Phase Evolution and Microstructure Analysis of CoCrFeNiMo High-Entropy Alloy for Electro-Spark-Deposited Coatings for Geothermal Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Processing
2.2. Microstructural Analyses
2.3. X-Ray Diffraction (XRD) Analysis
2.4. Electrochemical Corrosion Testing
2.5. Hardness Measurement
3. Results
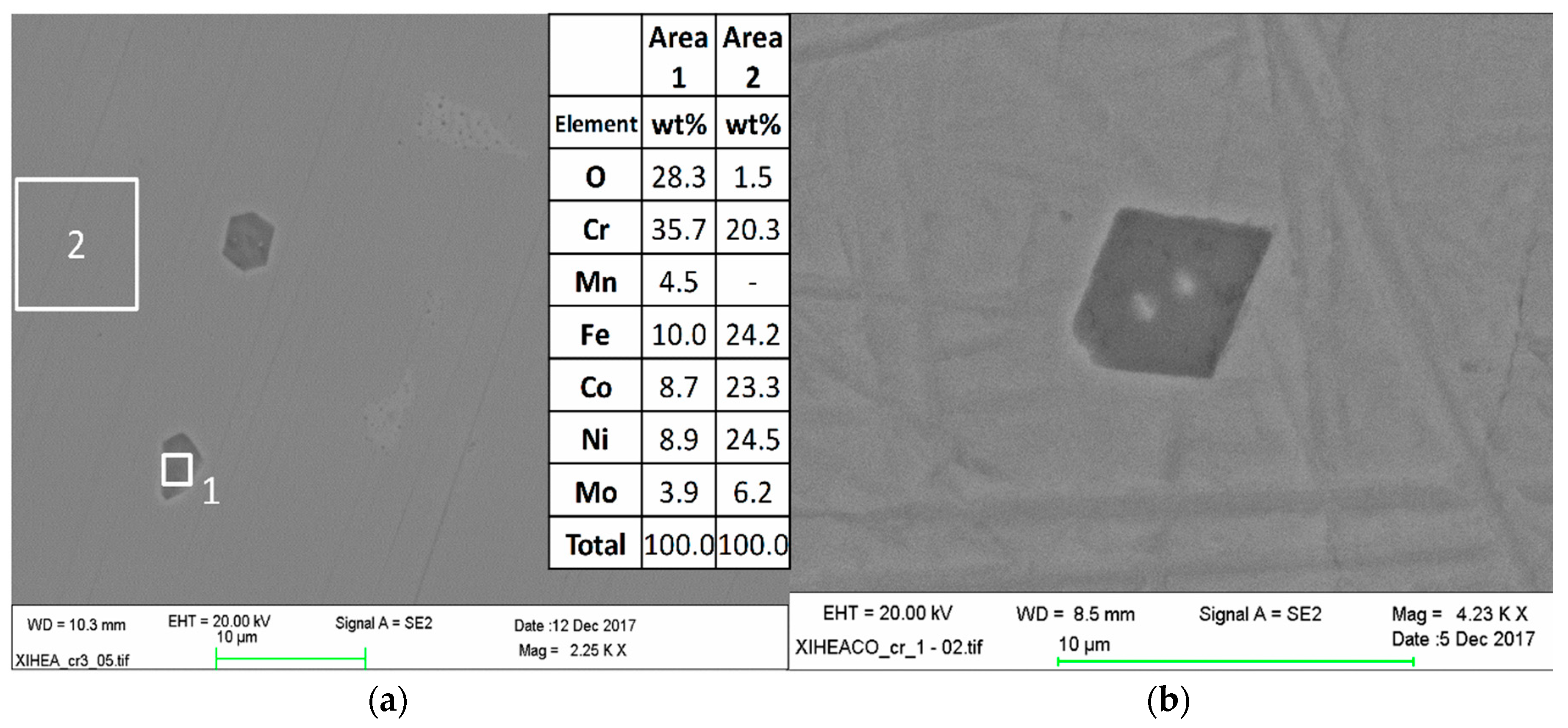
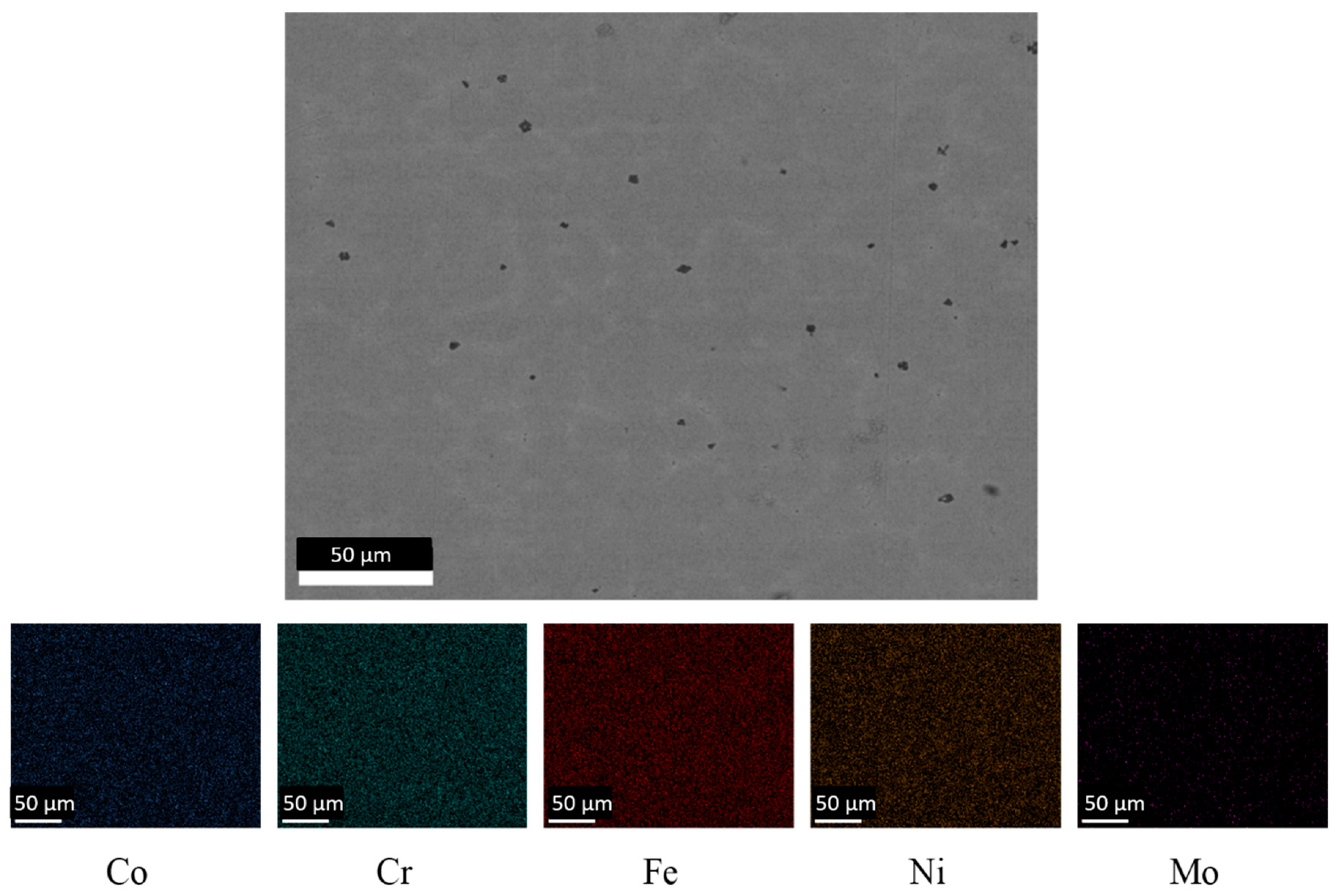
3.1. Microstructures of As-Cast CoCrFeNiMo
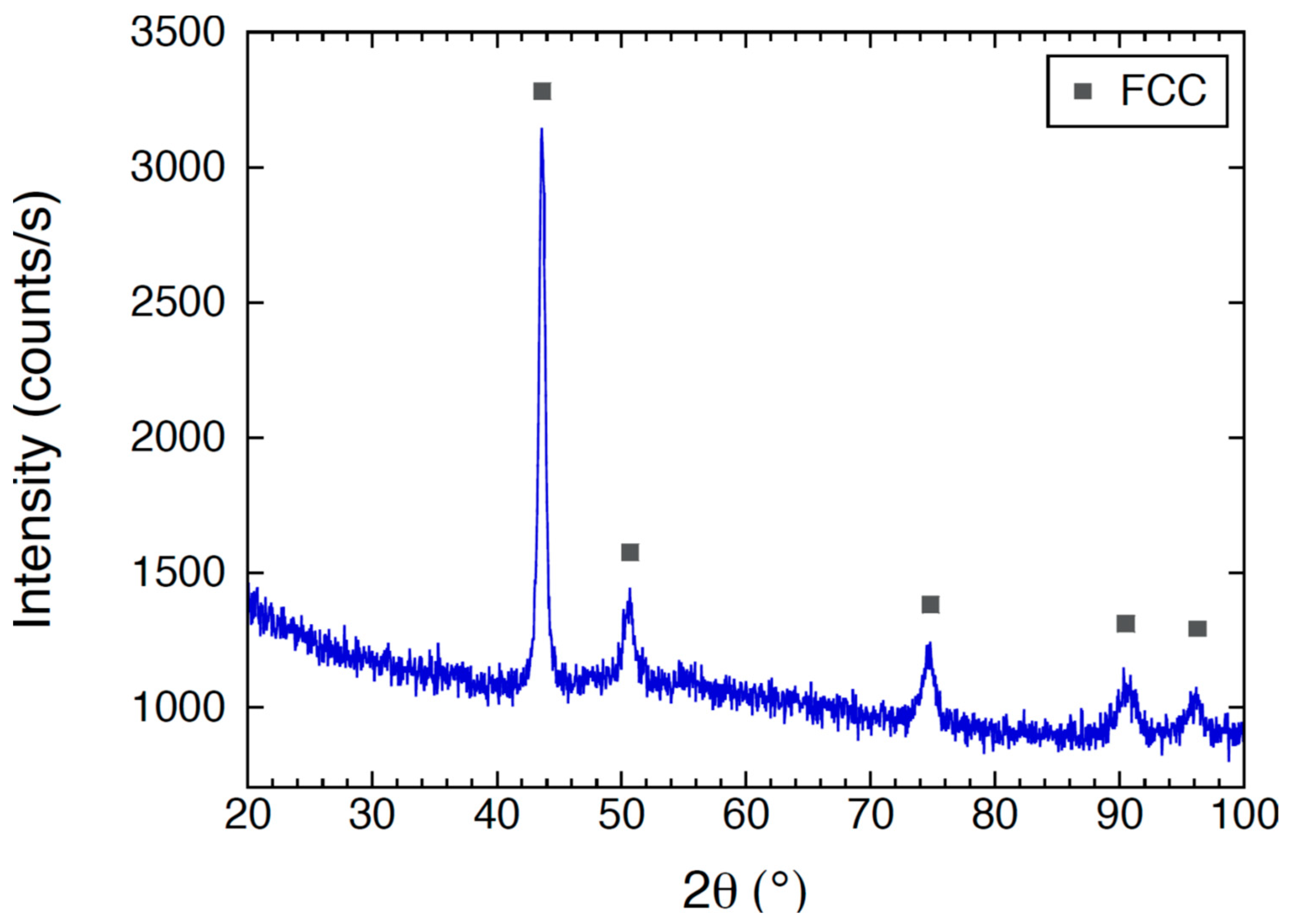
3.2. XRD Results for As-Cast CoCrFeNiMo
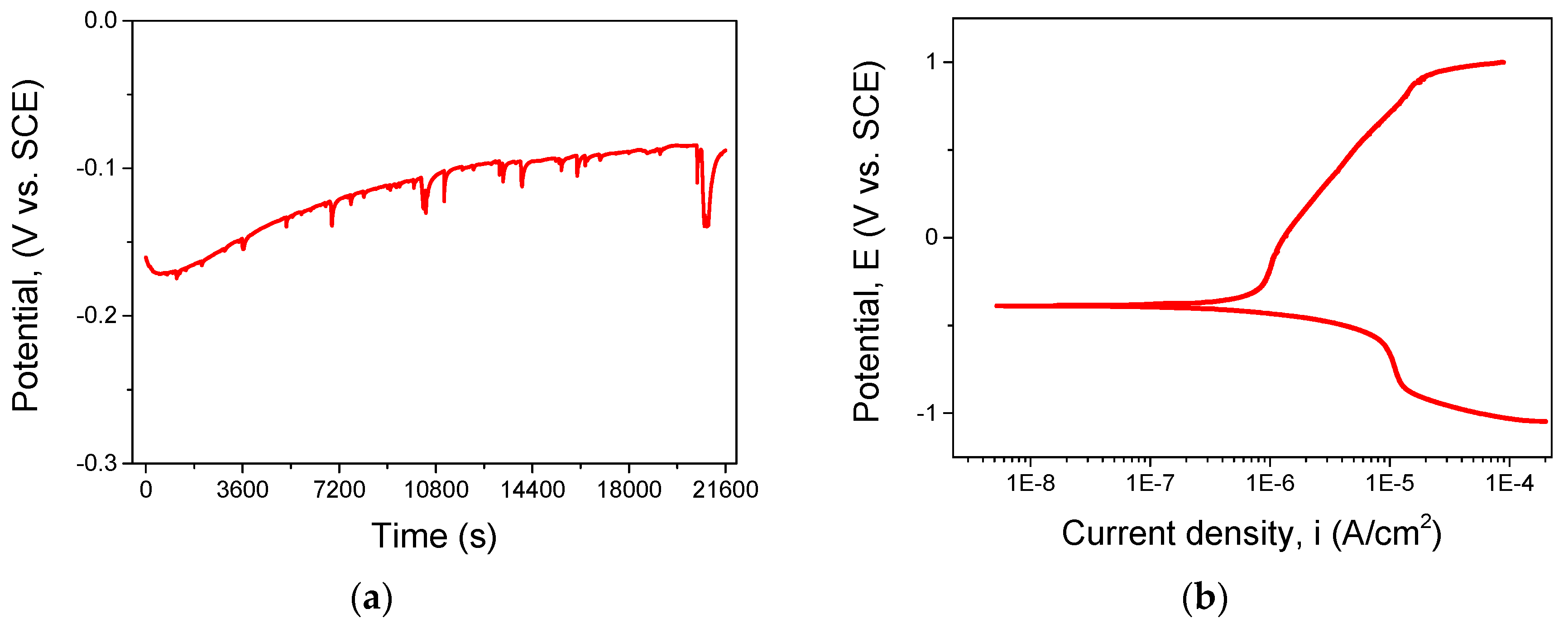
3.3. Corrosion Behavior of As-Cast CoCrFeNiMo
3.4. Hardness Measurements of As-Cast CoCrFeNiMo
3.5. Microstructural and Chemical Composition Analysis of ESD-Prepared CoCrFeNiMo Coatings
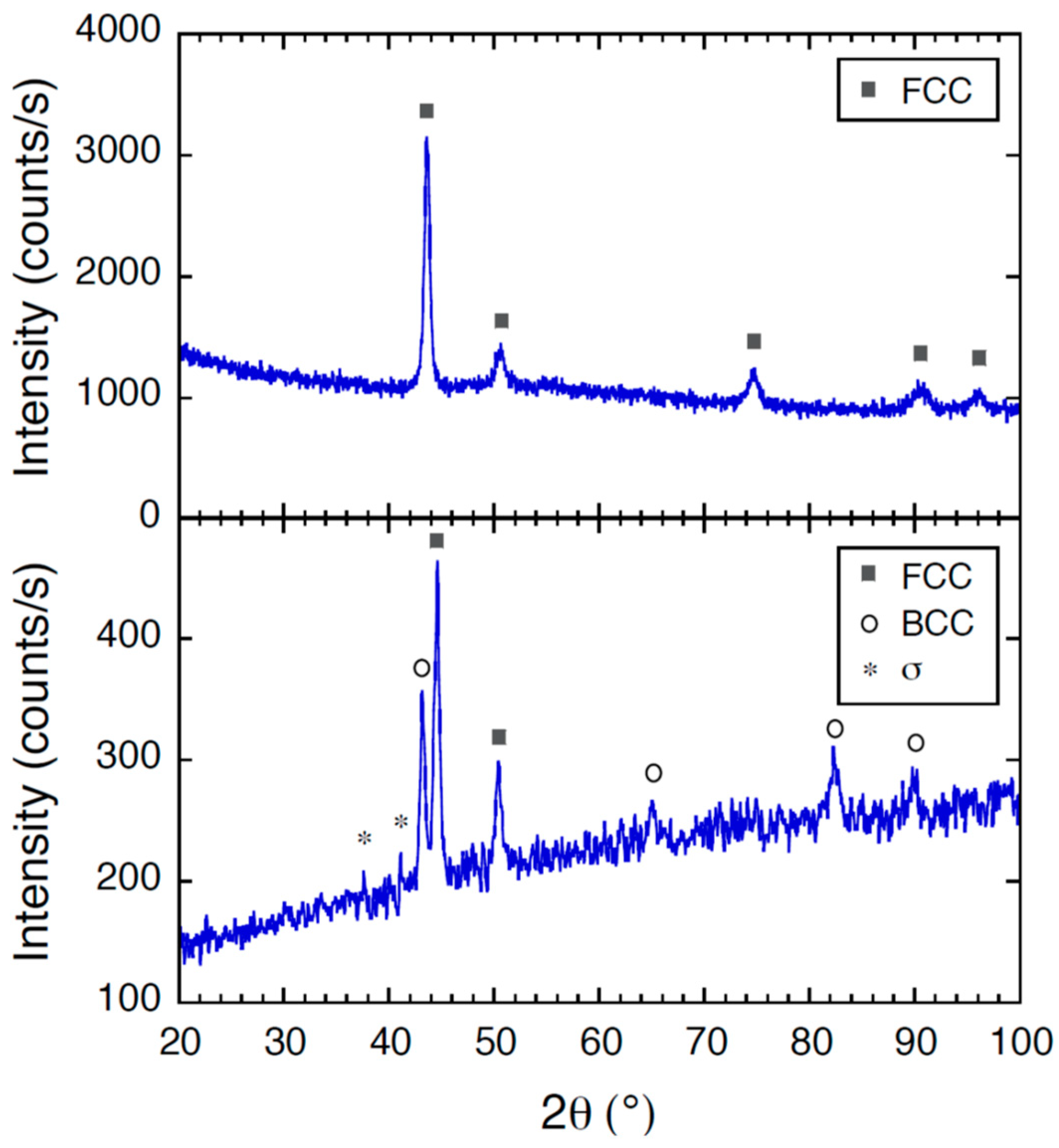
3.6. XRD Analysis of CoCrFeNiMo Coating
4. Discussion
5. Conclusions
- Vacuum-arc-melted CoCrFeNiMo HEA was fabricated and used for producing an electro-spark-deposited coating.
- Testing of the as-cast material revealed high hardness (593 HV) and low corrosion rates (0.0072 mm/year), which is promising for the high wear and corrosion resistance needed for the harsh geothermal environment.
- Adherent and dense coating was obtained, but some variance in the distribution of elements was observed in the coating, with Cr increasing with the distance from the substrate.
- The crystal structure of the as-cast electrode CoCrFeNiMo material was identified as FCC with XRD, but additional phases were formed in the CoCrFeNiMo coating, such as BCC and potentially the σ phase.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, W.H.; Lu, Z.P.; He, J.Y.; Luan, J.H.; Wang, Z.J.; Liu, B.; Liu, Y.; Chen, M.W.; Liu, C.T. Ductile CoCrFeNiMox high entropy alloys strengthened by hard intermetallic phases. Acta Mater. 2016, 116, 332–342. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, H.; Spolenak, R. Ultra strong ductile and stable high-entropy alloys at small scales. Nat. Commun. 2015, 6, 7748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Stocks, G.M.; Jin, K.; Lu, C.; Bei, H.; Sales, B.C.; Wang, L.; Beland, L.K.; Stoller, R.E.; Samolyuk, G.D.; et al. Influence of chemical disorder on energy dissipation and defect evolution in concentrated solid solution alloys. Nat. Commun. 2015, 6, 8736. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H. Three strategies for the design of advanced high-entropy alloys. Entropy 2016, 18, 252. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: formation of solid solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Guo, S.; Hu, Q.; Ng, C.; Liu, C.T. More than entropy in high-entropy alloys: Forming solid solutions or amorphous phase. Intermet. 2013, 41, 96–103. [Google Scholar] [CrossRef]
- Ren, M.X.; Li, B.S.; Fu, H.Z. Formation condition of solid solution type high entropy alloy. Trans. Nonferrous Metall. Soc. China 2013, 23, 991–995. [Google Scholar] [CrossRef]
- Liu, W.H.; Wu, Y.; He, J.Y.; Zhang, Y.; Liu, C.T.; Lu, Z.P. The phase competition and stability of high-entropy alloys. JOM 2014, 66, 1973–1983. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, S.; Liu, C.T. Phase selection in high-entropy alloys: From nonequilibrium to equilibrium. JOM 2014, 66, 1966–1972. [Google Scholar] [CrossRef]
- Karlsdottir, S.N. Corrosion, scaling and material selection in geothermal energy production. In Comprehensive Renewable Energy, 1st ed.; Sayigh, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 239–256. [Google Scholar]
- Karlsdottir, S.N.; Hjaltason, S.M.; Ragnarsdottir, K.R. Corrosion behaviour of materials in hydrogen sulphide abatement system at Hellisheiði geothermal power plant. Geothermics 2017, 70, 222–229. [Google Scholar] [CrossRef]
- Matsuda, H. Maintenance for reliable geothermal turbine. GRC Trans. 2006, 30, 755–760. [Google Scholar]
- Brown, A.; Müller, S.; Dobrotková, Z. Renewable Energy: Markets and Prospects by Technology; IEA: Paris, France, 2011; 66p. [Google Scholar]
- Kudryashov, A.E.; Potanin, A.Y.; Lebedev, D.N.; Sukhorukova, I.V.; Shtansky, D.V.; Levashov, E.A. Structure and properties of Cr-Al-Si-B coatings produced by pulsed electrospark deposition on a nickel alloy. Surf. Coat. Technol. 2016, 285, 278–288. [Google Scholar] [CrossRef]
- Xie, Y.J.; Wang, M.C. Isothermal oxidation behaviour of electrospark deposited MCrAlX-type coatings on a Ni-based superalloy. J. Alloys Compd. 2009, 480, 454–461. [Google Scholar] [CrossRef]
- Xie, Y.J.; Wang, M.C. Epitaxial MCrAlY coating on a Ni-base superalloy produced by electrospark deposition. Surf. Coat. Technol. 2006, 201, 3564–3570. [Google Scholar] [CrossRef]
- Podchernyaeva, I.A.; Panasyuk, A.D.; Teplenko, M.A.; Podol’skii, V.I. Protective coatings on heat-resistant nickel alloys (Review). Powder Metall. Met. Ceram. 2000, 39, 434–444. [Google Scholar] [CrossRef]
- Csáki, I.; Stefanoiu, R.; Karlsdottir, S.N.; Geambazu, L.E. Corrosion behavior in geothermal steam of CoCrFeNiMo high entropy alloy. In Proceedings of the CORROSION 2018, Phoenix, AZ, USA, 15–19 April 2018. [Google Scholar]
- Stern, M. A method for determining corrosion rates from linear polarization data. Corrosion 1958, 14, 60–64. [Google Scholar] [CrossRef]
- Sheir, L.L.; Jarman, R.A.; Burstein, G.T. Corrosion: Corrosion Control; Butterworth-Heinemann: Boston, MA, USA, 1994; pp. 19–38. [Google Scholar]
- ASTM G4-01(2014): Standard Guide for Conducting Corrosion Tests in Field Applications; ASTM International: West Conshohocken, PA, USA, 2008.
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-resistant high entropy alloys: A review. Metals 2017, 7, 43. [Google Scholar] [CrossRef]
- Sohi, M.H.; Ebrahimi, M.; Raouf, A.H.; Mahboubi, F. Comparative study of the corrosion behavior of plasma nitrocarburised AISI 4140 steel before and after post-oxidation. Mater. Des. 2010, 31, 4432–4437. [Google Scholar] [CrossRef]
- Praveen, S.; Murty, B.S.; Kottada, R.S. Alloying behaviour in multi-component AlCoCrCuFe and NiCoCrCuFe high entropy alloys. Mater. Sci. Eng. 2012, 534, 8389. [Google Scholar] [CrossRef]
- Liu, W.H.; Wu, Y.; He, J.Y.; Nieh, T.G.; Lu, Z.P. Grain growth and the Hall-Petch relationship in a high-entropy FeCrNiCoMn alloy. Scr. Mater. 2013, 68, 526–529. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef]
- Zhu, C.; Lu, Z.P.; Nieh, T.G. Incipient plasticity and dislocation nucleation of FeCoCrNiMn high-entropy alloy. Acta Mater. 2013, 61, 2993–3001. [Google Scholar] [CrossRef]
- Tsai, K.Y.; Tsai, M.H.; Yeh, J.W. Sluggish diffusion in Co-Cr-Fe-Mn-Ni high entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Shun, T.T.; Chang, L.Y.; Shiu, M.H. Microstructure and mechanical properties of multiprincipal component CoCrFeNiMox alloys. Mater. Charact. 2012, 70, 63–67. [Google Scholar] [CrossRef]
- Hansen, M. Constitution of Binary Alloys, Metallurgy and Metalurgical Enginerring Series; McGraw-Hill: New York, NY, USA, 1958; pp. 966–982. [Google Scholar]
- Tsai, M.-H.; Tsai, K.-Y.; Tsai, C.-W.; Lee, C.; Juan, C.-C.; Yeh, J.-W. Criterion for Sigma phase formation in Cr- and V-containing high-entropy alloys. Mater. Res. Lett. 2013, 1, 207–212. [Google Scholar] [CrossRef]
- Zhu, J.M.; Fu, H.M.; Zhang, H.F.; Wang, A.M.; Li, H.; Hu, Z.Q. Microstructures and compressive properties of multicomponent AlCoCrFeNiMox alloys. Mater. Sci. Eng. 2010, 527, 6975–6979. [Google Scholar] [CrossRef]
- Ye, X.; Ma, M.; Cao, Y.; Liu, W.; Gu., Y. The property research on high-entropy alloy AlxFeCoNiCuCr coating by laser cladding. Phys. Procedia 2011, 12, 303–312. [Google Scholar] [CrossRef]
- Ast, J.; Ghidelli, M.; Durst, K.; Göken, M.; Sebastiani, M.; Korsunsky, A.M. A review of experimental approaches to fracture toughness evaluation at the micro-scale. Mater. Des. 2019, 173, 107762. [Google Scholar] [CrossRef]
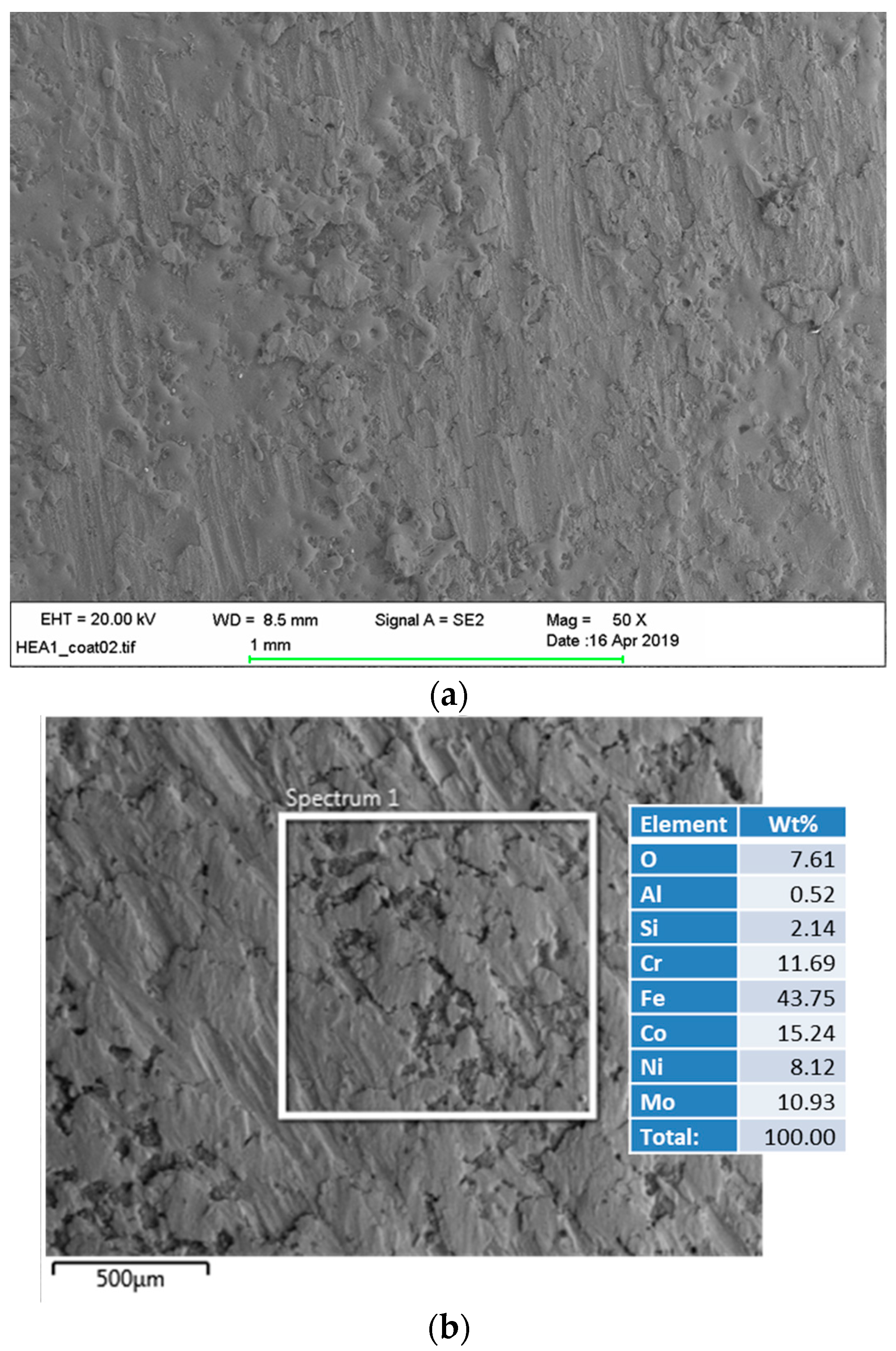
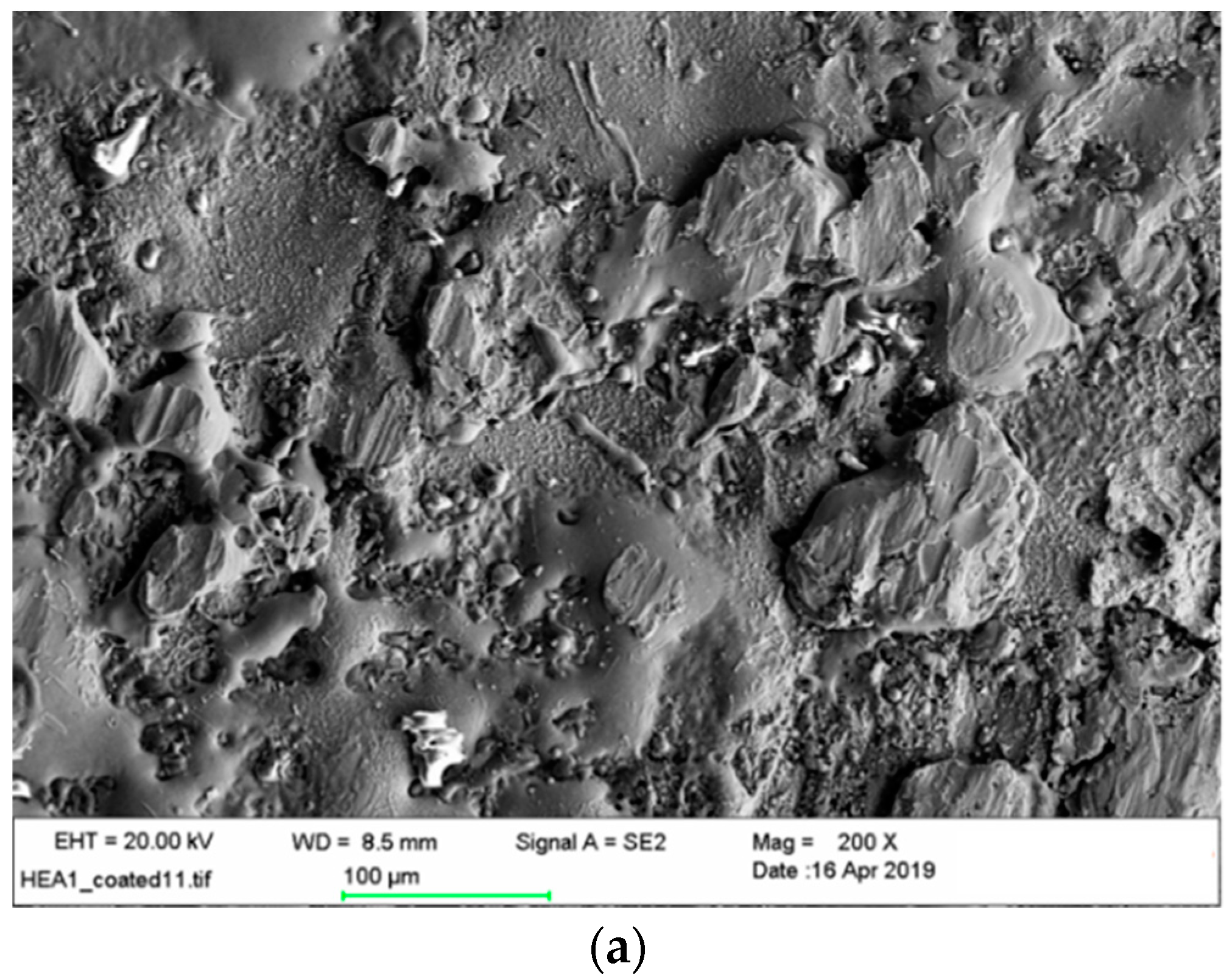
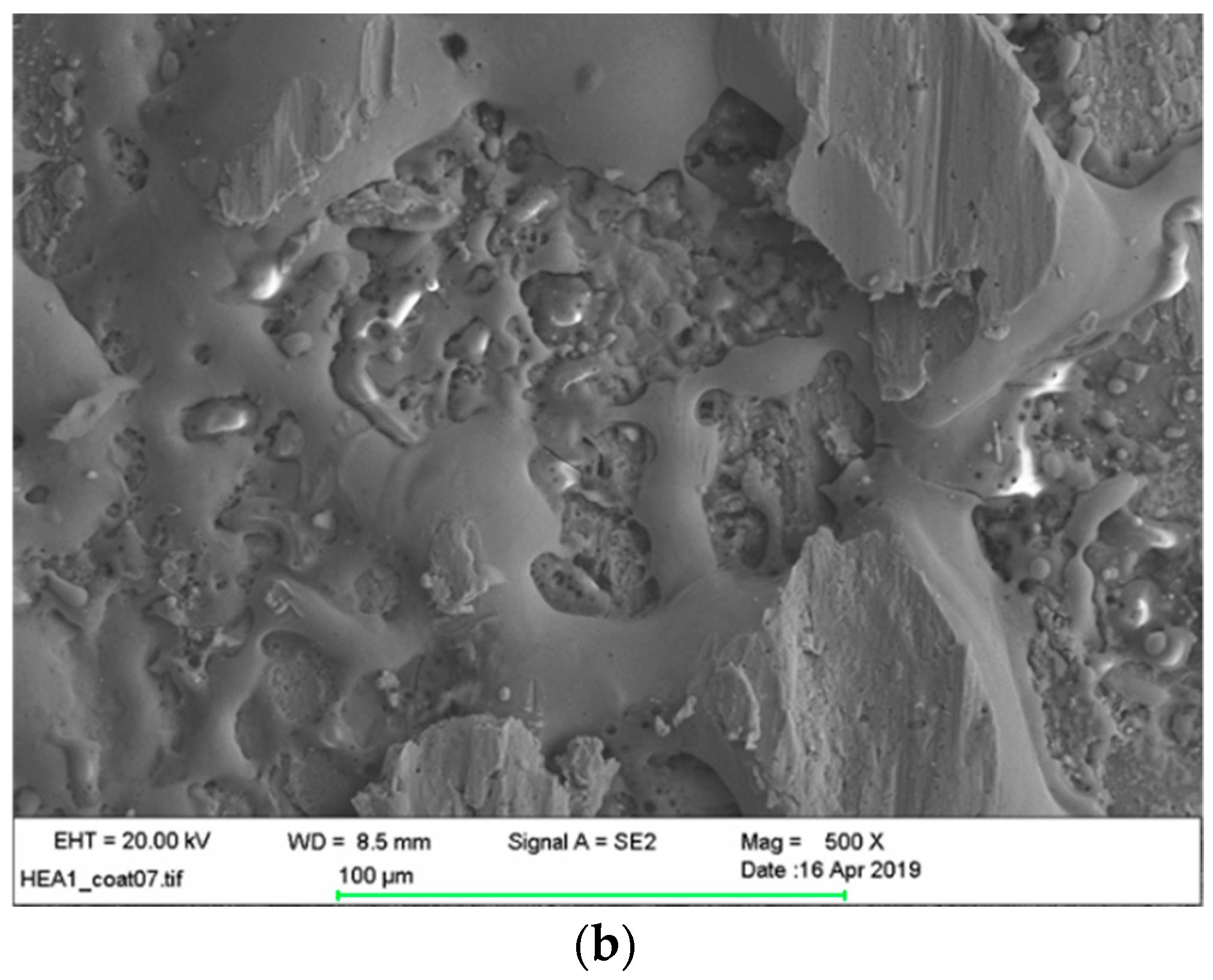
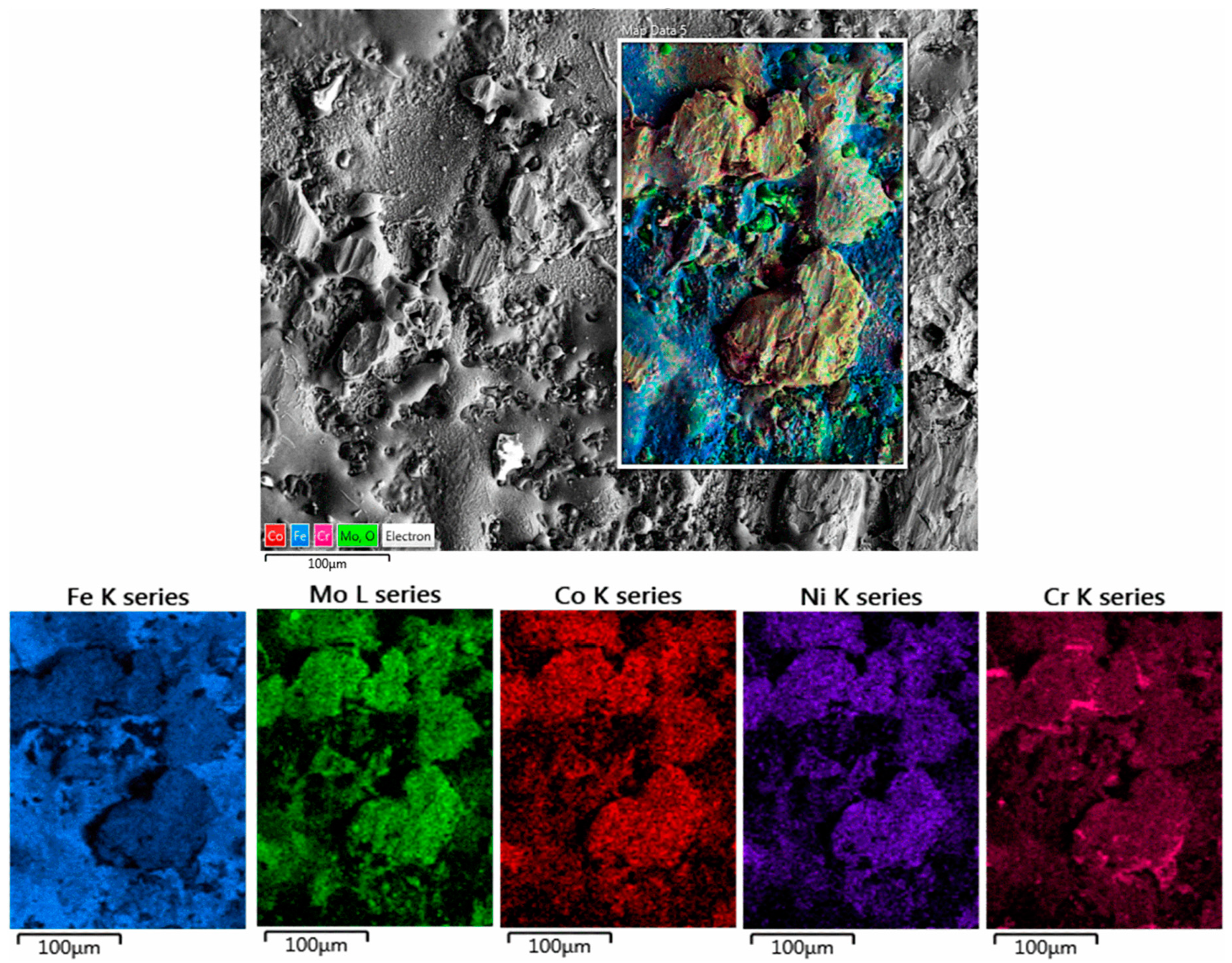
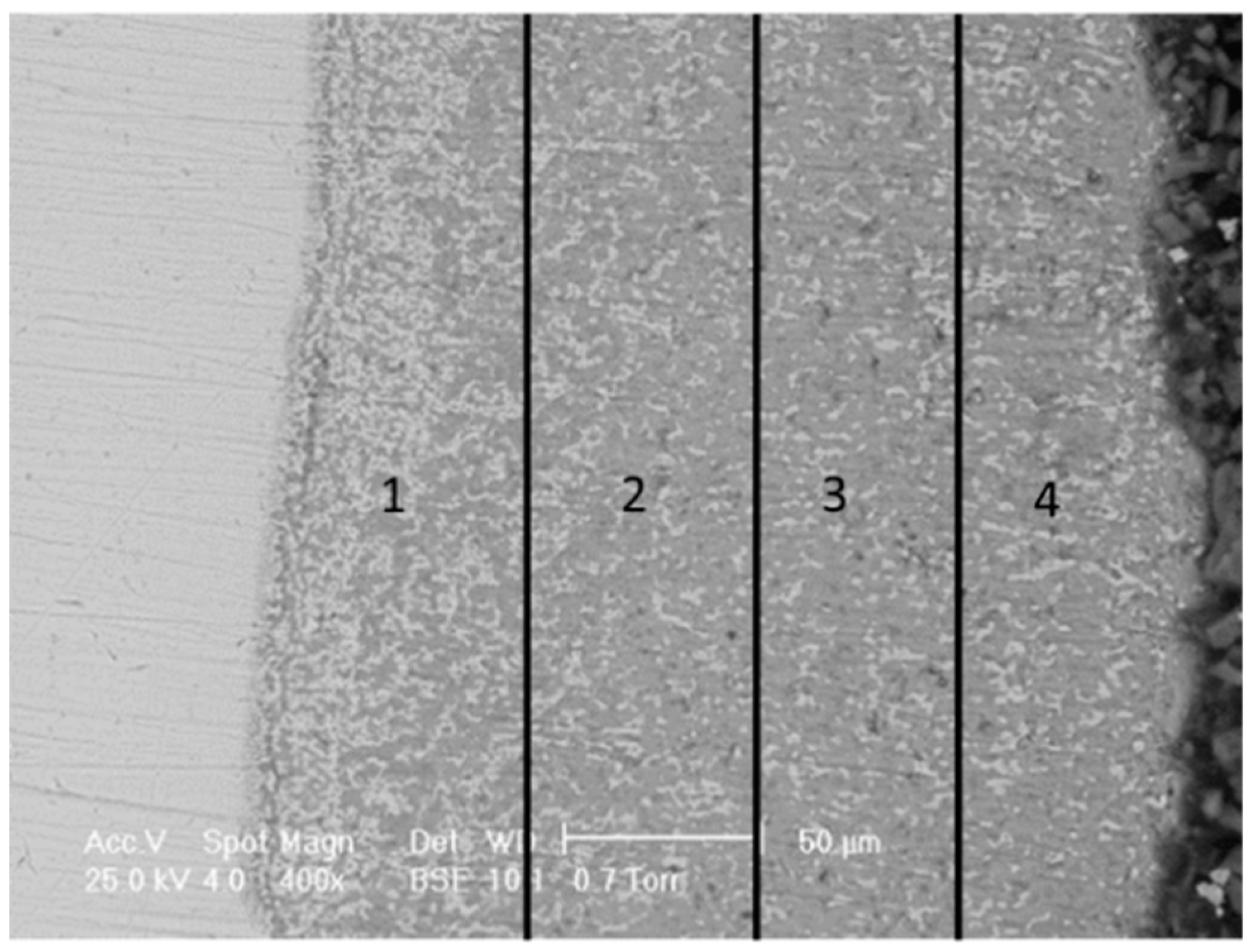
| Elements | Co | Cr | Fe | Ni | Mo | Si | Ca | Ti | O |
|---|---|---|---|---|---|---|---|---|---|
| Substrate | - | 16.5 | 66.4 | 10.4 | 3.9 | 0.8 | 0.9 | 1.0 | - |
| Area 1 | 3.2 | 17.7 | 44.7 | 10.9 | 4.3 | - | - | - | 19.9 |
| Area 2 | 1.6 | 18.6 | 37.0 | 9.8 | 4.9 | - | - | - | 28.1 |
| Area 3 | 1.6 | 18.8 | 34.2 | 10.6 | 5.0 | - | - | - | 29.7 |
| Area 4 | 2.4 | 18.5 | 29.4 | 11.7 | 6.8 | - | - | - | 31.2 |
| Elements (at%) | Co | Cr | Fe | Ni | Mo |
|---|---|---|---|---|---|
| CoCrFeNiMo | 20 | 20 | 20 | 20 | 20 |
| Sample | Capacitance (µF) | Voltage (V) | Frequency (Hz) | Atmosphere |
|---|---|---|---|---|
| CoCrFeNiMo | 20 | 100 | 3.5 | Argon |
| Sample | Eoc (mV) | Ecorr (mV) | icorr (µA/cm2) | βc (mV) | βa (mV) | Rp (kΩ × cm2) | CR (mm/year) |
|---|---|---|---|---|---|---|---|
| VAR_HEA | −88 | −360 | 0.017 | 207.13 | 92.96 | 1748.61 | 0.0072 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karlsdottir, S.N.; Geambazu, L.E.; Csaki, I.; Thorhallsson, A.I.; Stefanoiu, R.; Magnus, F.; Cotrut, C. Phase Evolution and Microstructure Analysis of CoCrFeNiMo High-Entropy Alloy for Electro-Spark-Deposited Coatings for Geothermal Environment. Coatings 2019, 9, 406. https://doi.org/10.3390/coatings9060406
Karlsdottir SN, Geambazu LE, Csaki I, Thorhallsson AI, Stefanoiu R, Magnus F, Cotrut C. Phase Evolution and Microstructure Analysis of CoCrFeNiMo High-Entropy Alloy for Electro-Spark-Deposited Coatings for Geothermal Environment. Coatings. 2019; 9(6):406. https://doi.org/10.3390/coatings9060406
Chicago/Turabian StyleKarlsdottir, Sigrun N., Laura E. Geambazu, Ioana Csaki, Andri I. Thorhallsson, Radu Stefanoiu, Fridrik Magnus, and Cosmin Cotrut. 2019. "Phase Evolution and Microstructure Analysis of CoCrFeNiMo High-Entropy Alloy for Electro-Spark-Deposited Coatings for Geothermal Environment" Coatings 9, no. 6: 406. https://doi.org/10.3390/coatings9060406
APA StyleKarlsdottir, S. N., Geambazu, L. E., Csaki, I., Thorhallsson, A. I., Stefanoiu, R., Magnus, F., & Cotrut, C. (2019). Phase Evolution and Microstructure Analysis of CoCrFeNiMo High-Entropy Alloy for Electro-Spark-Deposited Coatings for Geothermal Environment. Coatings, 9(6), 406. https://doi.org/10.3390/coatings9060406







