Effect of High-Temperature Calcined Wheat Straw Powder after Lignin Removal on Properties of Waterborne Wood Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Preparation of Coatings
2.3. Performance Test
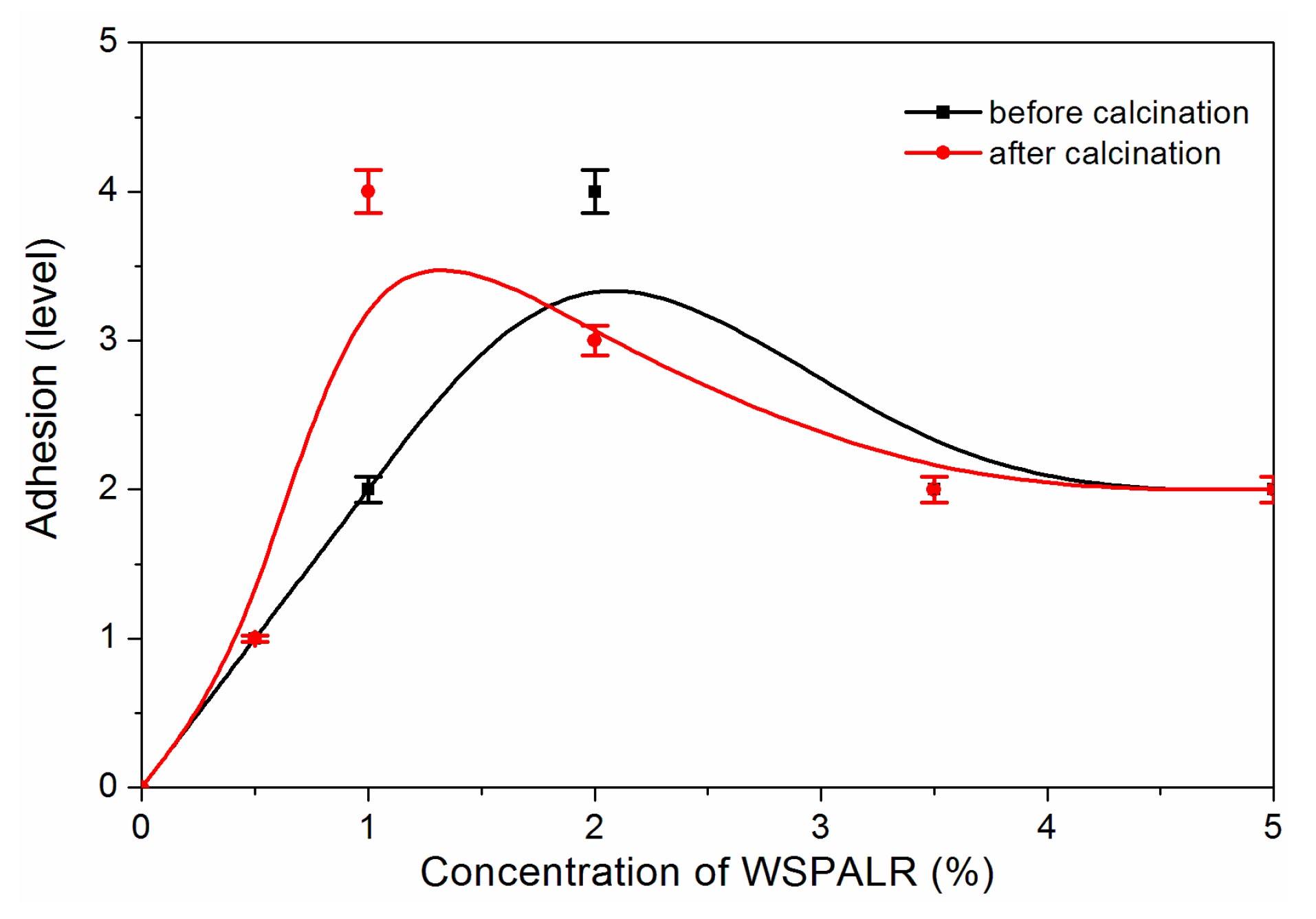
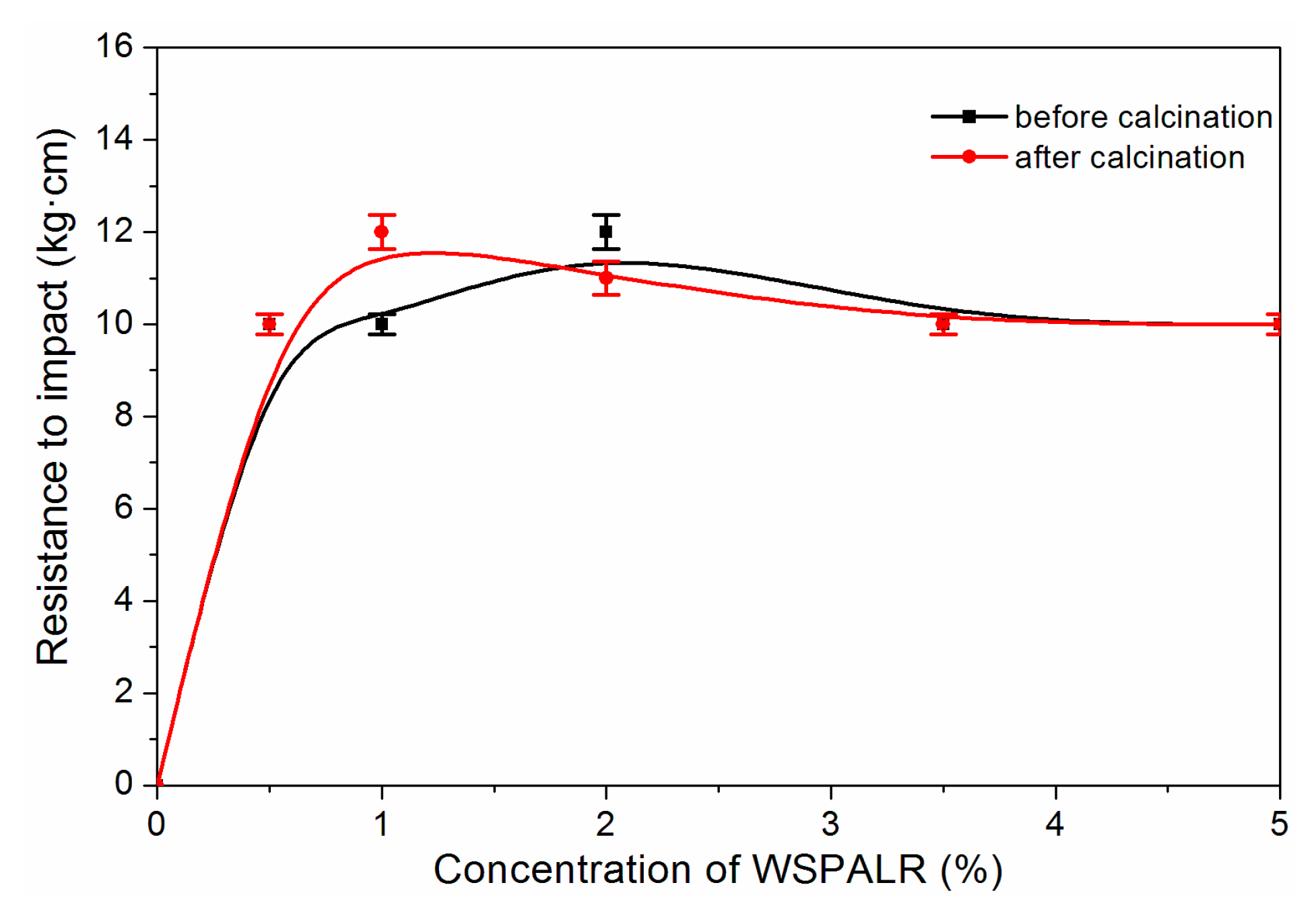
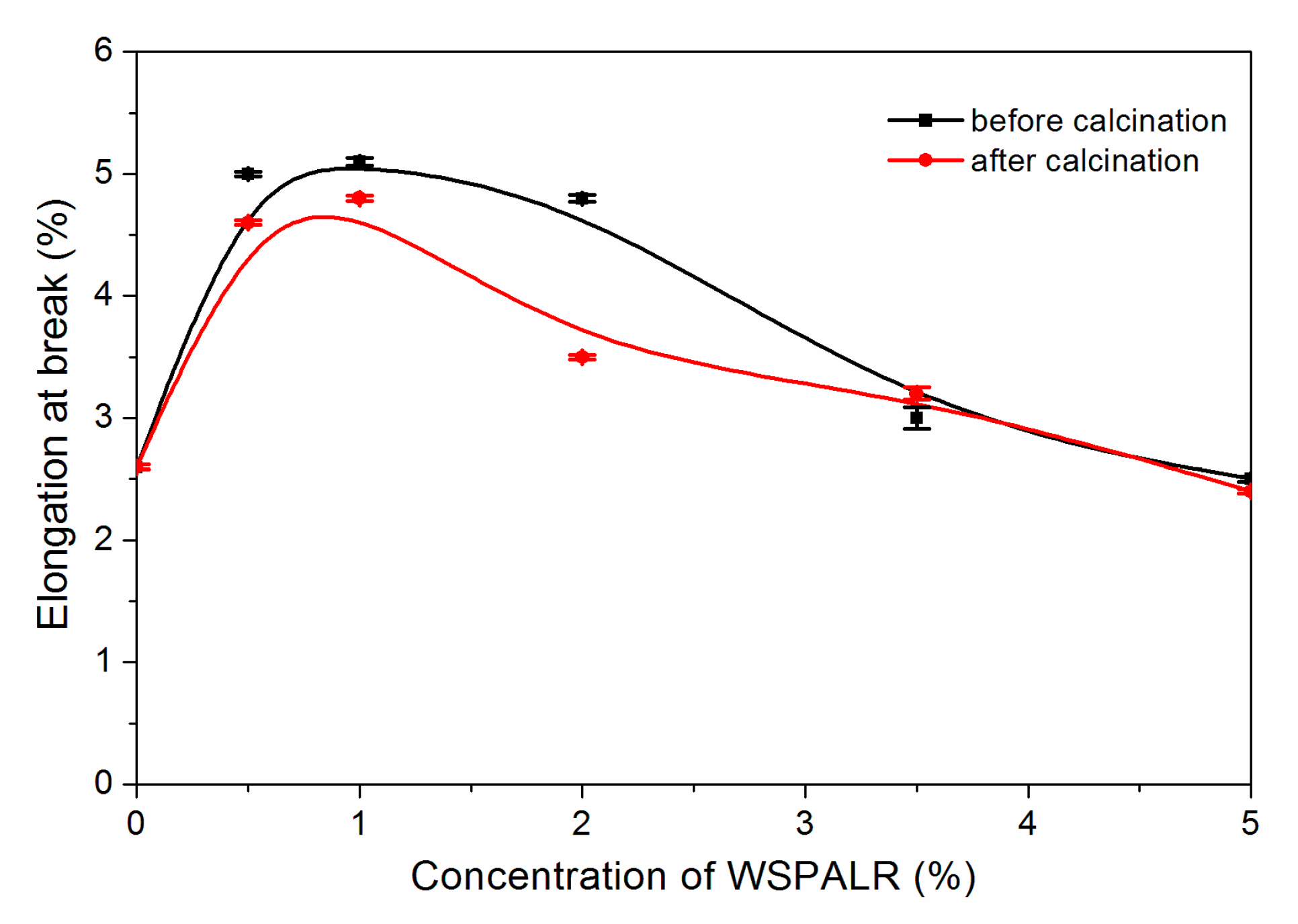
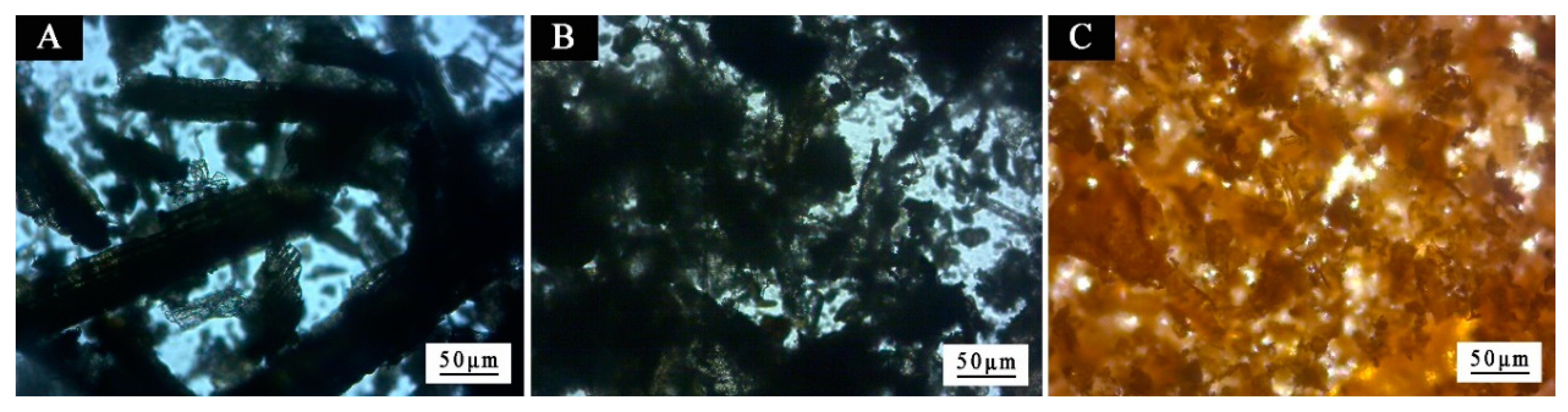
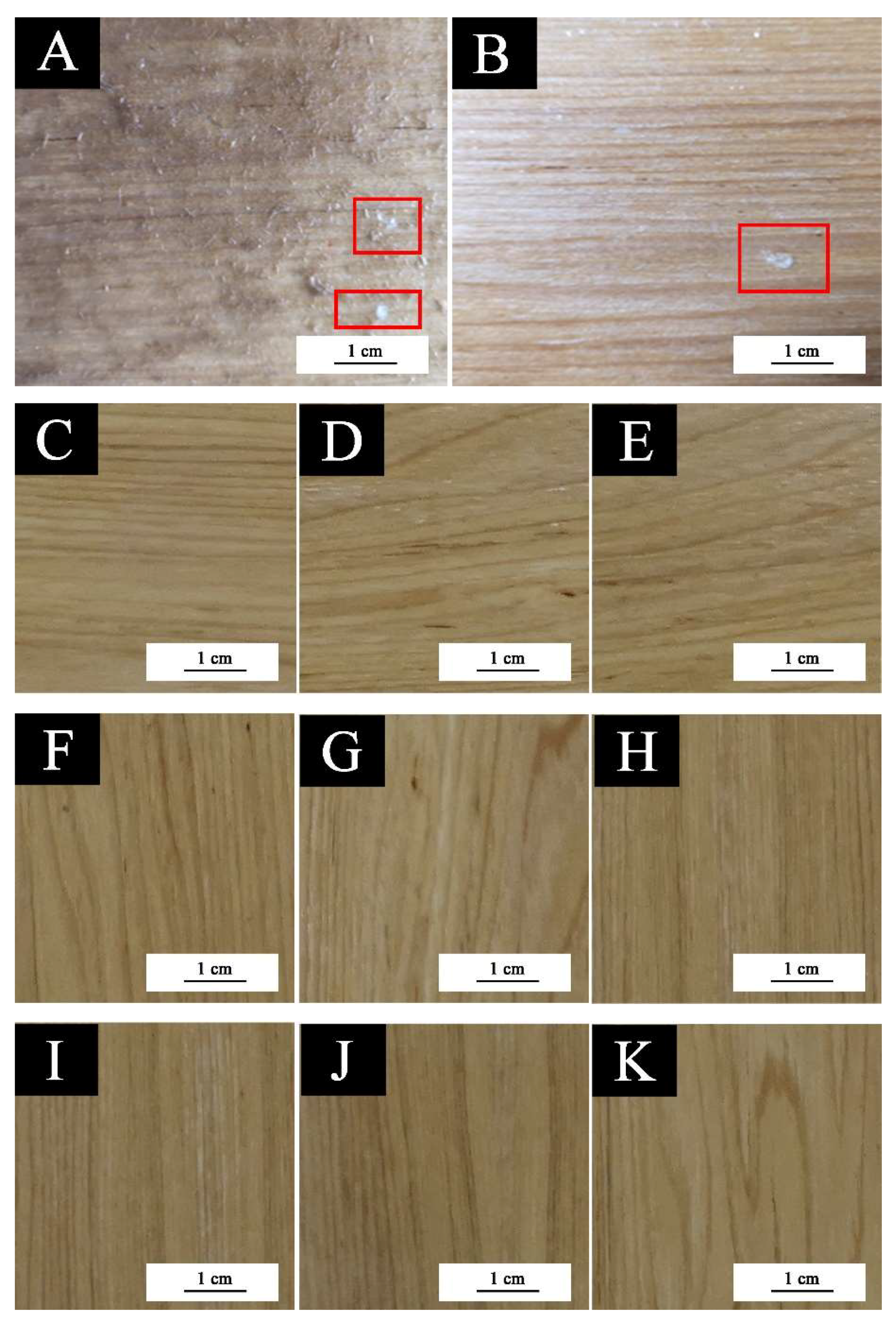
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Omel’chenko, S.I.; Tryhub, S.O.; Laskovenko, N.M. Prospects of investigation and production of waterborne paintwork materials. Mater. Sci. 2001, 37, 790–801. [Google Scholar] [CrossRef]
- Noreen, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Saif, M.J. Recent trends in environmentally friendly water-borne polyurethane coatings: A review. Korean J. Chem. Eng. 2016, 33, 388–400. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Y.; Zhang, T.; Dai, Y.; Zhang, D.; Qiu, F.; Yu, Z.; Yang, P. Synthesis of UV-curing waterborne polyurethane-acrylate coating and its photopolymerization kinetics using FT-IR and photo-DSC methods. Prog. Org. Coat. 2018, 122, 10–18. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; He, Y.; Tong, Z.; Shen, Z.; Li, X.; Li, B. Corrosion protection of galvanized sheet by maleic anhydride-g-liquid polybutadiene environmental friendly coatings. Prog. Org. Coat. 2008, 63, 195–200. [Google Scholar] [CrossRef]
- Xu, J.; Qiu, F.; Rong, X.; Dai, Y.; Yang, D. Preparation and surface pigment protection application of stone substrate on UV-curable waterborne polyurethane-acrylate coating. J. Polym. Mater. 2014, 31, 287–303. [Google Scholar]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Yang, D.; Wan, H.; Wang, X.; Liu, Z. Use of fungal metabolites to protect wood-based panels against mold infection. Biocontrol 2007, 52, 427–436. [Google Scholar] [CrossRef]
- Xu, W.; Wu, Z.; Zhang, J. Compressive creep and recovery behaviors of seat cushions in upholstered furniture. Wood Fiber Sci. 2015, 47, 431–444. [Google Scholar]
- Williams, H. The EU SERVOWOOD project-predicting the service life of exterior wood coatings. Surf. Coat. Int. 2015, 98, 271–273. [Google Scholar]
- Gu, Y.; Wu, Z.; Zhang, J. Load-deflection behavior of rattan chair seats. Wood Fiber Sci. 2016, 48, 13–24. [Google Scholar]
- Xu, H.; Qiu, F.; Wang, Y.; Yang, D.; Wu, W.; Chen, Z.; Zhu, J. Preparation, mechanical properties of waterborne polyurethane and crosslinked polyurethane-acrylate composite. J. Appl. Polym. Sci. 2012, 124, 958–968. [Google Scholar] [CrossRef]
- Lu, R.; Wan, Y.; Honda, T.; Ishimura, T.; Kamiya, Y.; Miyakoshi, T. Design and characterization of modified urethane lacquer coating. Prog. Org. Coat. 2006, 57, 215–222. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Sun, X. Epoxidized and acrylated epoxidized camelina oils for ultraviolet-curable wood coatings. J. Am. Oil Chem. Soc. 2018, 95, 1307–1318. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, S.; Qian, J. Synthesis and properties of a novel UV-curable waterborne hyperbranched polyurethane. J. Coat. Technol. Res. 2014, 11, 319–328. [Google Scholar] [CrossRef]
- Balakrishnan, T.; Sathiyanarayanan, S.; Mayavan, S. Advanced anticorrosion coating materials derived from sunflower oil with bifunctional properties. ACS Appl. Mater. Interfaces 2015, 7, 19781–19788. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Li, B.; Hu, T.; Yang, Y.; Li, L.; Zhang, J. Environmentally benign and durable superhydrophobic coatings based on SiO2 nanoparticles and silanes. J. Colloid. Interface Sci. 2019, 542, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zhao, W.; Wu, Y.; Dong, J.; Zhou, K.; Lu, G.; Pu, J. Anti-corrosion behaviors of epoxy composite coatings enhanced via graphene oxide with different aspect ratios. Prog. Org. Coat. 2019, 127, 70–79. [Google Scholar] [CrossRef]
- Kozlova, A.A.; Kondrashov, E.K.; Deev, I.S. Protective properties of paint and lacquer coatings based on a fluorine-containing film-forming material. Prot. Met. Phys. Chem. 2016, 52, 1181–1186. [Google Scholar] [CrossRef]
- Verma, G.; Dhoke, S.K.; Khanna, A.S. Polyester based-siloxane modified waterborne anticorrosive hydrophobic coating on copper. Surf. Coat. Technol. 2012, 212, 101–108. [Google Scholar] [CrossRef]
- Chen, C.; He, Y.; Xiao, G.; Zhong, F.; Li, H.; Wu, Y.; Chen, J. Synergistic effect of graphene oxide@phosphate-intercalated hydrotalcite for improved anti-corrosion and self-healable protection of waterborne epoxy coating in salt environments. J. Mater. Chem. C 2019, 7, 2318–2326. [Google Scholar] [CrossRef]
- Xiong, X.; Bao, Y.; Guo, W.; Fang, L.; Wu, Z. Preparation and application of high performance corn starch glue in straw decorative panel. Wood Fiber Sci. 2018, 50, 88–95. [Google Scholar]
- Podgorski, L.; Reynaud, C.; Montibus, M. Fungal growth on coated wood exposed outdoors: Influence of coating pigmentation, cardinal direction, and inclination of wood surfaces. Coatings 2019, 9, 27. [Google Scholar] [CrossRef]
- Hubmann, M.; Kong, X.H.; Curtis, J.M. Kinetic stabilization of cellulose nanocrystals in a photocurable prepolymer for application as an adhesion promoter in UV-curable coatings. Prog. Org. Coat. 2019, 129, 101–115. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, X.; Wu, X.; Wang, S.; Lu, C. Cellulose nanocrystals mediated assembly of graphene in rubber composites for chemical sensing applications. Carbohydr. Polym. 2016, 140, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Auclair, N.; Kaboorani, A.; Riedl, B.; Landry, V.; Hosseinaei, O.; Wang, S. Influence of modified cellulose nanocrystals (CNC) on performance of bionanocomposite coatings. Prog. Org. Coat. 2018, 123, 27–34. [Google Scholar] [CrossRef]
- Hasan, M.; Karal, M.A.; Levadnyy, V.; Yamazaki, M. Mechanism of initial stage of pore formation induced by antimicrobial peptide magainin 2. Langmuir 2018, 34, 3349–3362. [Google Scholar] [CrossRef]
- GB/T 1723-1993 Viscosimetry of Coatings; Standardization Administration of the People’s Republic of China: Beijing, China, 1993.
- Rosello, J.; Soriano, L.; Santamarina, M.P.; Akasaki, J.L.; Monzo, J.; Paya, J. Rice straw ash: A potential pozzolanic supplementary material for cementing systems. Ind. Crop. Prod. 2017, 103, 39–50. [Google Scholar] [CrossRef]
- GB/T 1732-93 Determination of Impact Resistance of Film; Standardization Administration of the People’s Republic of China: Beijing, China, 1993; pp. 418–420.
- GB/T 1720-89 Determination of Adhesion of Film; Standardization Administration of the People’s Republic of China: Beijing, China, 1979.
- GB/T 1741-2007 Test Method for Determining the Resistance of Paints Film to Mold; Standardization Administration of the People’s Republic of China: Beijing, China, 2007.
- Tatzber, M.; Stemmer, M.; Splegel, H.; Katziberger, C.; Haberhauer, G.; Mentler, A.; Gerzabek, M.H. FTIR-spectroscopic characterization of humic acids and humin fractions obtained by advanced NaOH, Na4P2O7, and Na2CO3 extraction procedures. J. Plant Nutr. Soil Sci. 2007, 170, 522–529. [Google Scholar] [CrossRef]
- Horgnies, M.; Willieme, P.; Gabet, O. Influence of the surface properties of concrete on the adhesion of coating: Characterization of the interface by peel test and FT-IR spectroscopy. Prog. Org. Coat. 2011, 72, 360–379. [Google Scholar] [CrossRef]
- Yang, J.; Deng, J.; Zhu, J.; Liu, W.; Zhou, M.; Li, D. Thermal polymerization of lacquer sap and its effects on the properties of lacquer film. Prog. Org. Coat. 2016, 94, 41–48. [Google Scholar] [CrossRef]
- Zhao, S.; Chang, H.; Chen, S.; Cui, J.; Yan, Y. High-performance and multifunctional epoxy composites filled with epoxide-functionalized graphene. Eur. Polym. J. 2016, 84, 300–312. [Google Scholar] [CrossRef]
- Avram, D.; Angelescu, N.; Ungureanu, D.N.; Ionita, I.; Bancuta, I.; Gheboianu, A.; Lungulescu, E.M. Study of phosphocalcic glasses from SiO2–CaO–P2O5 system with and without silver II. The bioactivity analysis by FTIR, SEM methods and microbiological study of silver-doped glasses. Revista De Chimie 2017, 68, 1188–1192. [Google Scholar]
- Yan, X.; Qian, X.; Lu, R.; Miyakoshi, T. Synergistic effect of addition of fillers on properties of interior waterborne UV-curing wood coatings. Coatings 2018, 8, 9. [Google Scholar] [CrossRef]
- Sarasini, F.; Tirillò, J.; Ferrante, L.; Sergi, C.; Sbardella, F.; Russo, P.; Simeoli, G.; Mellier, D.; Calzolari, A. Effect of temperature and fiber type on impact behavior of thermoplastic fiber metal laminates. Compos. Struct. 2019, 223, 110961. [Google Scholar] [CrossRef]
- Zarges, J.C.; Minkley, D.; Feldmann, M.; Heim, H.P. Fracture toughness of injection molded, man-made cellulose fiber reinforced polypropylene. Compos. Part A Appl. Sci. 2017, 98, 147–158. [Google Scholar] [CrossRef]
- Igarza, E.; Pardo, S.G.; Abad, M.J.; Cano, J.; Galante, M.J.; Pettarin, V.; Bernal, C. Structure-fracture properties relationship for polypropylene reinforced with fly ash with and without maleic anhydride functionalized isotactic polypropylene as coupling agent. Mater. Des. 2014, 55, 85–92. [Google Scholar] [CrossRef]
- GB/T 11186.3-1989 Method of Measurement of Coating Color. Part III: Calculation of Chromatic Aberration; Standardization Administration of the People’s Republic of China: Beijing, China, 1990.
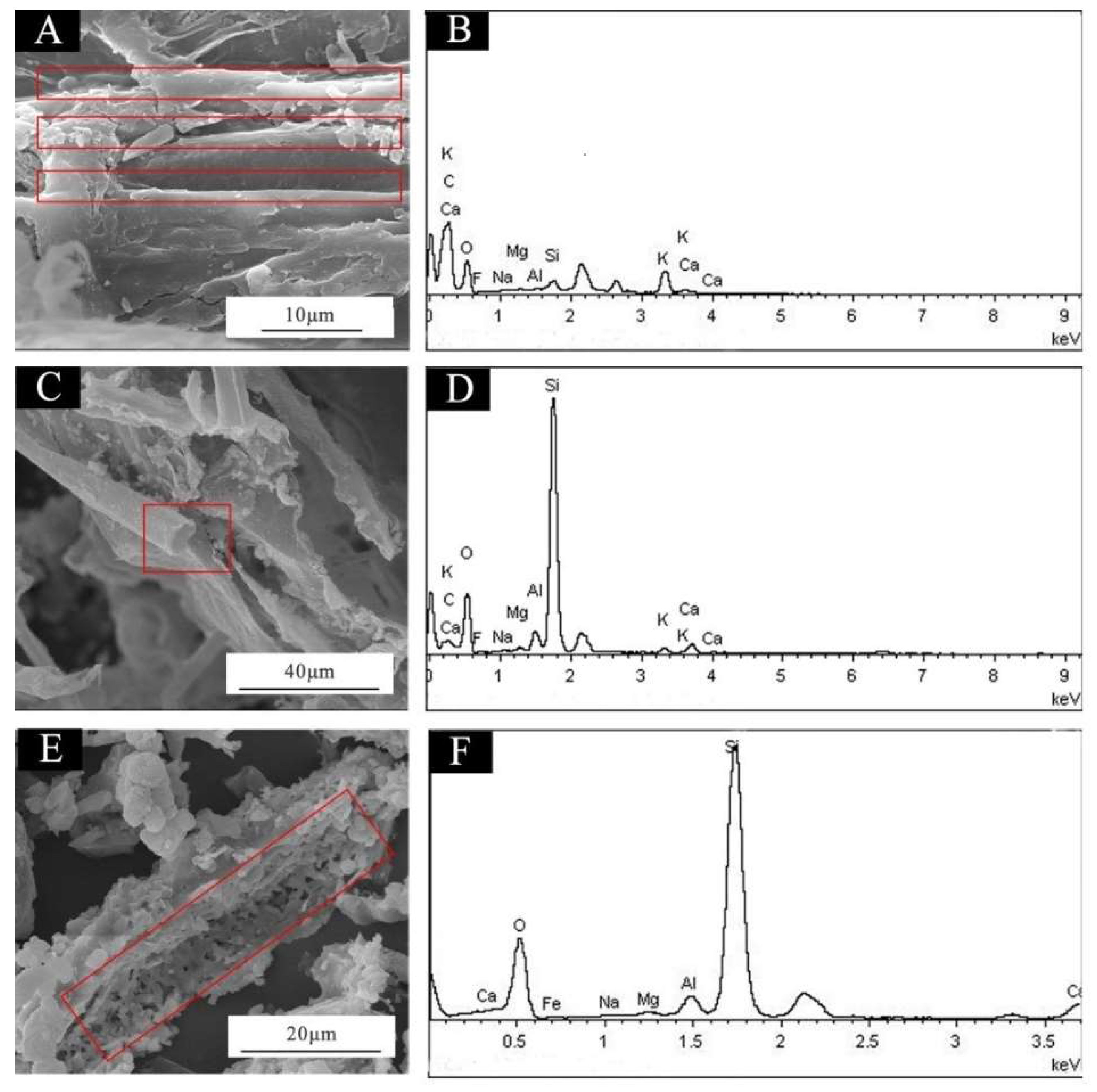
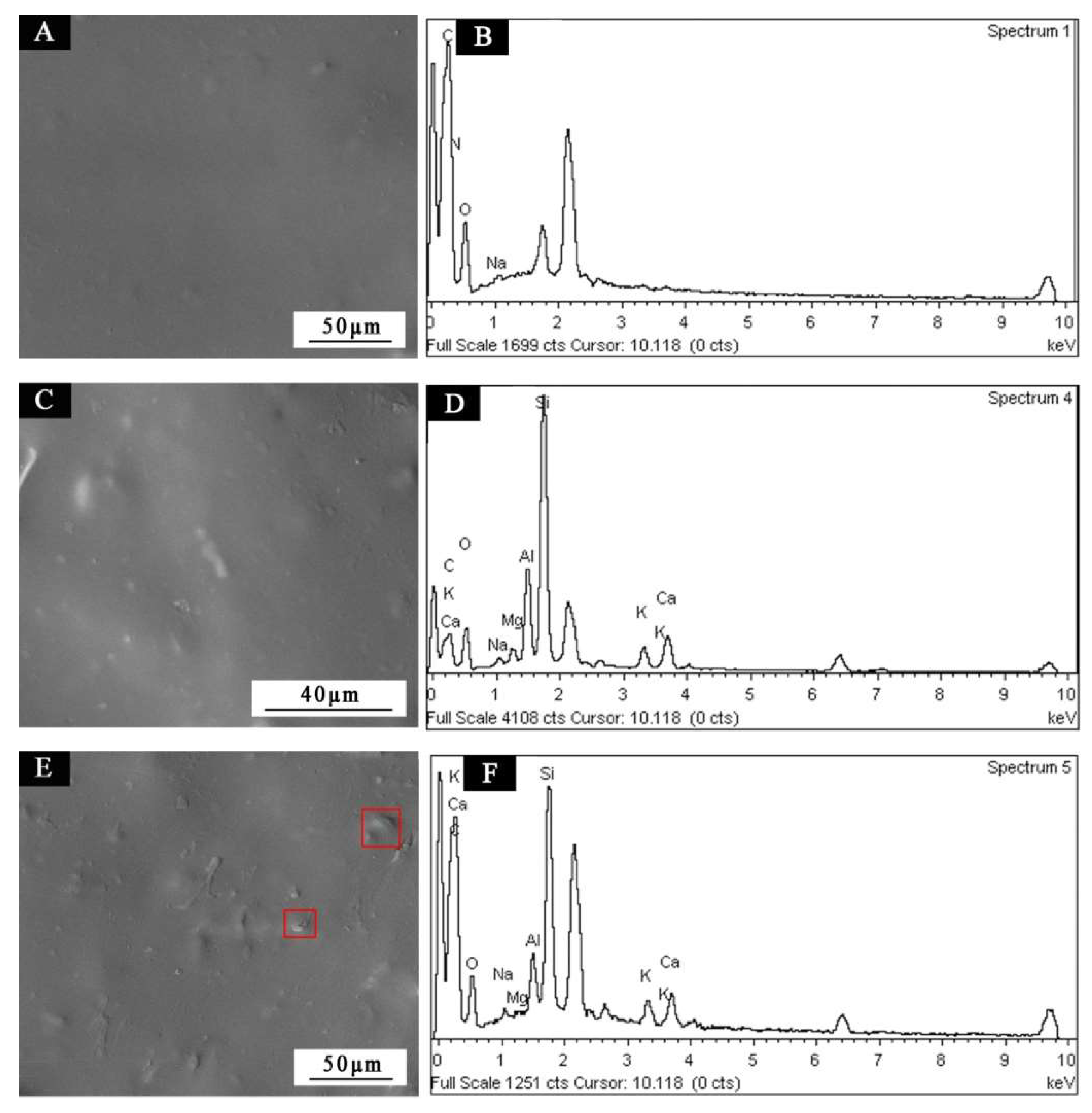


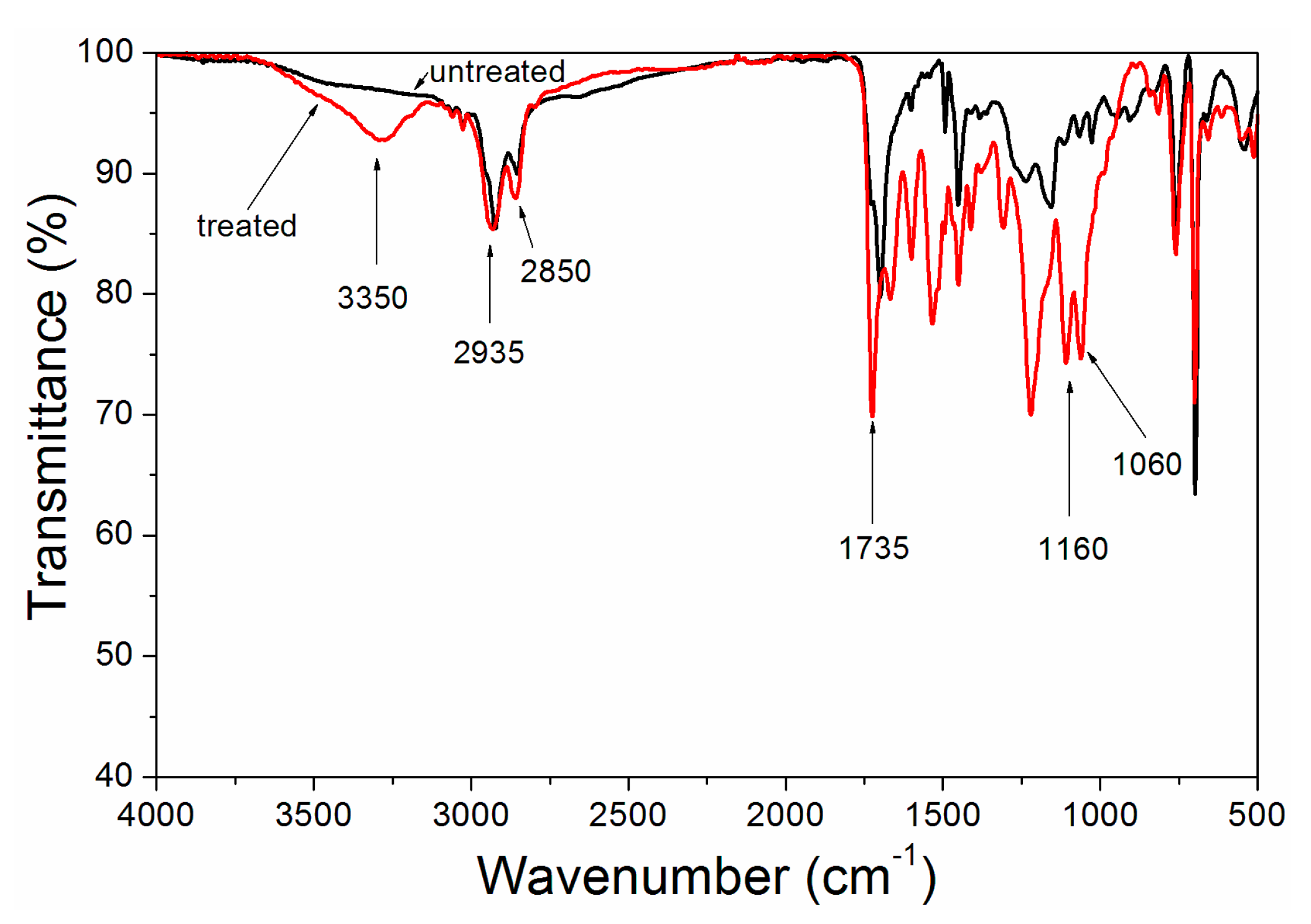
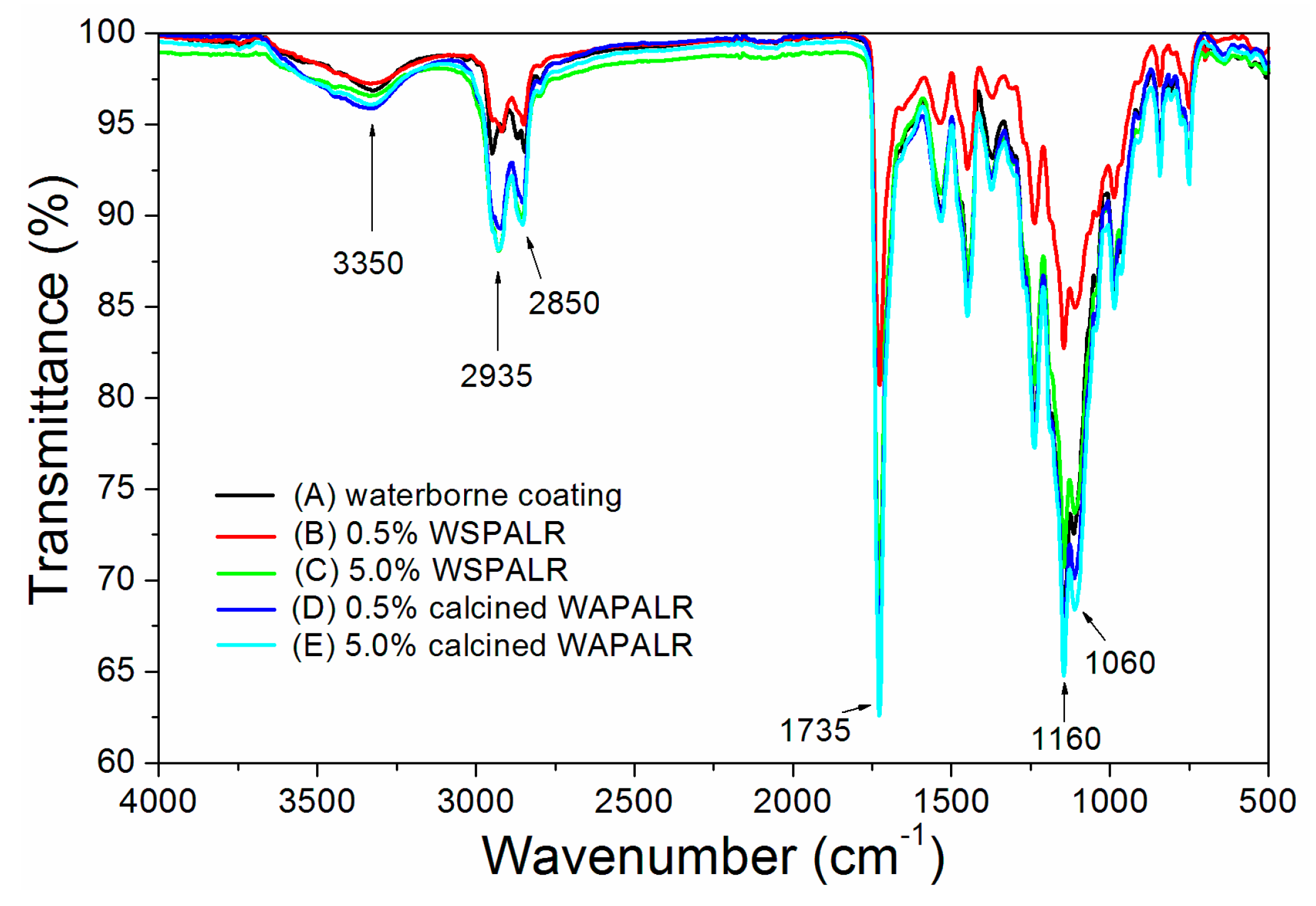

| Sample | Concentration (%) | WSPALR (g) | Calcined WSPALR (g) | Waterborne Wood Coating (g) |
|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 100.0 |
| 2 | 0.5 | 0.5 | 0 | 99.5 |
| 3 | 1.0 | 1.0 | 0 | 99.0 |
| 4 | 2.0 | 2.0 | 0 | 98.0 |
| 5 | 3.5 | 3.5 | 0 | 96.5 |
| 6 | 5.0 | 5.0 | 0 | 95.0 |
| 7 | 0.5 | 0 | 0.5 | 99.5 |
| 8 | 1.0 | 0 | 1.0 | 99.0 |
| 9 | 2.0 | 0 | 2.0 | 98.0 |
| 10 | 3.5 | 0 | 3.5 | 96.5 |
| 11 | 5.0 | 0 | 5.0 | 95.0 |
| Sample | m2 (g) | m1 (g) | Sicalcined (%) | Sicoating (%) |
|---|---|---|---|---|
| 1 | 0 | 0.79 ± 0 | 0 | 0 |
| 2 | 0.01 ± 0 | 0.80 ± 0 | 38.00 ± 0 | 0.46 ± 0.02 |
| 3 | 0.02 ± 0 | 0.82 ± 0 | 38.00 ± 0.01 | 0.91 ± 0.02 |
| 4 | 0.04 ± 0 | 0.84 ± 0.01 | 38.00 ± 0.01 | 1.75 ± 0.04 |
| 5 | 0.07 ± 0 | 0.87 ± 0.01 | 38.00 ± 0.01 | 2.96 ± 0.27 |
| 6 | 0.10 ± 0 | 0.91 ± 0.01 | 38.00 ± 0.01 | 4.06 ± 0.21 |
| 7 | 0.02 ± 0 | 0.81 ± 0 | 38.00 ± 0.02 | 0.71 ± 0.02 |
| 8 | 0.03 ± 0 | 0.82 ± 0 | 38.00 ± 0.01 | 1.39 ± 0.04 |
| 9 | 0.06 ± 0 | 0.84 ± 0 | 38.00 ± 0 | 2.71 ± 0.09 |
| 10 | 0.11 ± 0 | 0.87 ± 0 | 38.00 ± 0.01 | 4.57 ± 0.24 |
| 11 | 0.15 ± 0 | 0.91 ± 0.03 | 38.00 ± 0.01 | 6.29 ± 0.21 |
| Concentration of WSPALR (%) | L | a* | b* | L’ | a*’ | b*’ | ΔL | Δa* | Δb* | ΔE |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 59.80 ± 0 | 13.00 ± 0.02 | 21.20 ± 0.02 | 59.30 ± 0.01 | 11.80 ± 0.01 | 21.20 ± 0.22 | 0.50 ± 0.01 | 1.20 ± 0.02 | 0 ± 0.21 | 1.30 ± 0.05 |
| 0.5 | 51.40 ± 0 | 13.20 ± 0.08 | 29.80 ± 0.02 | 51.20 ± 0.02 | 12.60 ± 0.03 | 30.60 ± 0 | 0.20 ± 0.02 | 0.60 ± 0.10 | −0.80 ± 0.02 | 1.00 ± 0.05 |
| 1.0 | 52.30 ± 0.04 | 13.20 ± 0 | 24.50 ± 0 | 51.30 ± 0 | 14.30 ± 0.06 | 23.10 ± 0 | 1.00 ± 0.04 | −1.10 ± 0.06 | 1.40 ± 0 | 2.0 ± 0.05 |
| 2.0 | 52.30 ± 0.02 | 14.60 ± 0 | 25.00 ± 0.02 | 52.30 ± 0 | 15.90 ± 0.03 | 25.20 ± 0 | 0 ± 0.02 | −1.30 ± 0.03 | −0.20 ± 0.02 | 1.3 ± 0.05 |
| 3.5 | 50.20 ± 0 | 13.10 ± 0.02 | 29.40 ± 0.02 | 49.80 ± 0.02 | 11.80 ± 0.03 | 29.60 ± 0 | 0.40 ± 0.02 | 1.30 ± 0.05 | −0.20 ± 0.02 | 1.4 ± 0.05 |
| 5.0 | 47.10 ± 0 | 20.00 ± 0.02 | 35.50 ± 0.05 | 47.70 ± 0.02 | 23.70 ± 0.03 | 43.00 ± 0.08 | −0.60 ± 0.02 | −3.70 ± 0.05 | −7.50 ± 0.04 | 8.4 ± 0.05 |
| Concentration of Calcined WSPALR (%) | L | a* | b* | L’ | a*’ | b*’ | ΔL | Δa* | Δb* | ΔE |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 59.80 ± 0 | 13.00 ± 0.02 | 21.20 ± 0.02 | 59.30 ± 0.01 | 11.80 ± 0.01 | 21.20 ± 0.22 | 0.50 ± 0.01 | 1.20 ± 0.02 | 0 ± 0.21 | 1.30 ± 0.05 |
| 0.5 | 63.90 ± 0.02 | 12.70 ± 0.02 | 36.80 ± 0 | 64.70 ± 0 | 12.10 ± 0 | 36.40 ± 0.11 | −0.80 ± 0.02 | 0.60 ± 0.02 | 0.40 ± 0.11 | 1.10 ± 0.05 |
| 1.0 | 66.20 ± 0.02 | 12.40 ± 0 | 30.80 ± 0 | 68.00 ± 0.02 | 10.70 ± 0.02 | 29.10 ± 0 | −1.80 ± 0.04 | 1.70 ± 0.02 | 1.70 ± 0 | 3.00 ± 0.05 |
| 2.0 | 60.70 ± 0.02 | 14.60 ± 0 | 34.00 ± 0.02 | 57.50 ± 0.04 | 14.80 ± 0.02 | 34.50 ± 0 | 3.20 ± 0.02 | −0.20 ± 0.02 | −0.50 ± 0.02 | 3.20 ± 0.05 |
| 3.5 | 69.50 ± 0 | 11.40 ± 0.02 | 33.70 ± 0.02 | 64.80 ± 0.02 | 8.60 ± 0.02 | 32.60 ± 0 | 4.70 ± 0.02 | 2.80 ± 0.04 | 1.10 ± 0.02 | 5.60 ± 0.05 |
| 5.0 | 65.90 ± 0.02 | 12.90 ± 0.02 | 33.50 ± 0 | 60.30 ± 0 | 13.30 ± 0.02 | 34.40 ± 0.11 | 5.60 ± 0.02 | −0.40 ± 0.01 | −0.90 ± 0.11 | 5.70 ± 0.05 |
| ΔE | Acceptability |
|---|---|
| 0–0.25 | Very small or not: ideal matching |
| 0.25–0.5 | Small: acceptable |
| 0.5–1.0 | Small to the medium: acceptable in some applications |
| 1.0–2.0 | Medium: acceptable in specific applications |
| 2.0–4.0 | Gaps: acceptable in specific applications |
| >4.0 | Very large: basically unacceptable |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Wang, L.; Qian, X. Effect of High-Temperature Calcined Wheat Straw Powder after Lignin Removal on Properties of Waterborne Wood Coatings. Coatings 2019, 9, 444. https://doi.org/10.3390/coatings9070444
Yan X, Wang L, Qian X. Effect of High-Temperature Calcined Wheat Straw Powder after Lignin Removal on Properties of Waterborne Wood Coatings. Coatings. 2019; 9(7):444. https://doi.org/10.3390/coatings9070444
Chicago/Turabian StyleYan, Xiaoxing, Lin Wang, and Xingyu Qian. 2019. "Effect of High-Temperature Calcined Wheat Straw Powder after Lignin Removal on Properties of Waterborne Wood Coatings" Coatings 9, no. 7: 444. https://doi.org/10.3390/coatings9070444
APA StyleYan, X., Wang, L., & Qian, X. (2019). Effect of High-Temperature Calcined Wheat Straw Powder after Lignin Removal on Properties of Waterborne Wood Coatings. Coatings, 9(7), 444. https://doi.org/10.3390/coatings9070444




