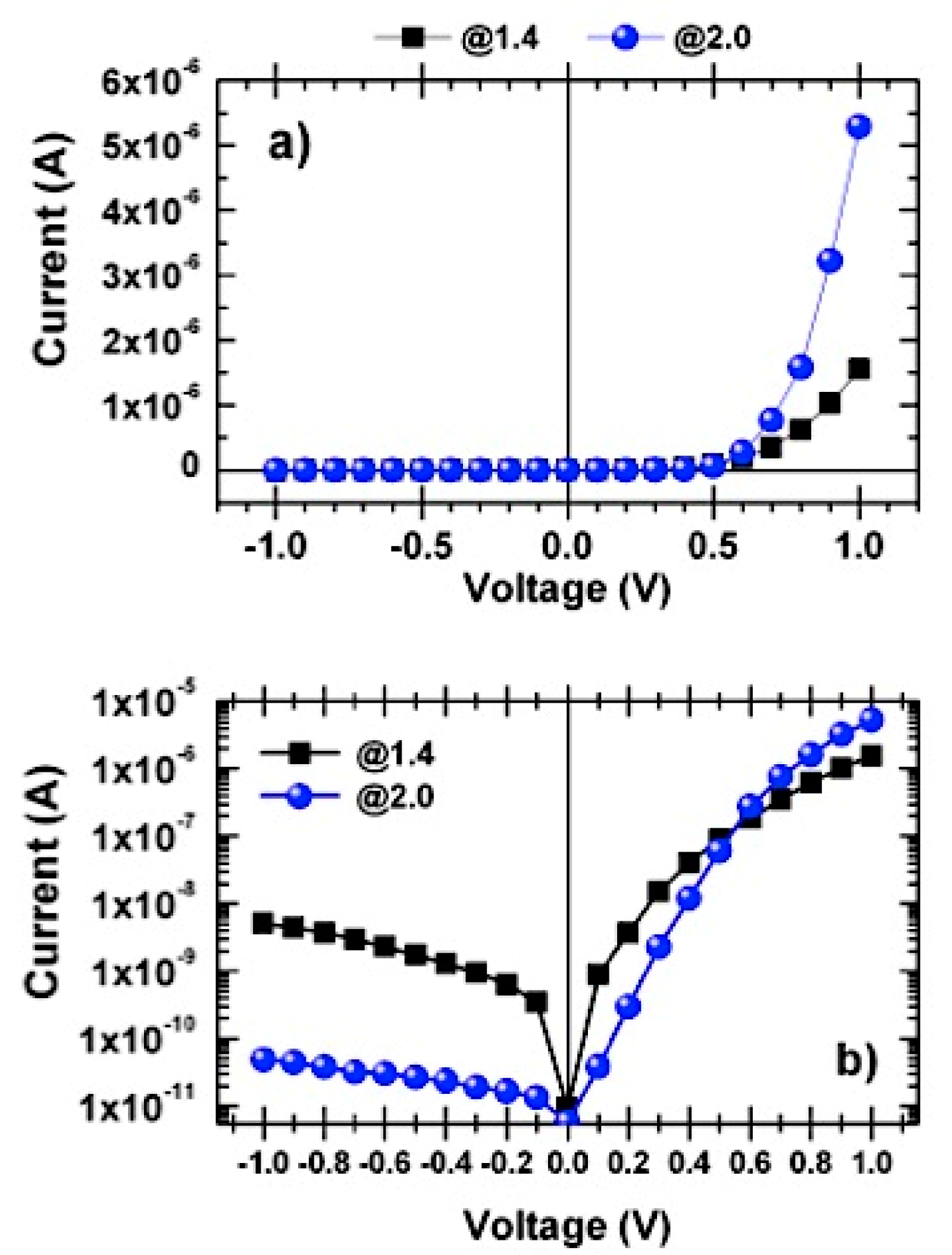
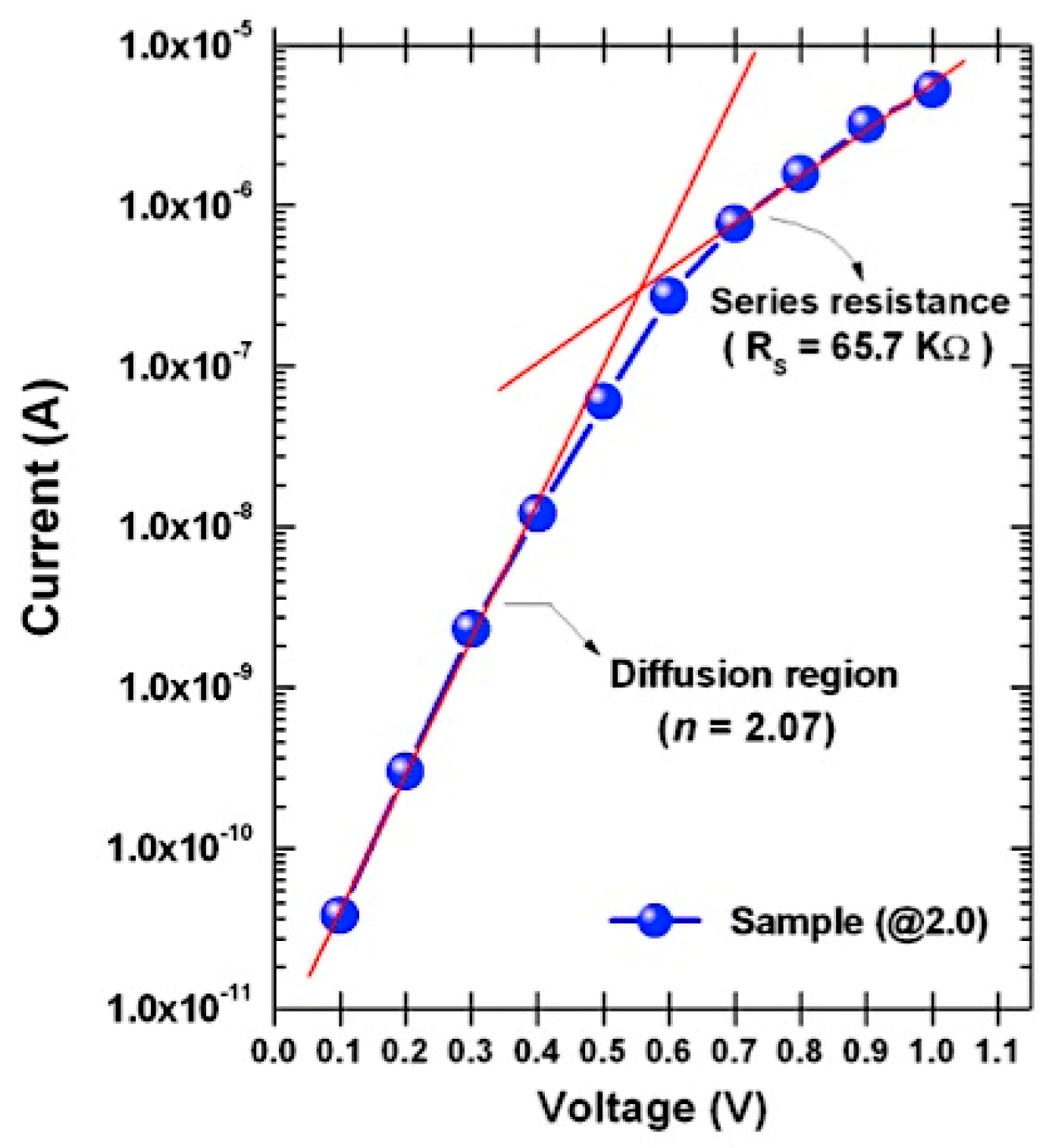
3.3. Current–Voltage Characteristics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- López, J.A.L.; Valerdi, D.E.V.; Lara, A.B.; Salgado, G.G.; Hernández-de la Luz, A.D.; Sánchez, A.M.; Flores Gracia, F.J.; Dominguez, M.A. Optical and compositional properties of SiOx films deposited by HFCVD: Effect of the hydrogen flow. J. Electron. Mater. 2017, 46, 2309. [Google Scholar] [CrossRef]
- Liao, N.; Xue, W.; Zhou, H.; Zhang, M. Investigation on high temperature fracture properties of amorphous silicon dioxide by large-scale atomistic simulations. J. Mater. Sci. Mater. Electron. 2013, 24, 1575–1579. [Google Scholar] [CrossRef]
- Jiew, C.; Chien, Y.; Yung, R. Development of a robust 2T-SONOS cell for embedded flash application. In Proceedings of the Non-Volatile Memory Technology Symposium (NVMTS, 12), Singapore, 31 October–2 November 2012; pp. 1–6. [Google Scholar]
- Leterrier, Y. Durability of nanosized oxygen-barrier coatings on polymers—Internal stresses. Prog. Mater. Sci. 2003, 48, 1–55. [Google Scholar] [CrossRef]
- Dennler, G.; Houdayer, A.; Segui, Y.; Wertheimer, M.R. Growth and structure of hyperthin SiO2 coatings on polymers. J. Vac. Sci. Technol. A 2001, 19, 2320–2327. [Google Scholar] [CrossRef]
- Madou, M.J. Fundamentals of Microfabrication: The Science of Miniaturization, 2nd ed.; CRC Press LCC: Boca Raton, FL, USA, 2002. [Google Scholar]
- Nicollian, E.H.; Brews, J.R. MOS (Metal Oxide Semiconductor) Physics and Technology; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Nakano, Y.; Jimbo, T. Electrical characterization of SiO2/n-GaN metal–insulator–semiconductor diodes. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2003, 21, 1364–1368. [Google Scholar] [CrossRef]
- Fedder, G.K. Mems fabrication. In Proceedings of the 2003 International Test Conference (ITC 2003): Breaking Test Interface Bottlenecks, Charlotte, NC, USA, 28 September–3 October 2003; Volume 1, pp. 691–698. [Google Scholar]
- Wright, J.T.; Carbaugh, D.J.; Haggerty, M.; Richard, A.L.; Ingram, D.C.; Jadwisienczak, W.M.; Rahman, F.; Kaya, S. Thermal oxidation of silicon in a residual oxygen atmosphere—The RESOX process—For self-limiting growth of thin silicon dioxide films. Semicond. Sci. Technol. 2016, 31, 105007. [Google Scholar] [CrossRef]
- Adams, A.C.; Smith, T.E.; Chang, C.C. The growth and characterization of very thin silicon dioxide films. J. Electrochem. Soc. 1980, 127, 1787–1794. [Google Scholar] [CrossRef]
- Boyd, I.W.; Wilson, J.I. A study of thin silicon dioxide films using infrared absorption techniques. J. Appl. Phys. 1982, 53, 4166–4172. [Google Scholar] [CrossRef]
- Jun, S.I.; McKnight, T.E.; Melechko, A.V.; Simpson, M.L.; Rack, P.D. Characterisation of reactively sputtered silicon oxide for thin-film transistor fabrication. Electron. Lett. 2005, 41, 822–823. [Google Scholar] [CrossRef]
- Carneiro, J.; Teixeira, V.; Martins, A.; Mendes, M.; Ribeiro, M.; Vieira, A.; Carneiro, J. Surface properties of doped and undoped TiO2 thin films deposited by magnetron sputtering. Vacuum 2009, 83, 1303–1306. [Google Scholar] [CrossRef]
- Sainty, W.G.; Netterfield, R.P.; Martin, P.J. Protective dielectric coatings produced by ion-assisted deposition. Appl. Opt. 1984, 23, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Alvisi, M.; De Tomasi, F.; Della Patria, A.; Di Giulio, M.; Masetti, E.; Perrone, M.R.; Protopapa, M.L.; Tepore, A. Ion assistance effects on electron beam deposited MgF2 films. J. Vac. Sci. Technol. A 2002, 20, 714–720. [Google Scholar] [CrossRef]
- Jaing, C.-C.; Shiao, M.-H.; Lee, C.-C.; Lu, C.-J.; Liu, M.-C.; Lee, C.-H.; Chen, H.-C. Effects of ion assistance and substrate temperature on optical characteristics and microstructure of MgF2 films formed by electron-beam evaporation. Jpn. J. Appl. Phys. 2006, 45, 5027–5029. [Google Scholar] [CrossRef]
- Megiris, C.E.; Glezer, J.H.E. Preparation of silicon dioxide films by low-pressure chemical vapor deposition on dense and porous alumina substrates. Chem. Eng. Sci. 1992, 47, 3925–3934. [Google Scholar] [CrossRef]
- Hayashi, S.; Tanimoto, S.; Yamamoto, K. Analysis of surface oxides of gas-evaporated Si small particles with infrared spectroscopy, high-resolution electron microscopy, and x-ray photoemission spectroscopy. J. Appl. Phys. 1990, 68, 5300–5308. [Google Scholar] [CrossRef]
- Teixeira, V.; Soares, P.; Martins, A.J.; Carneiro, J.; Cerqueira, F.; Cerqueira, M.F. Nanocomposite metal amorphous-carbon thin films deposited by hybrid PVD and PECVD technique. J. Nanosci. Nanotechnol. 2009, 9, 4061–4066. [Google Scholar] [CrossRef] [PubMed]
- Sellers, J. Asymmetric bipolar pulsed DC: The enabling technology for reactive PVD. Surf. Coat. Technol. 1998, 98, 1245–1250. [Google Scholar] [CrossRef]
- Kelly, P.; Beevers, C.; Henderson, P.; Arnell, R.; Bradley, J.; Bäcker, H. A comparison of the properties of titanium-based films produced by pulsed and continuous DC magnetron sputtering. Surf. Coat. Technol. 2003, 174, 795–800. [Google Scholar] [CrossRef]
- Studenyak, I.; Kranjčec, M.; Kurik, M. Urbach rule in solid state physics. Int. J. Opt. Appl. 2014, 4, 76–83. [Google Scholar]
- Schottky, W. Zur Halbleitertheorie der Sperrschicht- und Spitzengleichrichter. Z. Phys. 1939, 113, 367–414. [Google Scholar] [CrossRef]
- Tung, R.T. The physics and chemistry of the Schottky barrier height. Appl. Phys. Rev. 2014, 1, 011304. [Google Scholar]
- Zhang, Z.; Yates, J.T., Jr. Band bending in semiconductors: Chemical and physical consequences at surfaces and interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.; Teixeira, V.; Portinha, A.; Magalhães, A.; Coutinho, P.; Tavares, C.J.; Newton, R.; Carneiro, J.; Magalhaẽs, A. Iron-doped photocatalytic TiO2 sputtered coatings on plastics for self-cleaning applications. Mater. Sci. Eng. B 2007, 138, 144–150. [Google Scholar] [CrossRef]
- Carneiro, J.O.; Teixeira, V.; Nascimento, J.H.O.; Neves, J.; Tavares, P.B.; Carneiro, J. Photocatalytic activity and UV-protection of TiO2 nanocoatings on poly(lactic acid) fibres deposited by pulsed magnetron sputtering. J. Nanosci. Nanotechnol. 2011, 11, 8979–8985. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barradas, N.; Jeynes, C.; Webb, R.; Kreissig, U.; Grötzschel, R. Unambiguous automatic evaluation of multiple ion beam analysis data with simulated annealing. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1999, 149, 233–237. [Google Scholar] [CrossRef]
- Musil, J.; Baroch, P.; Vlček, J.; Nam, K.; Han, J. Reactive magnetron sputtering of thin films: Present status and trends. Thin Solid Film. 2005, 475, 208–218. [Google Scholar] [CrossRef]
- Ohring, M. The Materials Science of Thin Films; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Sigmund, P. Theory of sputtering. I. Sputtering yield of amorphous and polycrystalline targets. Phys. Rev. 1969, 184, 383. [Google Scholar] [CrossRef]
- Kudriavtsev, Y.; Villegas, A.; Godines, A.; Asomoza, R. Calculation of the surface binding energy for ion sputtered particles. Appl. Surf. Sci. 2005, 239, 273–278. [Google Scholar] [CrossRef]
- Ay, F.; Aydinly, A. Comparative investigation of hydrogen bonding in silicon based PECVD grown dielectrics for optical waveguides. Opt. Mater. 2004, 26, 33–46. [Google Scholar] [CrossRef]
- Lin, G.-R.; Lin, C.-J.; Lin, C.-K.; Chou, L.-J.; Chueh, Y.-L. Oxygen defect and Si nanocrystal dependent white-light and near-infrared electroluminescence of Si-implanted and plasma-enhanced chemical-vapor deposition-grown Si-rich SiO2. J. Appl. Phys. 2005, 97, 094306. [Google Scholar] [CrossRef]
- Pai, P.G.; Chao, S.S.; Takagi, Y.; Lucovsky, G. Infrared spectroscopic study of silicon oxide (SiOx) films produced by plasma enhanced chemical vapor deposition. J. Vac. Sci. Technol. A 1986, 4, 689–694. [Google Scholar] [CrossRef]
- Vázquez-Valerdi, D.E.; Luna-López, J.A.; Carrillo-López, J.; García-Salgado, G.; Benítez-Lara, A.; Espinosa-Torres, N.D. Compositional and optical properties of SiOx films and (SiOx/SiOy) junctions deposited by HFCVD. Nanoscale Res. Lett. 2014, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Morioka, T.; Tsuda, N.; Koike, C.; Kaito, C.; Saito, Y.; Kimura, S. Study of the structure of silica film by infrared spectroscopy and electron diffraction analyses. Mon. Not. R. Astron. Soc. 1998, 299, 78–82. [Google Scholar] [CrossRef]
- Ogata, Y.; Niki, H.; Sakka, T.; Iwasaki, M. Oxidation of porous silicon under water vapor environment. J. Electrochem. Soc. 1995, 142, 1595–1601. [Google Scholar] [CrossRef]
- Gupta, P.; Dillon, A.; Bracker, A.; George, S. FTIR studies of H2O and D2O decomposition on porous silicon surfaces. Surf. Sci. 1991, 245, 360–372. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Akl, A.A. Influence of composition on optical and dispersion parameters of thermally evaporated non-crystalline Cd50S50-xSex thin films. J. Alloys Compd. 2015, 648, 280–290. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Urbach, F. The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Malainho, E.; Vasilevskiy, M.I.; Alpuim, P.; Filonovich, S.A. Dielectric function of hydrogenated amorphous silicon near the optical absorption edge. J. Appl. Phys. 2009, 106, 073110. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Akl, A.A. Effect of Se addition on optical and electrical properties of chalcogenide CdSSe thin films. Superlattices Microstruct. 2016, 89, 153–169. [Google Scholar] [CrossRef]
- Kazmersky, L.L. (Ed.) Polycrystalline and Amorphous Thin Films and Devices; Academic Press: New York, NY, USA, 1980; p. 135. [Google Scholar]
- Nekrashevich, S.S.; Gritsenko, V.A. Electronic structure of silicon dioxide (a review). Phys. Solid State 2014, 56, 207–222. [Google Scholar] [CrossRef]
- Mott, N.F.; Davis, E.A. Electronic Processes in Non-Crystalline Materials; Oxford University Press: Oxford, UK, 1971. [Google Scholar]
- Fadel, M.; Fayek, S.; Abou-Helal, M.; Ibrahim, M.; Shakra, A. Structural and optical properties of SeGe and SeGeX (X = In, Sb and Bi) amorphous films. J. Alloys Compd. 2009, 485, 604–609. [Google Scholar] [CrossRef]
- Choi, Y.-R.; Zheng, M.; Bai, F.; Liu, J.; Tok, E.-S.; Huang, Z.; Sow, C.-H. Laser-induced greenish-blue photoluminescence of mesoporous silicon nanowires. Sci. Rep. 2014, 4, 4940. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, M.; Chelikowsky, J. Electron states in α-quartz (SiO2). Solid State Commun. 1977, 21, 381–384. [Google Scholar] [CrossRef]
- Calabrese, E.; Fowler, W.B. Electronic energy-band structure of α quartz. Phys. Rev. B 1978, 18, 2888–2896. [Google Scholar] [CrossRef]
- Butler, M.A. Prediction of flatband potentials at semiconductor-electrolyte interfaces from atomic electronegativities. J. Electrochem. Soc. 1978, 125, 228–232. [Google Scholar] [CrossRef]
- Morrison, S.R. Electrochemistry at Semiconductor and Oxidized Metal Electrodes; Plenum Press: New York, NY, USA, 1980. [Google Scholar]
- Chen, X.Y.; Lu, Y.F.; Tang, L.J.; Wu, Y.H.; Cho, B.J.; Xu, X.J.; Dong, J.R.; Song, W.D. Annealing and oxidation of silicon oxide films prepared by plasma-enhanced chemical vapor deposition. J. Appl. Phys. 2005, 97, 014913. [Google Scholar] [CrossRef]
- Bo, H.; Ma, Z.Q.; Jing, X.; Lei, Z.; Sheng, Z.N.; Feng, L.; Cheng, S.; Ling, S.; Jie, M.X.; Yue, Z.C.; et al. Structural, electrical and optical properties of AZO/SiO2/p-Si SIS heterojunction prepared by magnetron sputtering. Opt. Appl. 2010, 40, 15–24. [Google Scholar]
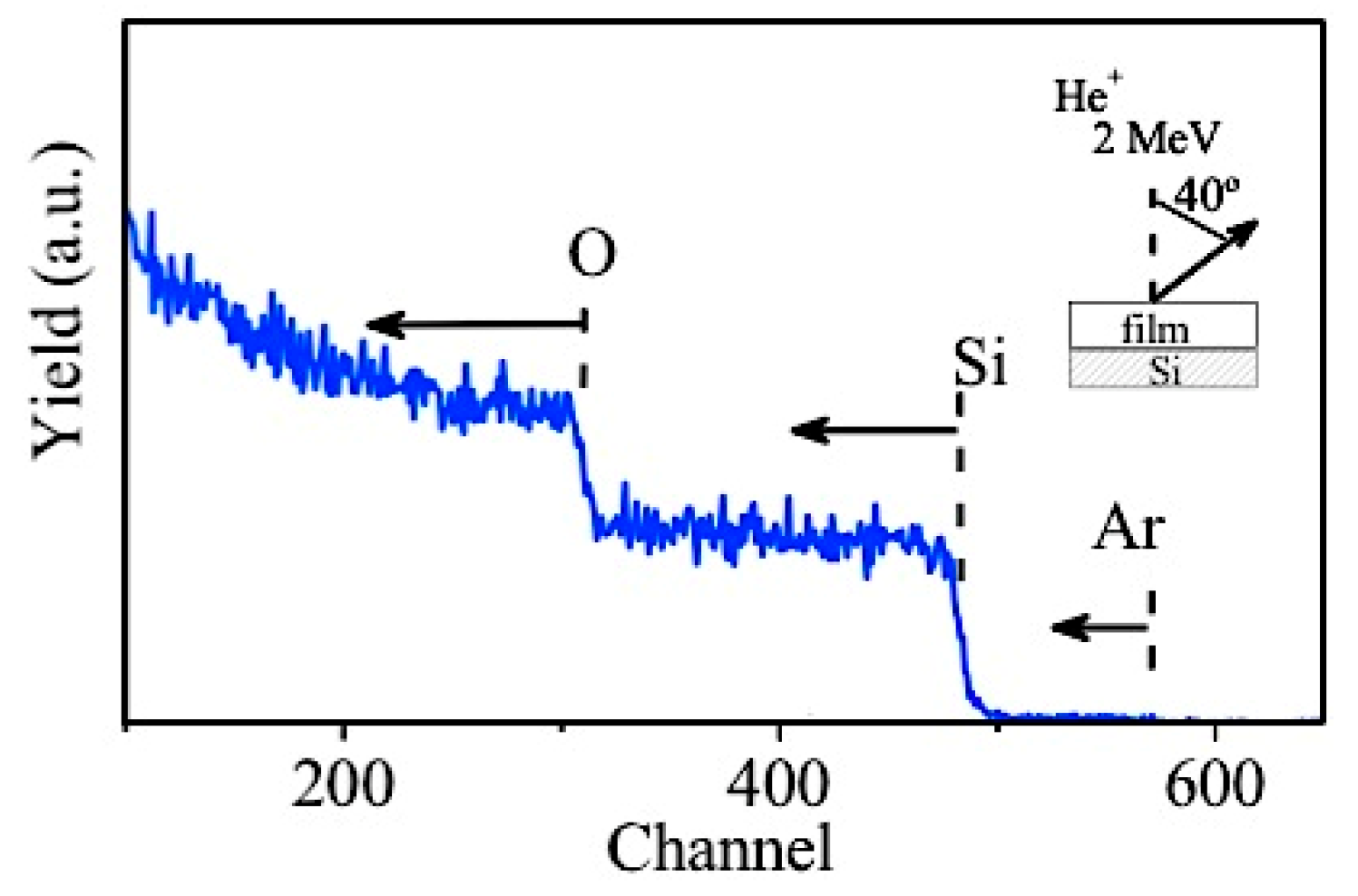
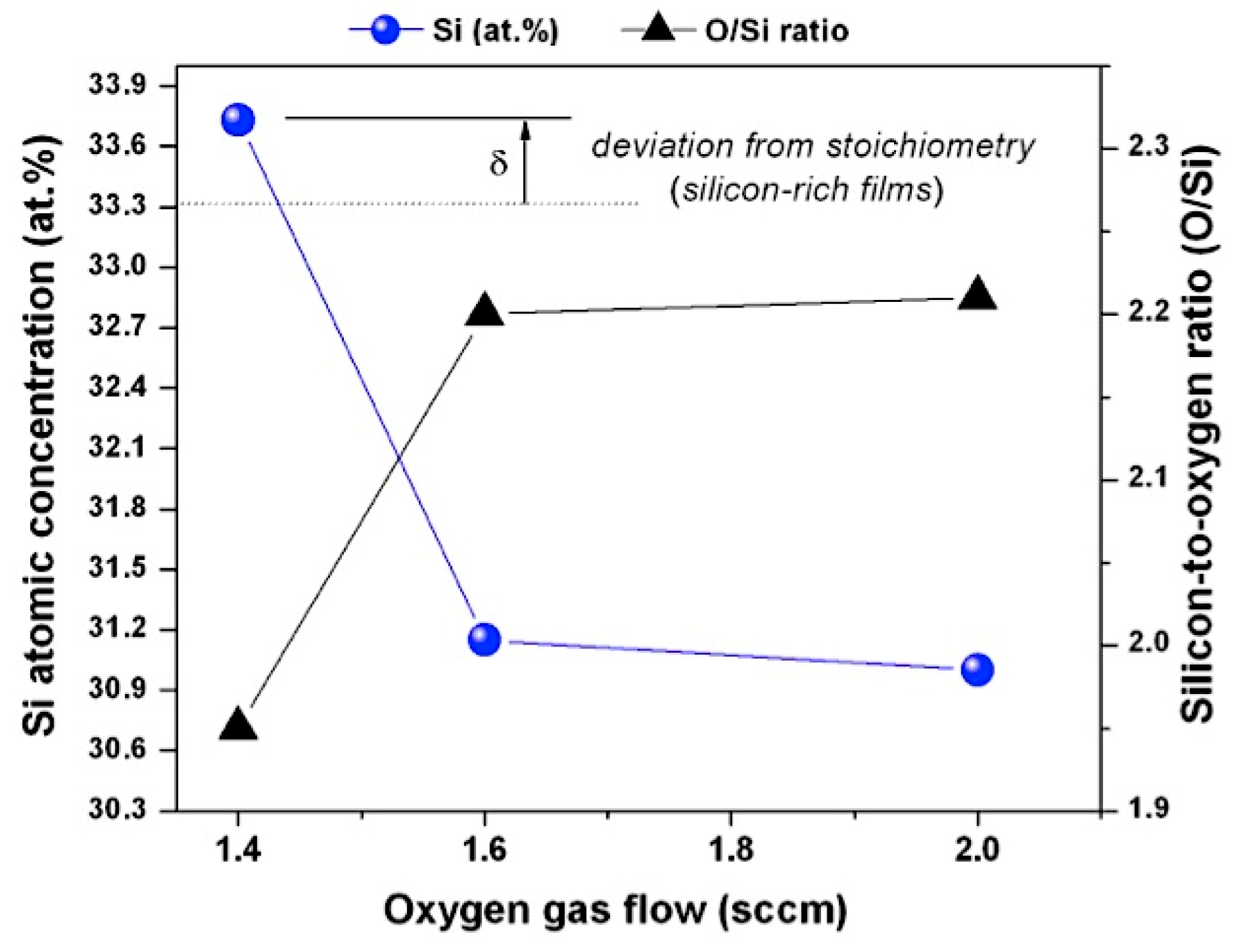
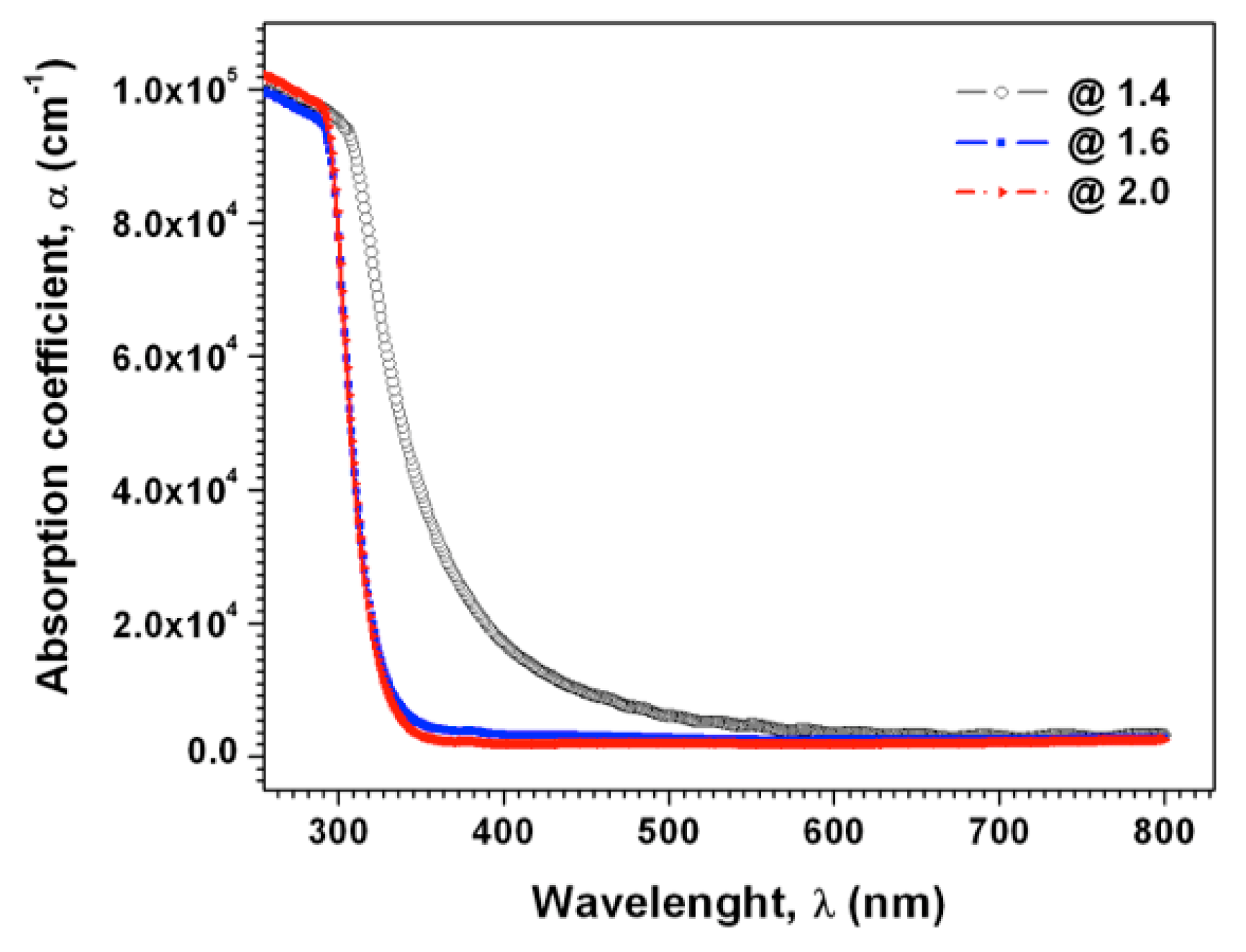

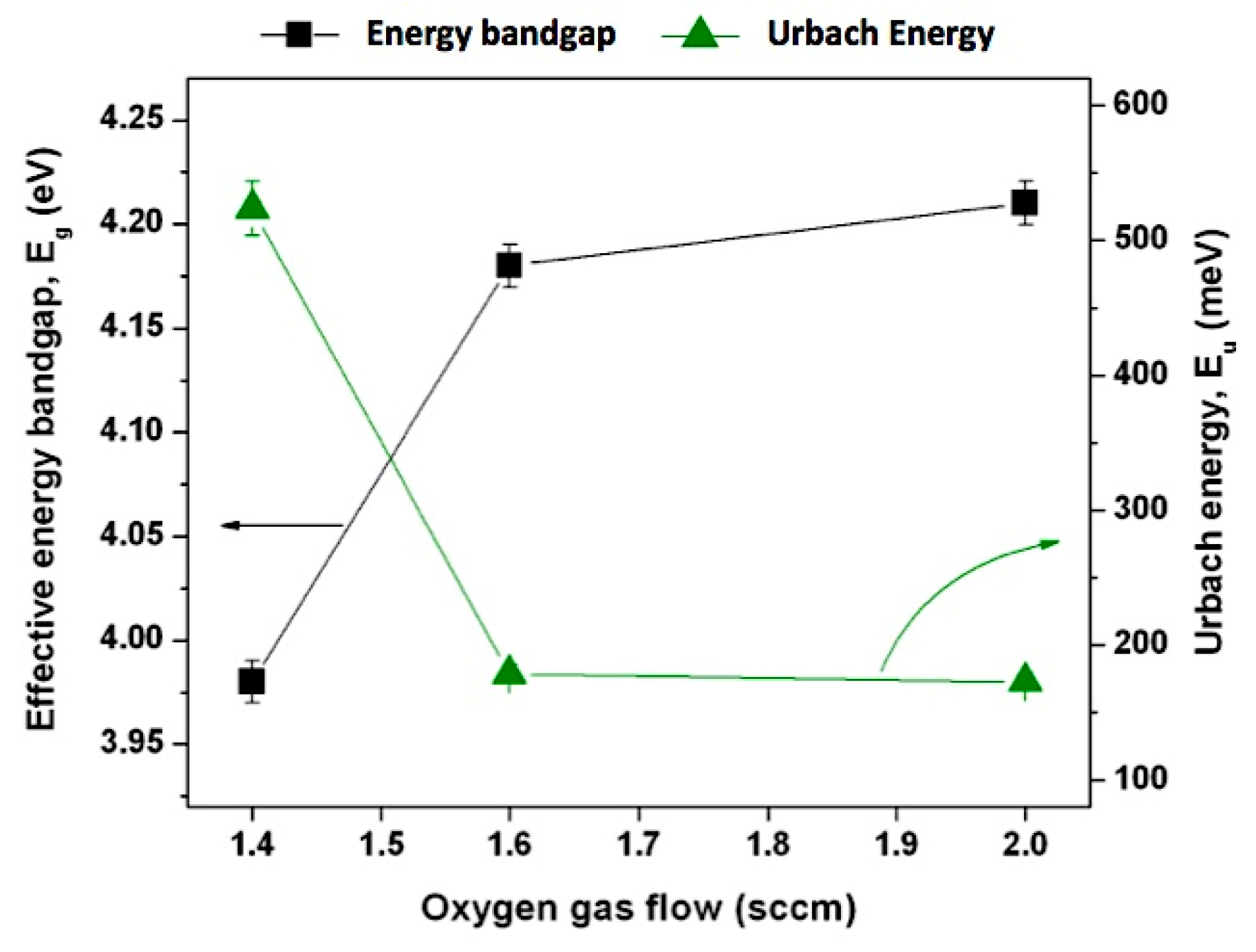

| Sample ID | Current (A) | Voltage (V) | Reverse Time (µs) | Frequency (kHz) | Gas Flow (sccm) | Reverse Phase (%) | |
|---|---|---|---|---|---|---|---|
| Ar | O2 | ||||||
| @1.4 | 0.35 | 385 | 5.0 | 60.0 | 8.0 | 1.4 | 30.0 |
| @1.6 | 217 | 1.6 | |||||
| @2.0 | 210 | 2.0 | |||||
| Vibration Mode | Peak Position (cm−1) | |||
|---|---|---|---|---|
| Sample ID | ||||
| @1.4 | @1.6 | @2.0 | SiO2 | |
| Si–O rocking | 397 | 438 | 440 | 461 |
| Si–O bending | 789 | 797 | 799 | 810 |
| Si–O stretching (on phase) | 932 | 1010 | 1013 | 1082 |
| Si–O stretching out of phase | 1140 | 1148 | 1150 | 1203 |
| Si–H wagging | 648 | – | – | – |
| Group | Cluster | Peak Number | Vibration Type | Wavenumber (cm−1) |
|---|---|---|---|---|
| SiH3 | OSiH3 | (3) | s-stretching | 2162 |
| SiH2 | OSiH2 | (2) | a-stretching | 2025 |
| SiH | OSiH | (1) | stretching | 1980 |
| O2SiH | (4) | stretching | 2376 |
| Sample ID | Eu (meV) | Eg (eV) | Steepness Parameter (σ) | Constant, α0 (cm−1) |
|---|---|---|---|---|
| @1.4 | 523.45 | 3.98 | 0.049 | 104.1 |
| @1.6 | 178.48 | 4.18 | 0.145 | 104.8 |
| @2.0 | 172.85 | 4.21 | 0.150 | 105.1 |
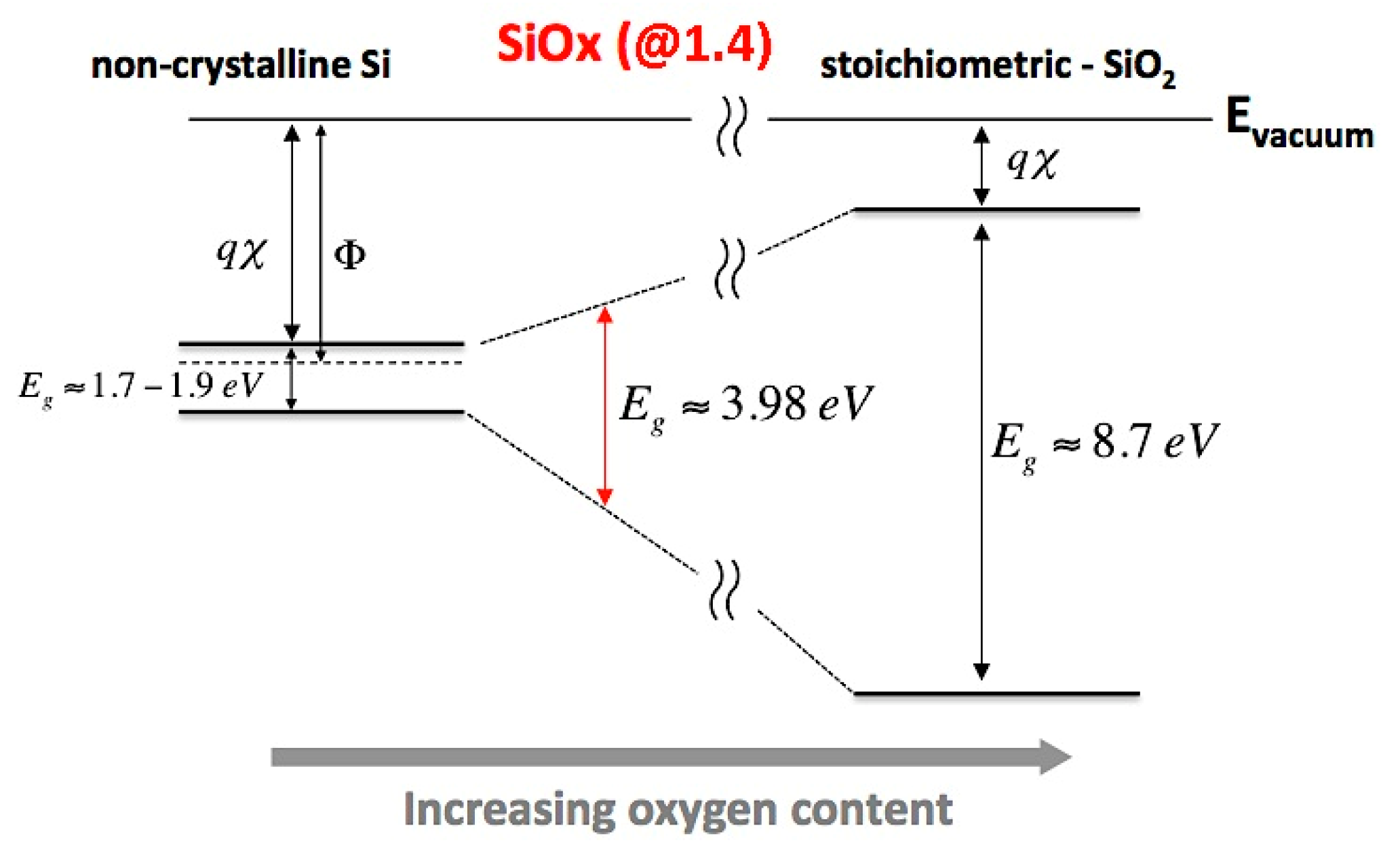
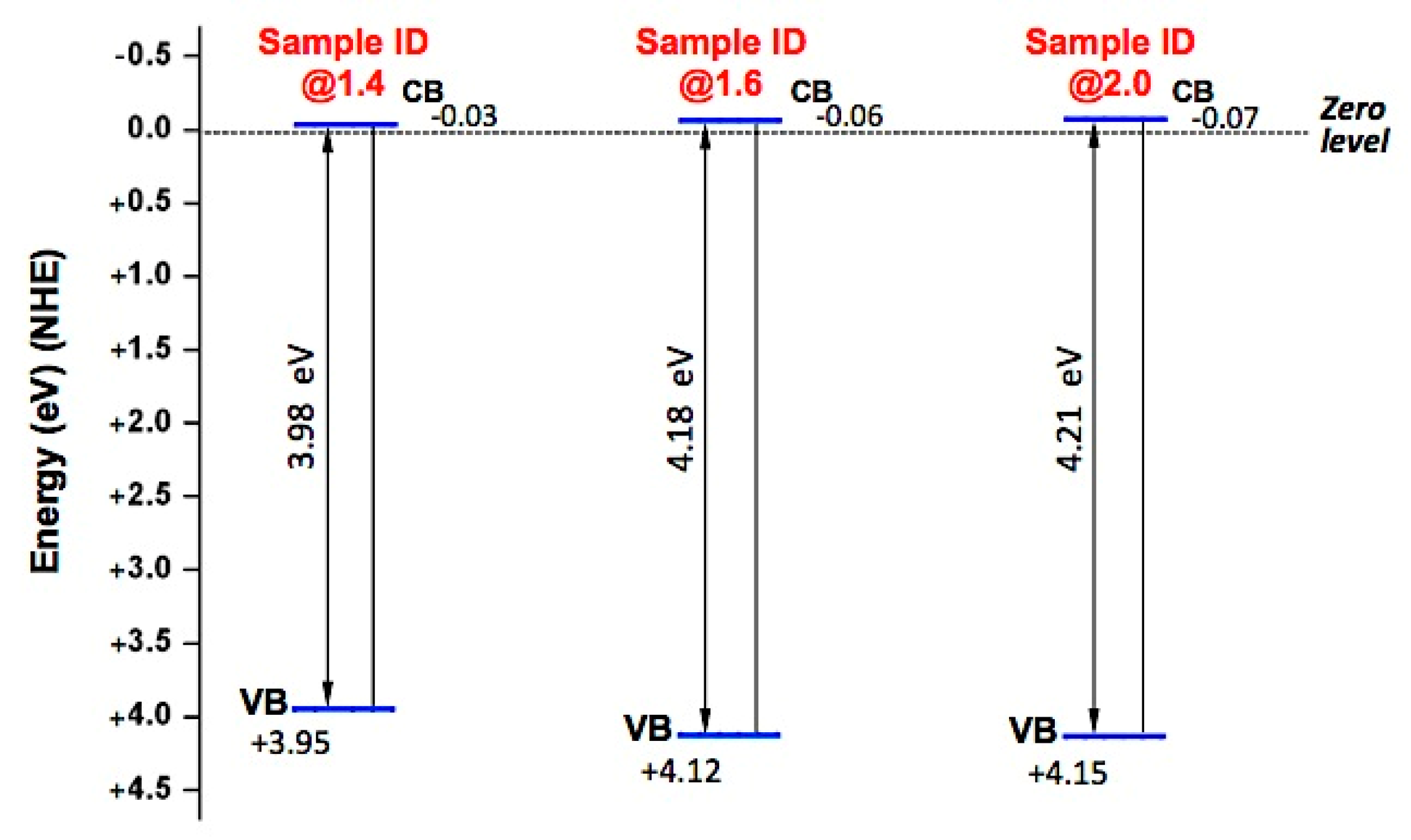
| Sample ID | Eg (eV) | X (eV) | (vs. NHE Scale) | (vs. NHE Scale) |
|---|---|---|---|---|
| @1.4 | 3.98 | 6.457 | −0.03 | 3.95 |
| @1.6 | 4.18 | 6.534 | −0.06 | 4.12 |
| @2.0 | 4.21 | 6.539 | −0.07 | 4.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carneiro, J.O.; Machado, F.; Rebouta, L.; Vasilevskiy, M.I.; Lanceros-Méndez, S.; Teixeira, V.; Costa, M.F.; Samantilleke, A.P. Compositional, Optical and Electrical Characteristics of SiOx Thin Films Deposited by Reactive Pulsed DC Magnetron Sputtering. Coatings 2019, 9, 468. https://doi.org/10.3390/coatings9080468
Carneiro JO, Machado F, Rebouta L, Vasilevskiy MI, Lanceros-Méndez S, Teixeira V, Costa MF, Samantilleke AP. Compositional, Optical and Electrical Characteristics of SiOx Thin Films Deposited by Reactive Pulsed DC Magnetron Sputtering. Coatings. 2019; 9(8):468. https://doi.org/10.3390/coatings9080468
Chicago/Turabian StyleCarneiro, Joaquim O., Filipe Machado, Luis Rebouta, Mikhail I. Vasilevskiy, Senen Lanceros-Méndez, Vasco Teixeira, Manuel F. Costa, and Anura P. Samantilleke. 2019. "Compositional, Optical and Electrical Characteristics of SiOx Thin Films Deposited by Reactive Pulsed DC Magnetron Sputtering" Coatings 9, no. 8: 468. https://doi.org/10.3390/coatings9080468
APA StyleCarneiro, J. O., Machado, F., Rebouta, L., Vasilevskiy, M. I., Lanceros-Méndez, S., Teixeira, V., Costa, M. F., & Samantilleke, A. P. (2019). Compositional, Optical and Electrical Characteristics of SiOx Thin Films Deposited by Reactive Pulsed DC Magnetron Sputtering. Coatings, 9(8), 468. https://doi.org/10.3390/coatings9080468










