Biofouling of FeNP-Coated SWRO Membranes with Bacteria Isolated after Pre-Treatment in the Sea of Cortez
Abstract
:1. Introduction
2. Materials and Methods
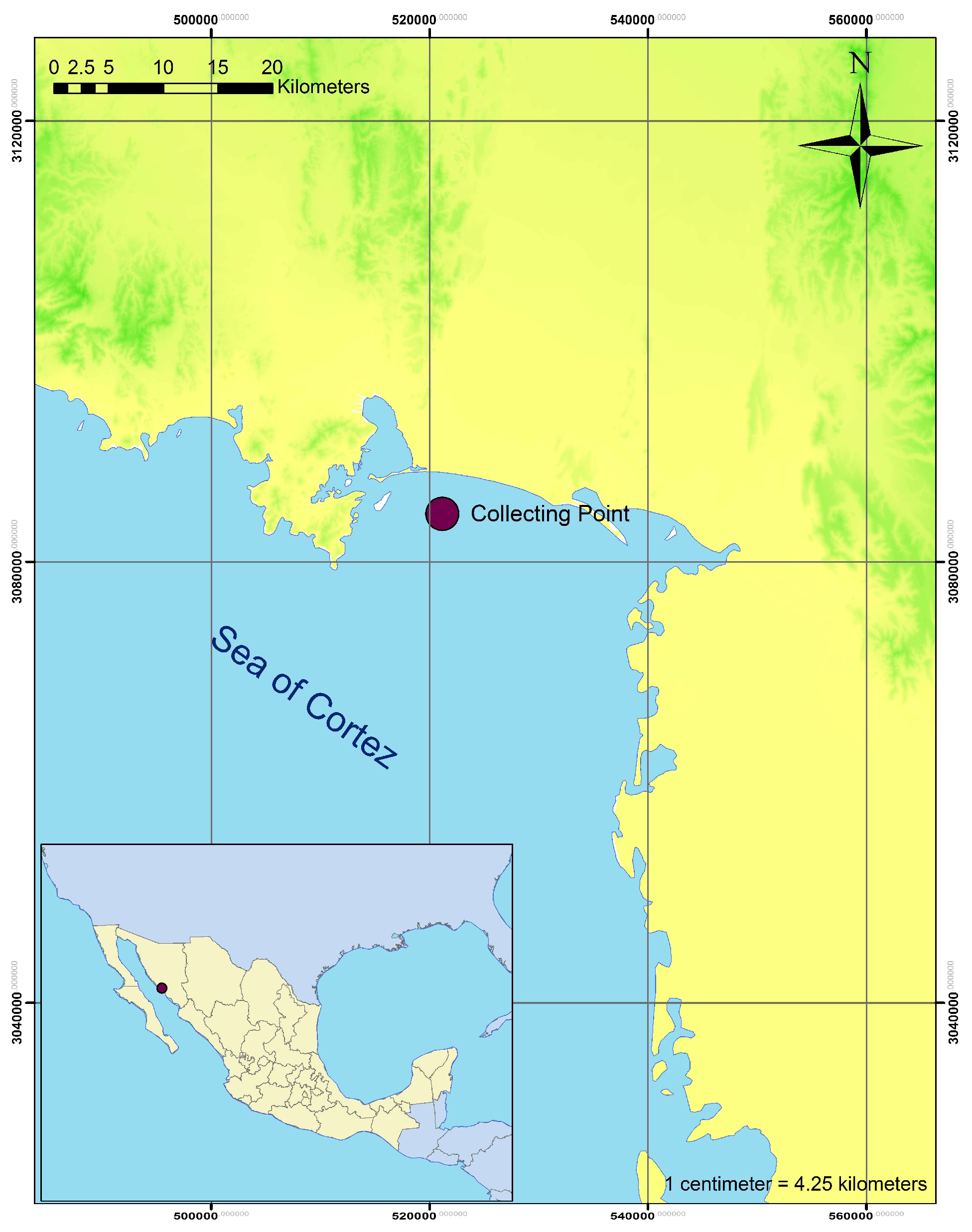
2.1. Sampling
2.2. Bacterial Isolation and Molecular Identification
2.3. Minimum Inhibitory Fe+2 Concentration
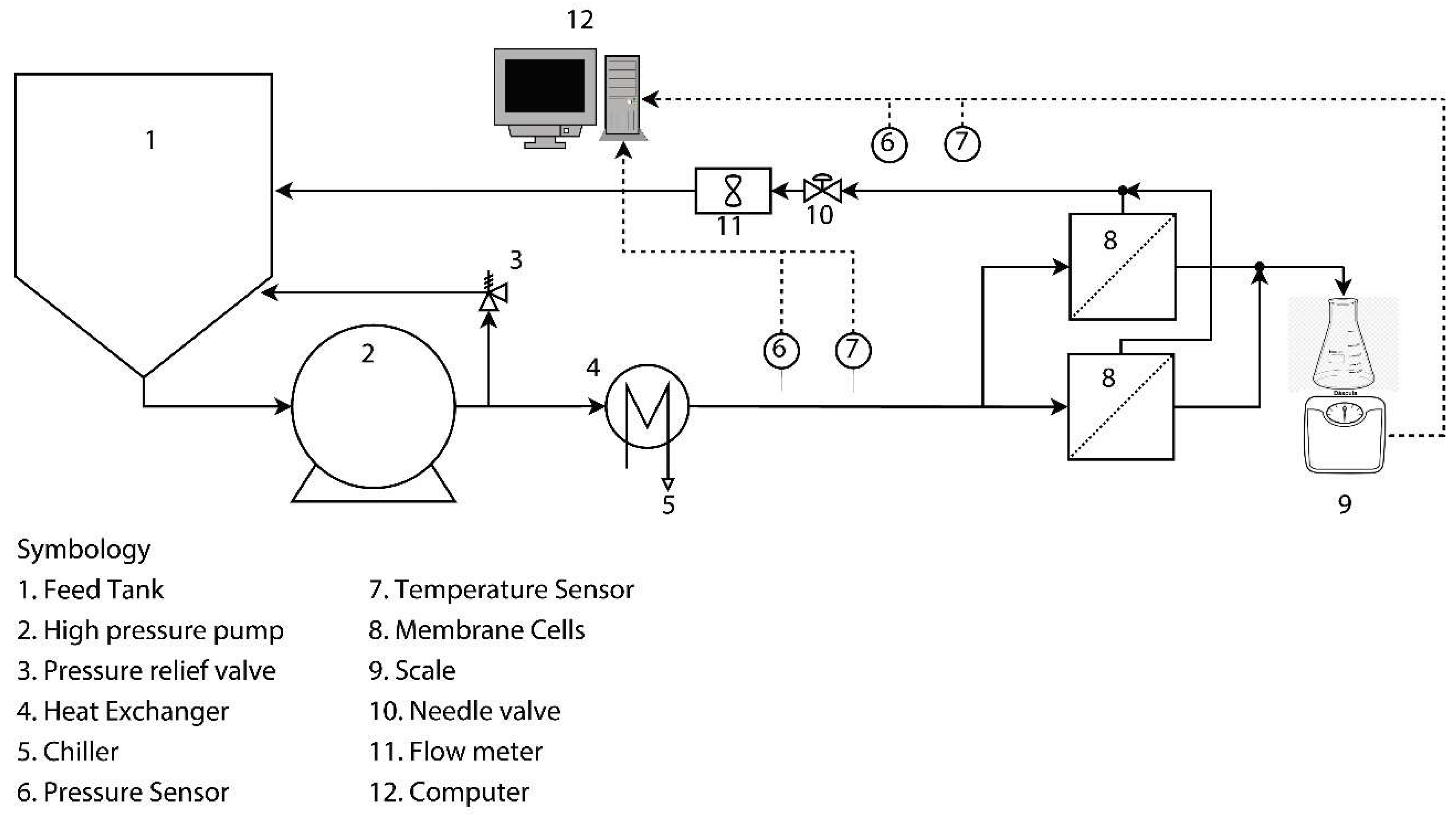
2.4. Accelerated Biofouling Test on RO Membranes
2.5. Statistical Analysis
3. Results and Discussion
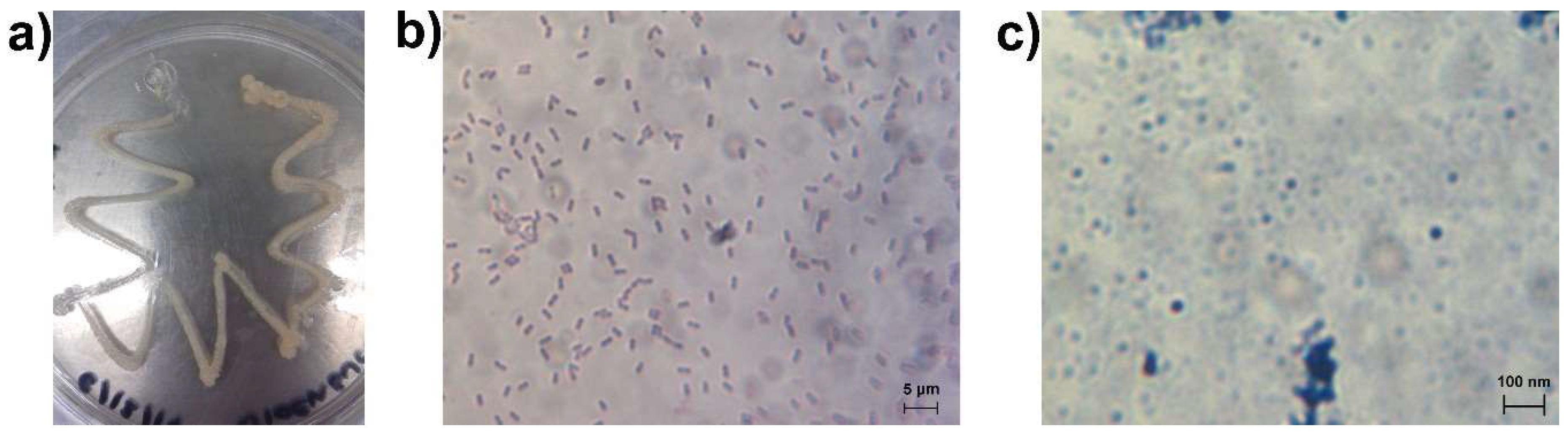
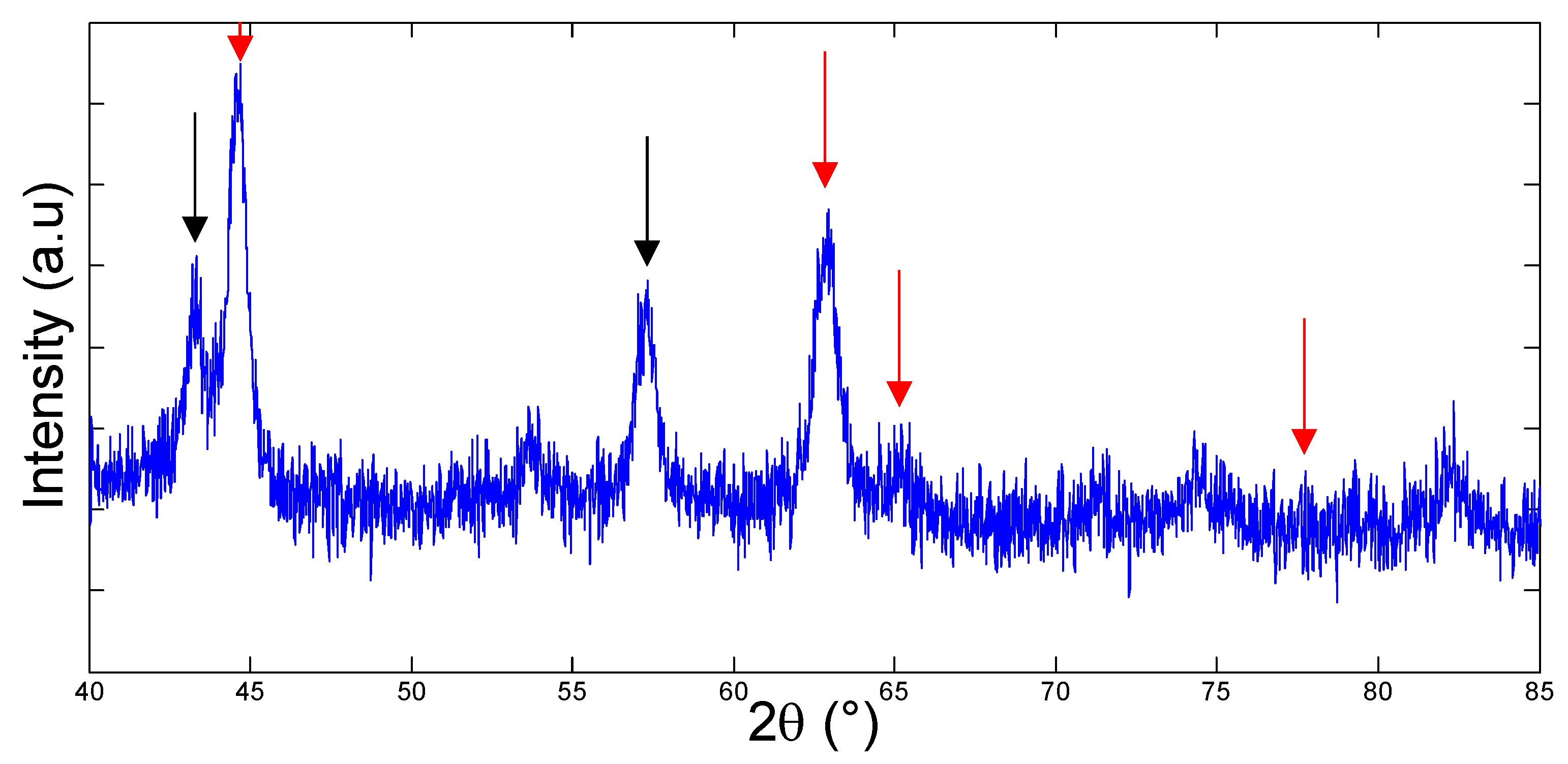
3.1. Bacteria Growth, Isolation and Molecular Identification
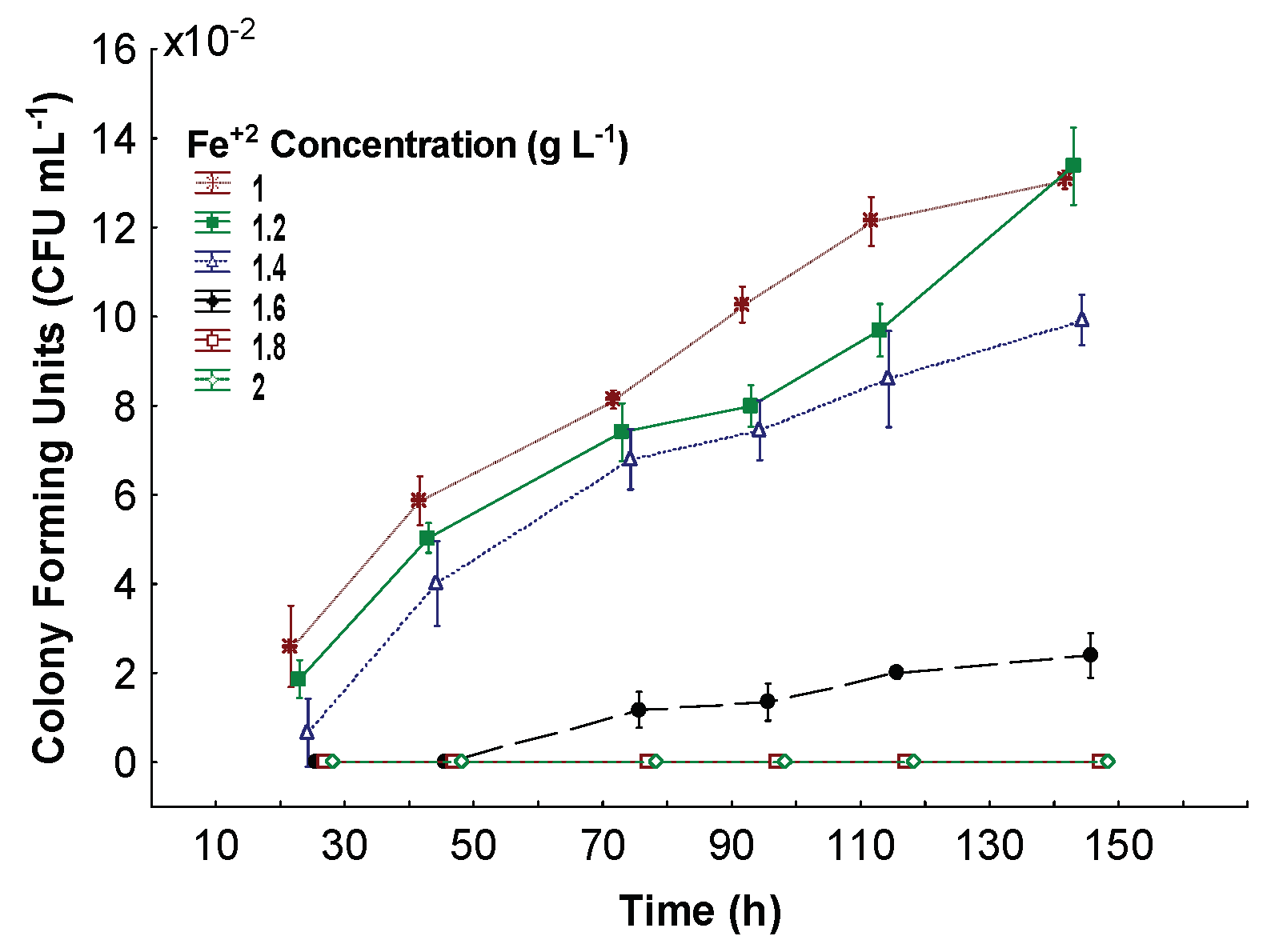
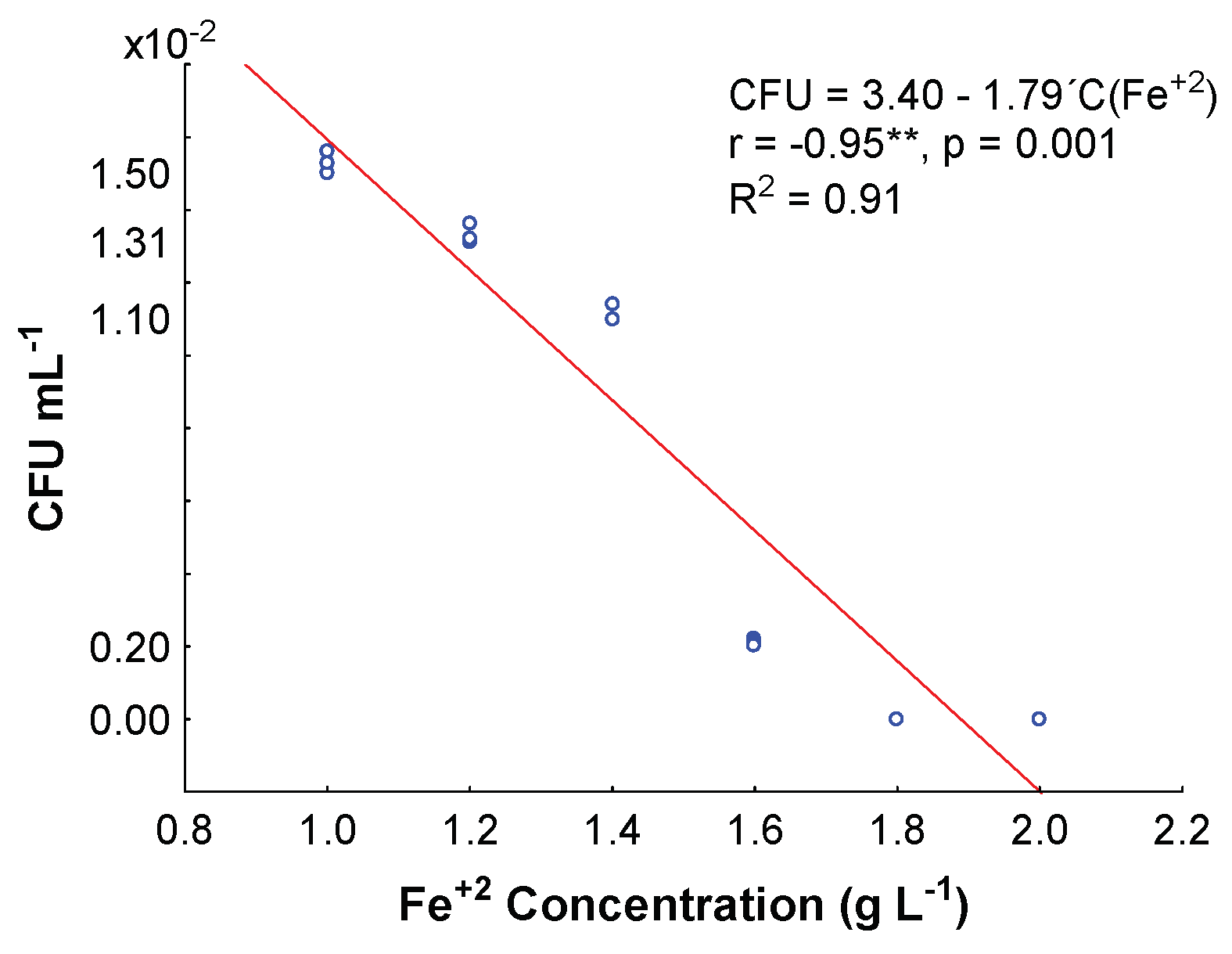
3.2. Minimum Inhibitory Fe+2 Concentration
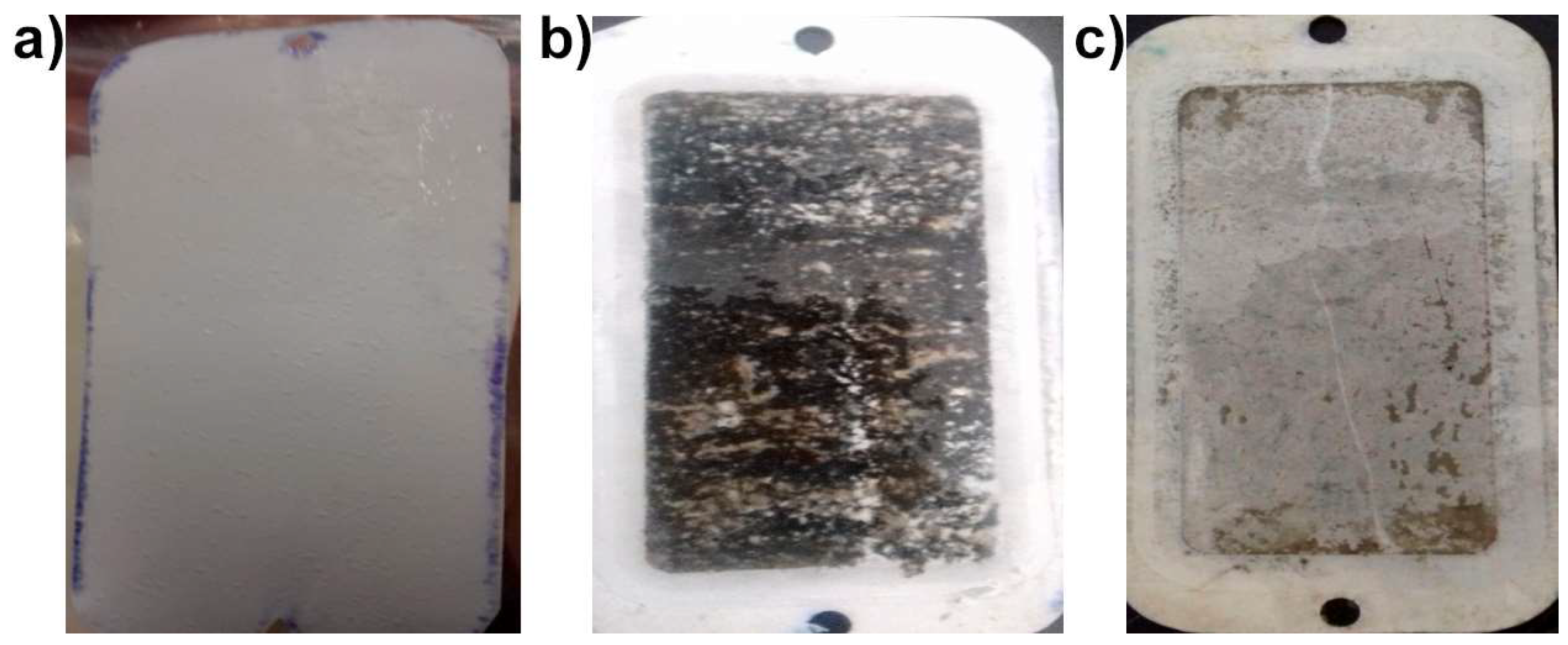
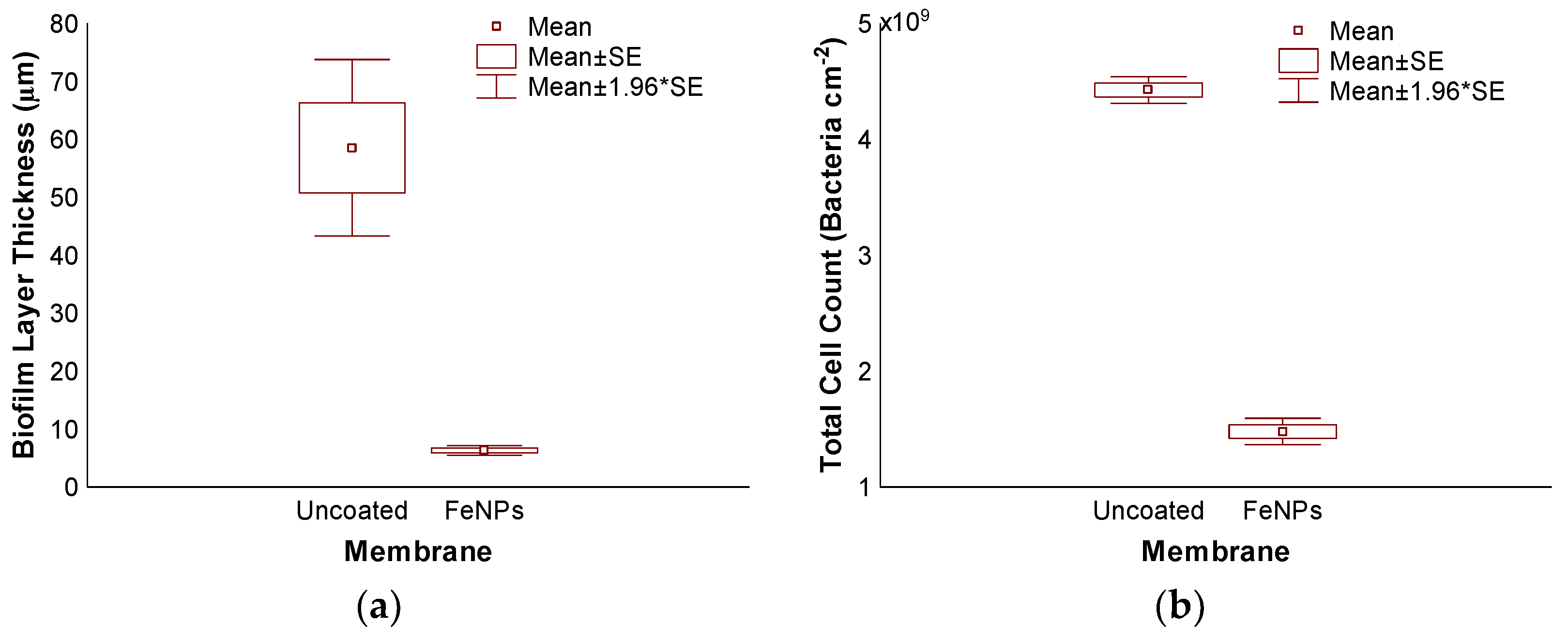
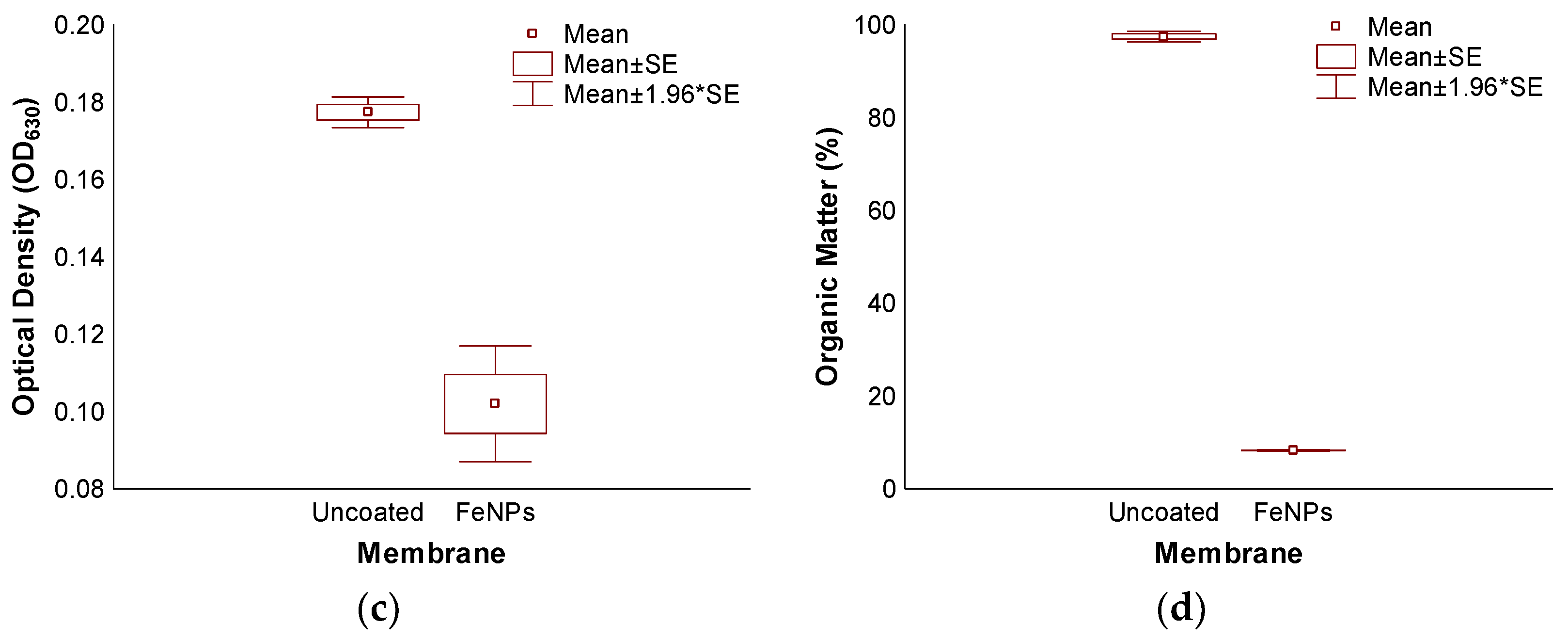
3.3. Accelerated Biofouling Test on RO Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramírez, N.; Sandoval, A.; Serrano, J. Las bacterias halófilas y sus aplicaciones biotecnológicas. Rev. Soc. Venez. Microbiol. 2004, 24, 12–23. [Google Scholar]
- Ayala Borda, P.V.; Vargas Calle, V.A.; Solis Valdivia, J.L. Producción de bacteriocinas por bacterias halófilas y halotelerantes de la laguna Chairkota, Potosí-Bolivia. Rev. Investig. Inform. Salud. 2017, 12, 42–51. [Google Scholar]
- Etesami, H.; Beattie, G. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Indira, D.; Das, B.; Balasubramanian, P.; Jayabalan, R. Sea Water as a reaction medium for bioethanol production. In Microbial Biotechnology; Patra, J.K., Das, G., Shin, H.-S., Eds.; Springer: Singapore, Singapore, 2018; pp. 171–192. [Google Scholar]
- Menasria, T.; Aguilera, M.; Hocine, H.; Benammar, L.; Ayachi, A.; Bachir, A.S.; Dekak, A.; Monteoliva-Sánchez, M. Diversity and bioprospecting of extremely halophilic archaea isolated from Algerian arid and semi-arid wetland ecosystems for halophilic-active hydrolytic enzymes. Microbiol. Res. 2018, 207, 289–298. [Google Scholar] [CrossRef]
- Poli, A.; Finore, I.; Romano, I.; Gioiello, A.; Lama, L.; Nicolaus, B. Microbial diversity in extreme marine habitats and their biomolecules. Microorganisms 2017, 5, 25. [Google Scholar] [CrossRef]
- Lee, J.; Kim, I.S. Microbial community in seawater reverse osmosis and rapid diagnosis of membrane biofouling. Desalination 2011, 273, 118–126. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, Y.; Lang, Y.; Wang, L.; Liu, B.; Zhang, Z. A comparative study on the impact of the carbon nanotubes-modified polydimethylsiloxane nanocomposites on the colonization dynamics of the pioneer biofilm communities. Int. Biodeterior. Biodegrad. 2018, 129, 195–201. [Google Scholar] [CrossRef]
- Veza, J.M.; Ortiz, M.; Sadhwani, J.J.; Gonzalez, J.E.; Santana, F.J. Measurement of biofouling in seawater: some practical tests. Desalination 2008, 220, 326–334. [Google Scholar] [CrossRef]
- Drioli, E.; Criscuoli, A.; Macedonio, F. Membrane-Based Desalination: An Integrated Approach (MEDINA); Iwa Publishing: London, UK, 2011. [Google Scholar]
- Powell, C.L.; Hilal, N.; Wright, C.J. Atomic force microscopy study of the biofouling and mechanical properties of virgin and industrially fouled reverse osmosis membranes. Desalination 2017, 404, 313–321. [Google Scholar] [CrossRef]
- García, A.; Quintero, Y.; Vicencio, N.; Rodríguez, B.; Ozturk, D.; Mosquera, E.; Corrales, T.P.; Volkmann, U.G. Influence of TiO2 nanostructures on anti-adhesion and photoinduced bactericidal properties of thin film composite membranes. RSC Adv. 2016, 6, 82941–82948. [Google Scholar] [CrossRef]
- Pérez-Sicairos, S.; Miranda-Ibarra, S.A.; Lin-Ho, S.W.; Álvarez-Sánchez, J.; Pérez-Reyes, J.C.; Corrales-López, K.A.; Morales-Cuevas, J.B. Membranas de nanofiltración, preparadas viá polimerización en interfase, dopadas con nanopartículas de ZnO: Efecto en su desempeño. Rev. Mex. Ing. Quim. 2016, 15, 961–975. [Google Scholar]
- Lee, S.Y.; Kim, H.J.; Patel, R.; Im, S.J.; Kim, J.H.; Min, B.R. Silver nanoparticles immobilized on thin film composite polyamide membrane: characterization, nanofiltration, antifouling properties. Polym. Adv. Technol. 2007, 18, 562–568. [Google Scholar] [CrossRef]
- García, A.; Rodríguez, B.; Oztürk, D.; Rosales, M.; Diaz, D.I.; Mautner, A. Incorporation of CuO nanoparticles into thin-film composite reverse osmosis membranes (TFC-RO) for antibiofouling properties. Polym. Bull. 2017, 75, 2053–2069. [Google Scholar] [CrossRef]
- Lang, Y.; Sun, Y.; Yu, M.; Ji, Y.; Wang, L.; Zhang, Z. Differential colonization dynamics of marine biofilm-forming eukaryotic microbes on different protective coating materials. Polymers 2019, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhuang, W.; Wang, X.; Yu, K.; Yang, S.; Xia, S. Potential effects of loading nano zero valent iron discharged on membrane fouling in an anoxic/oxic membrane bioreactor. Water Res. 2017, 111, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Armendáriz-Ontiveros, M.M.; García, A.G.; De Los Santos Villalobos, S.; Weihs, G.F. Biofouling performance of RO membranes coated with Iron NPs on graphene oxide. Desalination 2019, 451, 45–58. [Google Scholar] [CrossRef]
- Armendariz Ontiveros, M.; Quintero, Y.; Llanquilef, A.; Morel, M.; Argentel Martínez, L.; García García, A.; Garcia, A. Anti-biofouling and desalination properties of thin film composite reverse osmosis membranes modified with copper and iron nanoparticles. Materials 2019, 12, 2081. [Google Scholar] [CrossRef] [PubMed]
- Ayache, C.; Manes, C.; Pidou, M.; Croue, J.P.; Gernjak, W. Microbial community analysis of fouled reverse osmosis membranes used in water recycling. Water Res. 2013, 47, 3291–3299. [Google Scholar] [CrossRef]
- Belila, A.; El-Chakhtoura, J.; Otaibi, N.; Muyzer, G.; Gonzalez-Gil, G.; Saikaly, P.; Van Loosdrecht, M.; Vrouwenvelder, J.; Saikaly, P.; Vrouwenvelder, H. Bacterial community structure and variation in a full-scale seawater desalination plant for drinking water production. Water Res. 2016, 94, 62–72. [Google Scholar] [CrossRef] [Green Version]
- GWI. Desalting Plant Inventory. In DesalData; Global Water Intelligence: Oxford, UK, 2015. [Google Scholar]
- CONAGUA. Taller del Proyecto de la Planta Desaladora para las Ciudades de Guaymas y Empalme, Estado de Sonora; CONAGUA: Mexico City, Mexico, 2017; pp. 1–71. [Google Scholar]
- Center, A.W.R.R. Field Manual for Water Quality Sampling; Arizona Water Resources research Center: Tucson, AZ, USA, 1995; pp. 6–37. [Google Scholar]
- Al-Ghamdi, M.; Ghaffour, N. Development of a Novel UF Membrane Cleaning Method Using Carbon Dioxide; IDA World Congress: Sao Paulo, Brazil, 2017. [Google Scholar]
- Ben-David, A.; Davidson, C.E. Estimation method for serial dilution experiments. J. Microbiol. Methods 2014, 107, 214–221. [Google Scholar] [CrossRef] [Green Version]
- La Cono, V.; Smedile, F.; La Spada, G.; Arcadi, E.; Genovese, M.; Ruggeri, G.; Genovese, L.; Giuliano, L.; Yakimov, M.M. Shifts in the meso- and bathypelagic archaea communities composition during recovery and short-term handling of decompressed deep-sea samples. Environ. Microbiol. Rep. 2015, 7, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, A.B.; Fulton, M.D. A Simplified method of staining endospores. Science 1933, 77, 194. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, S.; Parra Cota, F.I.; Herrera Sepúlveda, A.; Valenzuela Aragón, B.; Estrada Mora, J.C. Colmena: colección de microorganismos edáficos y endófitos nativos, para contribuir a la seguridad alimentaria nacional. Rev. Mex. Cienc. Agric. 2018, 9, 191–202. [Google Scholar]
- Raeder, U.; Broda, P. Rapid preparation of DNA from filamentous fungi. Lett. Appl. Microbiol. 1985, 1, 17–20. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; A Pelletier, D.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa-Rodríguez, E.; Parra-Cota, F.; Castro-Longoria, E.; López-Cervantes, J.; Santos-Villalobos, S.D.L. Bacillus subtilis TE3: A promising biological control agent against bipolaris sorokiniana, the causal agent of spot blotch in wheat (Triticum turgidum L. subsp. durum). Boil. Control. 2019, 132, 135–143. [Google Scholar] [CrossRef]
- Baltazar, S.E.; García, A.; Romero, A.H.; Rubio, M.A.; Arancibia-Miranda, N.; Altbir, D. Surface rearrangement of nanoscale zerovalent iron: the role of pH and its implications in the kinetics of arsenate sorption. Environ. Technol. 2014, 35, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Arancibia-Miranda, N.; Baltazar, S.E.; García, A.; Muñoz-Lira, D.; Sepúlveda, P.; Rubio, M.A.; Altbir, D. Nanoscale zero valent supported by Zeolite and Montmorillonite: Template effect of the removal of lead ion from an aqueous solution. J. Hazard. Mater. 2016, 301, 371–380. [Google Scholar] [CrossRef]
- Carrera, M.; Zandomeni, R.; FitzGibbon, J.; Sagripanti, J.-L. Difference between the spore sizes of Bacillus anthracis and other Bacillus species. J. Appl. Microbiol. 2007, 102, 303–312. [Google Scholar] [CrossRef]
- Guo, W.; Cui, P.; Chen, X. Complete genome of Bacillus sp. Pc3 isolated from the Antarctic seawater with antimicrobial activity. Mar. Genom. 2015, 20, 1–2. [Google Scholar] [CrossRef]
- Angert, E.R. Alternatives to binary fission in bacteria. Nat. Rev. Genet. 2005, 3, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Nogi, Y.; Soda, K.; Oikawa, T. Flavobacterium frigidimaris sp. nov., isolated from Antarctic seawater. Syst. Appl. Microbiol. 2005, 28, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Siefert, J.L.; Larios-Sanz, M.; Nakamura, L.K.; Slepecky, R.A.; Paul, J.H.; Moore, E.R.B.; Fox, G.E.; Jurtshuk, J.P. Phylogeny of Marine Bacillus Isolates from the Gulf of Mexico. Curr. Microbiol. 2000, 41, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Methods 2013, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Arancibia-Miranda, N.; Baltazar, S.E.; García, A.; Romero, A.H.; Rubio, M.A.; Altbir, D. Lead removal by nano-scale zero valent iron: Surface analysis and pH effect. Mater. Res. Bull. 2014, 59, 341–348. [Google Scholar] [CrossRef]
- Samad, N.S.A.; Amid, A.; Jimat, D.N.; Shukor, A.N.A. Isolation and identification of halophilic bacteria producing halotolerant protease. Sci. Herit. J. 2017, 1, 7–9. [Google Scholar] [CrossRef]
- Yadav, A.N.; Verma, P.; Kumar, M.; Pal, K.K.; Dey, R.; Gupta, A.; Padaria, J.C.; Gujar, G.T.; Kumar, S.; Suman, A.; et al. Diversity and phylogenetic profiling of niche-specific Bacilli from extreme environments of India. Ann. Microbiol. 2015, 65, 611–629. [Google Scholar] [CrossRef]
- Dinali, R.; Ebrahiminezhad, A.; Manley-Harris, M.; Ghasemi, Y.; Berenjian, A. Iron oxide nanoparticles in modern microbiology and biotechnology. Crit. Rev. Microbiol. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Kharangate-Lad, A.; Bhosle, S. Effect of zerovalent iron (Zvi) nanoparticles on siderophores produced by halophilic and halotolerant adhered bacteria from the mangrove ecosystem. BAOJ Chem. 2017, 3, 24. [Google Scholar]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.J.; Wang, J.K.; Ferguson, S.; Wang, T.; Bao, Y.; Hao, H.X. Mechanism, synthesis and modification of nano zerovalent iron in water treatment. Nanoscale 2016, 8, 9962–9975. [Google Scholar] [CrossRef] [PubMed]

| Temperature (°C) | Electrical Conductivity (µS cm−1) | Total Dissolved Solids (mg L−1) | Salt Fraction (%) | Dissolved Oxygen (mg L−1) | pH |
|---|---|---|---|---|---|
| 24.1 ± 0.3 | 48,100 ± 350 | 34.99 ± 0.1 | 3.56 ± 0.0 | 8.42 ± 0.1 | 8.1 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armendáriz-Ontiveros, M.M.; Fimbres Weihs, G.A.; de los Santos Villalobos, S.; Salinas-Rodriguez, S.G. Biofouling of FeNP-Coated SWRO Membranes with Bacteria Isolated after Pre-Treatment in the Sea of Cortez. Coatings 2019, 9, 462. https://doi.org/10.3390/coatings9070462
Armendáriz-Ontiveros MM, Fimbres Weihs GA, de los Santos Villalobos S, Salinas-Rodriguez SG. Biofouling of FeNP-Coated SWRO Membranes with Bacteria Isolated after Pre-Treatment in the Sea of Cortez. Coatings. 2019; 9(7):462. https://doi.org/10.3390/coatings9070462
Chicago/Turabian StyleArmendáriz-Ontiveros, Maria Magdalena, Gustavo A. Fimbres Weihs, Sergio de los Santos Villalobos, and Sergio G. Salinas-Rodriguez. 2019. "Biofouling of FeNP-Coated SWRO Membranes with Bacteria Isolated after Pre-Treatment in the Sea of Cortez" Coatings 9, no. 7: 462. https://doi.org/10.3390/coatings9070462
APA StyleArmendáriz-Ontiveros, M. M., Fimbres Weihs, G. A., de los Santos Villalobos, S., & Salinas-Rodriguez, S. G. (2019). Biofouling of FeNP-Coated SWRO Membranes with Bacteria Isolated after Pre-Treatment in the Sea of Cortez. Coatings, 9(7), 462. https://doi.org/10.3390/coatings9070462







