The Antibacterial Properties and Safety of a Nanoparticle-Coated Parquet Floor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
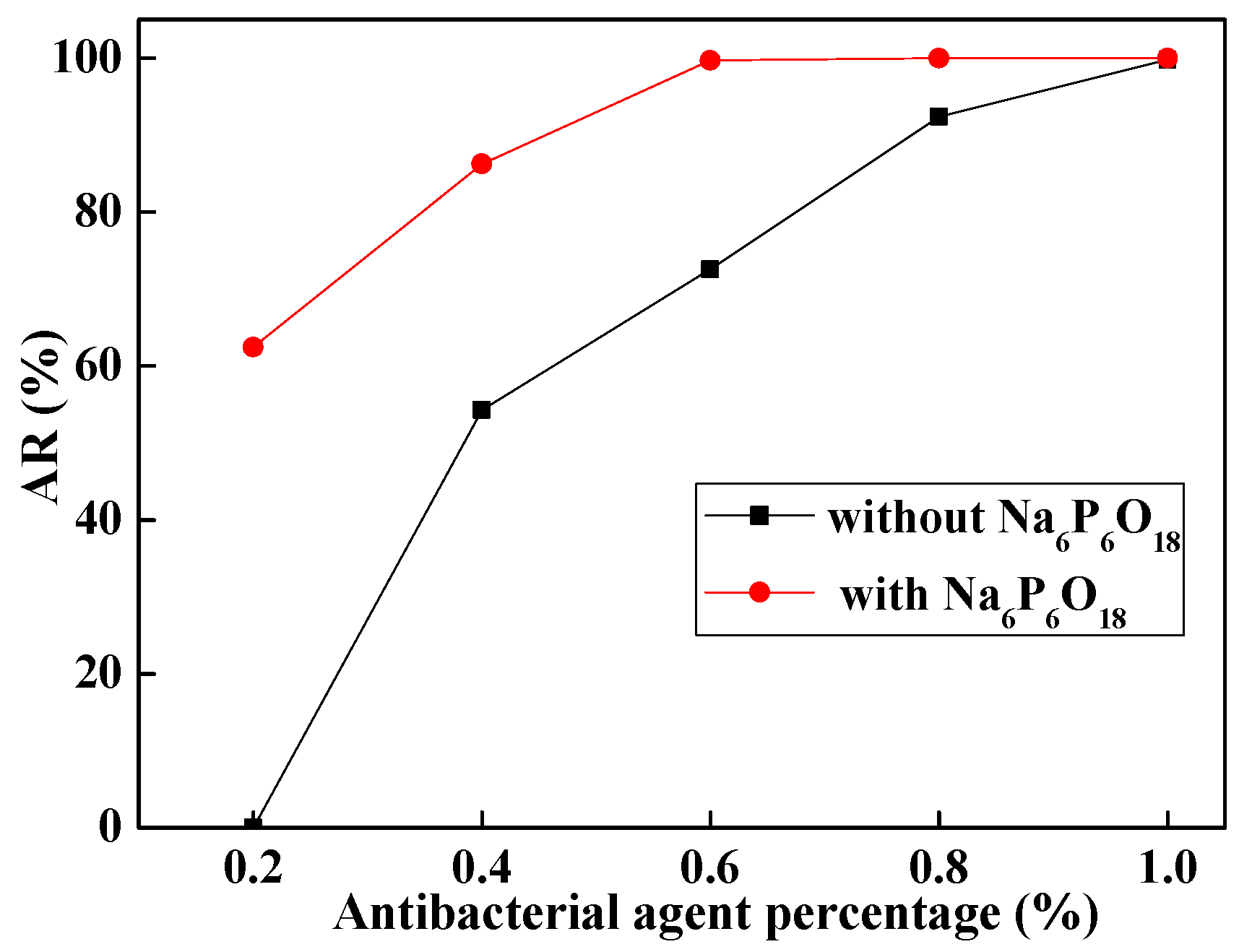
2.2. Selection of Antibacterial Agent
2.3. Selection of Dispersing Agent
2.4. Preparation of Antibacterial Cedar Parquet Floor
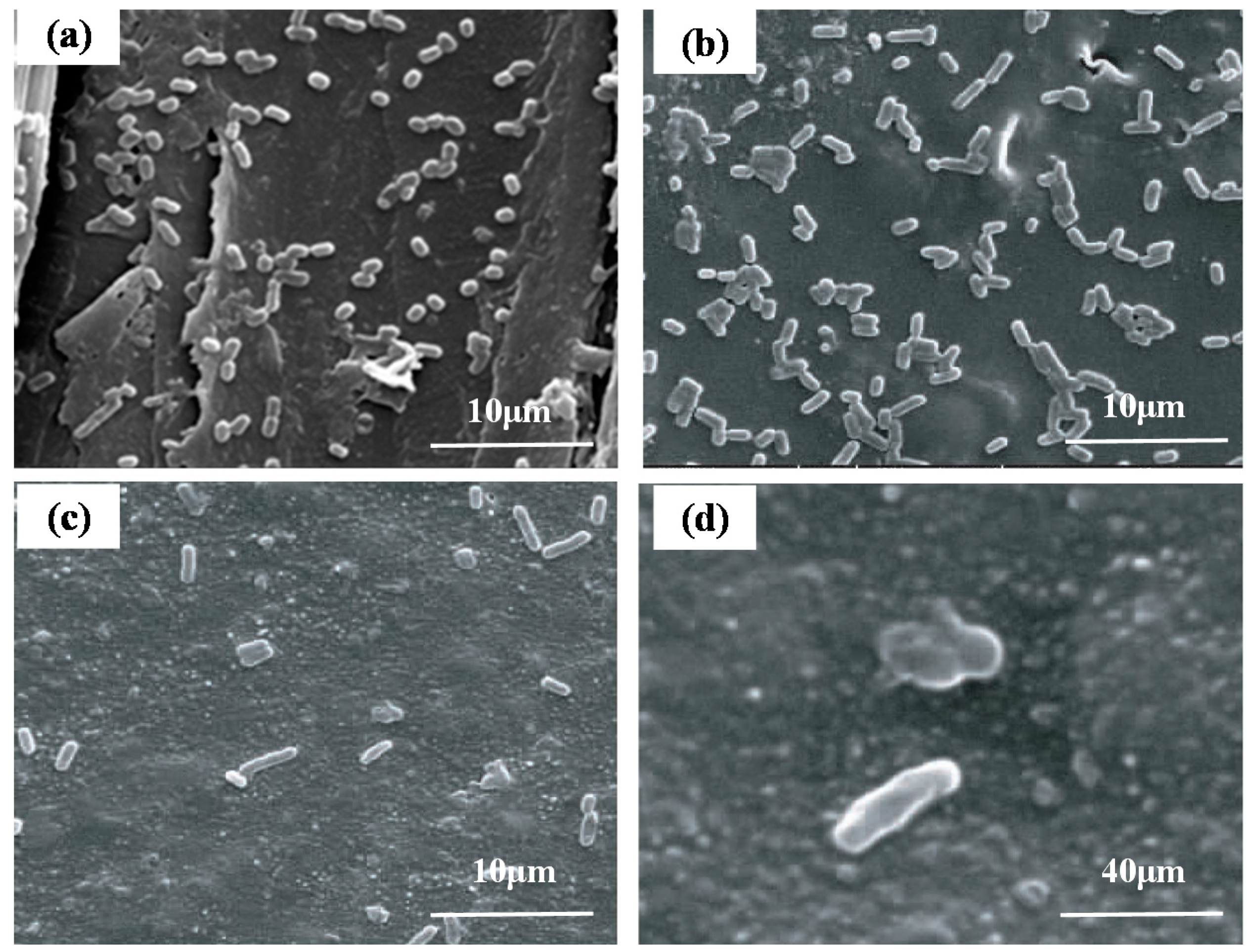
2.5. Antibacterial Properties Analysis of the Cedar Parquet Floor
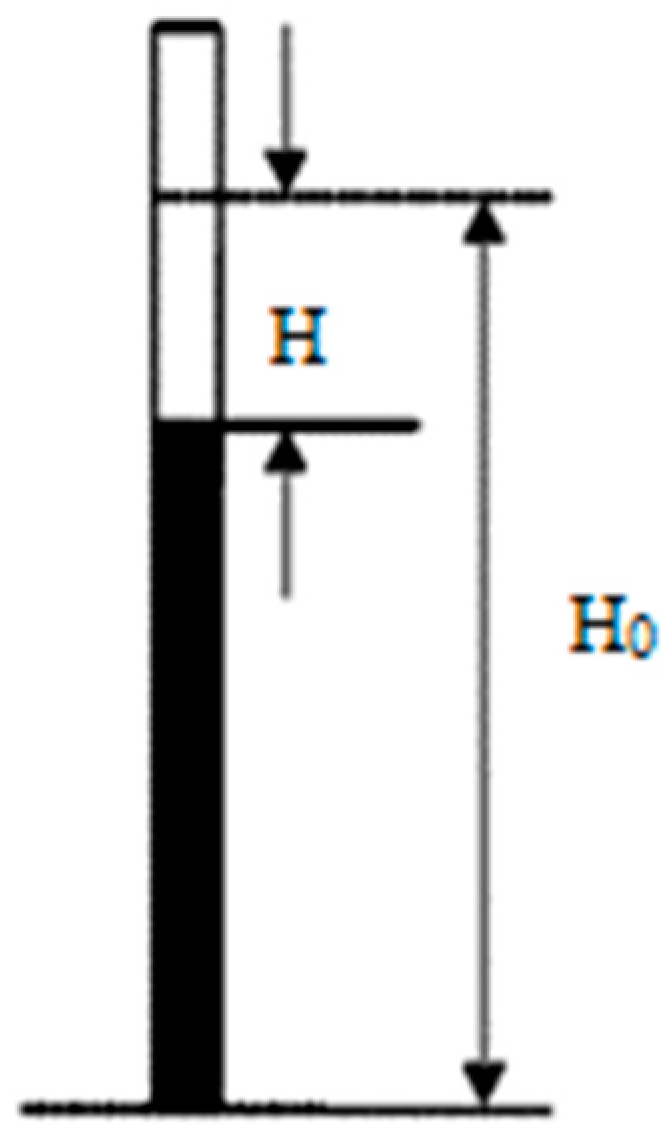
2.6. Free Formaldehyde Content (FFC) and Total Volatile Organic Compounds (TVOC) Tests of the Cedar Parquet Floor
2.7. Cytotoxicity Tests of the Cedar Parquet Floor
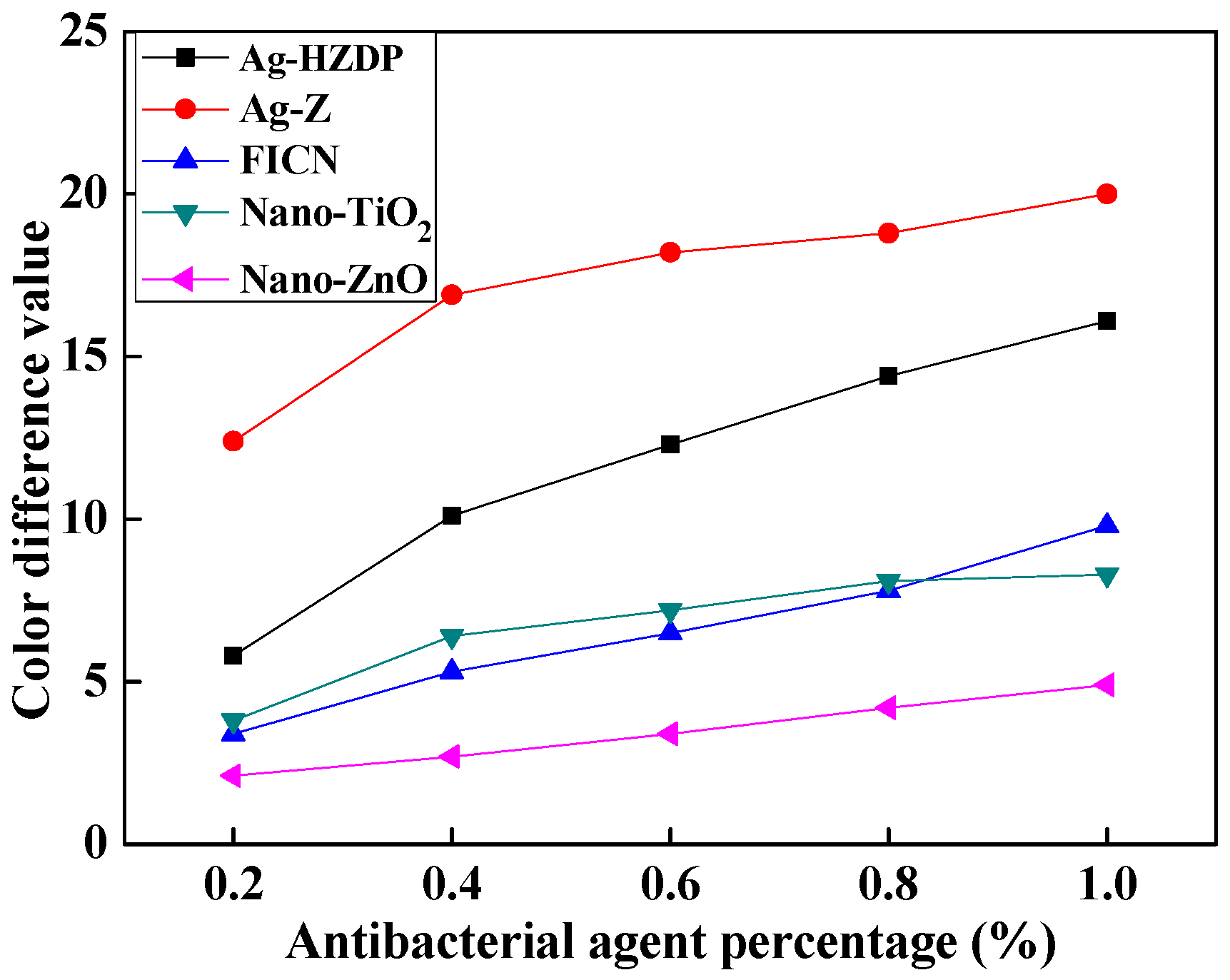
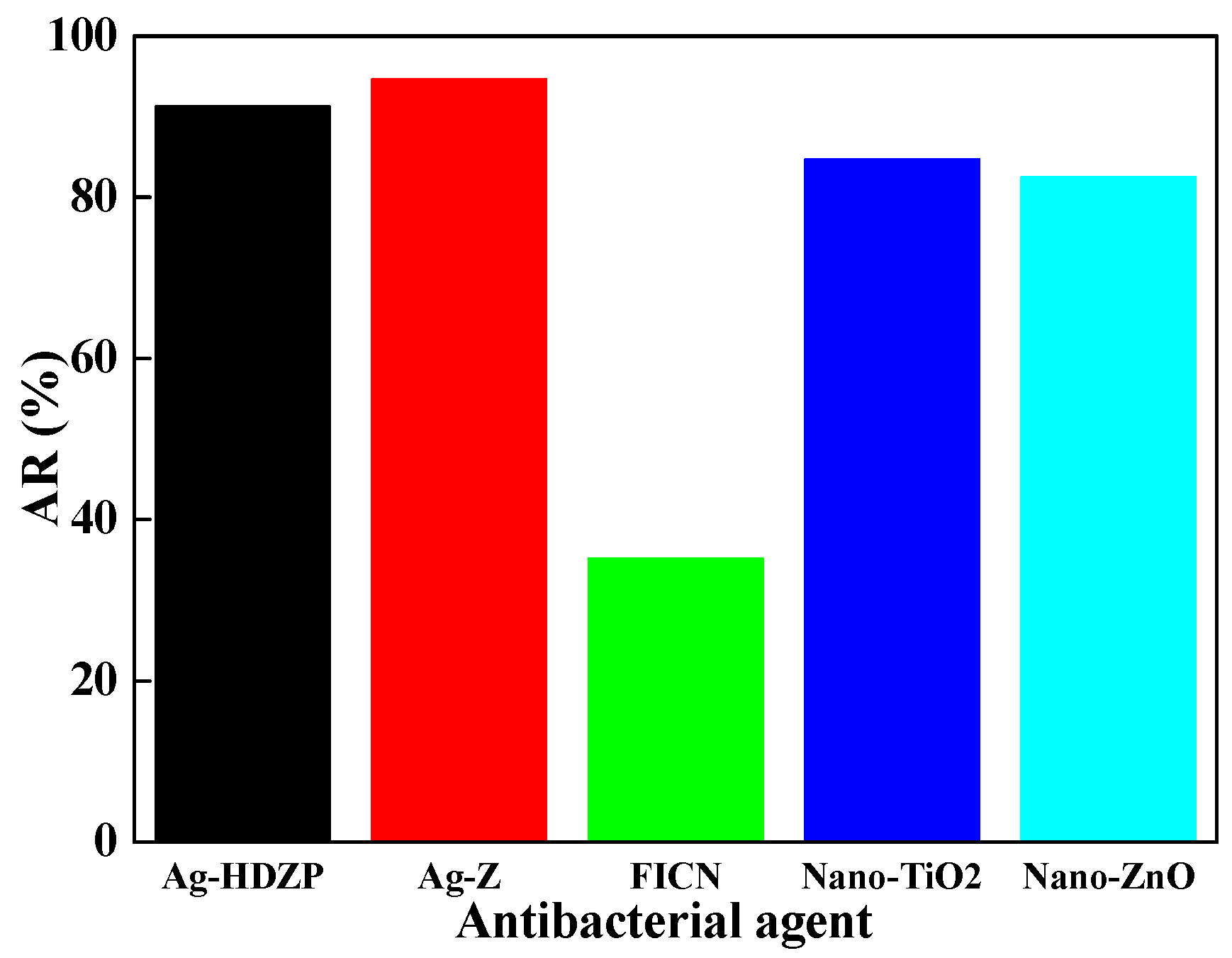
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yamulki, S.; Morison, J.I.L. Annual greenhouse gas fluxes from a temperate deciduous oak forest floor. Forestry 2017, 90, 541–552. [Google Scholar] [CrossRef]
- Zhang, H.Y.; He, Q.; Lu, X.N.; Pizzi, A.; Mei, C.T.; Zhan, X.X. Energy release rate measurement of welded bamboo joints. J. Renew. Mater. 2018, 6, 450–456. [Google Scholar] [CrossRef]
- MacFarlane, D.W.; Meyer, S.P. Characteristics and distribution of potential ash tree hosts for emerald ash borer. For. Ecol. Manag. 2005, 213, 15–24. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Pizzi, A.; Zhou, X.H.; Lu, X.N.; Wang, Z.Q. The study of linear vibrational welding of moso bamboo. J. Adhes. Sci. Technol. 2018, 32, 1–10. [Google Scholar] [CrossRef]
- De Figueiredo Latorraca, J.V.; Teixeira, D.E.; Batista, D.C. Overlay of Eucalyptus urophylla cement-bonded particleboard for application as flooring panels. For. Prod. J. 2009, 59, 65–69. [Google Scholar]
- Salca, E.-A. Black alder (Alnus glutinosa L.)—A resource for value-added products in furniture industry under European screening. Curr. For. Rep. 2019, 5, 41–54. [Google Scholar] [CrossRef]
- Huang, W.; Wilkes, A.; Sun, X.; Terheggen, A. Who is importing forest products from Africa to China? An analysis of implications for initiatives to enhance legality and sustainability. Environ. Dev. Sustain. 2013, 15, 339–354. [Google Scholar] [CrossRef]
- Hesser, F.; Wohner, B.; Meints, T.; Stern, T.; Windsperger, A. Integration of LCA in R&D by applying the concept of payback period: Case study of a modified multilayer wood parquet. Int. J. Life Cycle Assess. 2017, 22, 307–316. [Google Scholar]
- Berti, S.; Burato, P.; Dionisi-Vici, P.; Allegretti, O. Orange wood for parquet and engineered flooring use. BioResources 2017, 13, 586–596. [Google Scholar] [CrossRef]
- Blanchet, P.; Beauregard, R.; Erb, A.; Lefebvre, M. Comparative study of four adhesives used as binder in engineered wood parquet flooring. For. Prod. J. 2003, 53, 89. [Google Scholar]
- Heudorf, U.; Angerer, J. Internal exposure to PAHs of children and adults living in homes with parquet flooring containing high levels of PAHs in the parquet glue. Int. Arch. Occup. Environ. Health. 2001, 74, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Saelzer, E.; Maack, J.; Moeck, T. Special impact sound insulation cases, Part 1: Laminate and parquet flooring, dry and raised floor constructions and terrace flooring. Bauphysik 2012, 34, 223–228. [Google Scholar]
- Derler, S.; Kausch, F.; Huber, R. Systematic patterns and random fluctuations in time series of coefficients of friction measured on floor surfaces. Saf. Sci. 2005, 43, 751–770. [Google Scholar] [CrossRef]
- Zigon, J.; Pizzi, A.; Zhang, H.; Sega, B.; Cop, M.; Sernek, M. The influence of heat and chemical treatments of beech wood on the shear strength of welded and UF bonded specimens. Eur. J. Wood Wood Prod. 2015, 73, 685–687. [Google Scholar] [CrossRef]
- Bruez, E.; Haidar, R.; Alou, M.T.; Vallance, J.; Bertsch, C.; Mazet, F.; Fermaud, M.; Deschamps, A.; Guerin-Dubrana, L.; Compant, S.; et al. Bacteria in a wood fungal disease: Characterization of bacterial communities in wood tissues of esca-foliar symptomatic and asymptomatic grapevines. Front. Microbiol. 2015, 6, 1137. [Google Scholar] [CrossRef] [PubMed]
- Milling, A.; Kehr, R.; Wulf, A.; Smalla, K. Survival of bacteria on wood and plastic particles: Dependence on wood species and environmental conditions. Holzforschung 2005, 59, 72–81. [Google Scholar] [CrossRef]
- Joycharat, N.; Thammavong, S.; Limsuwan, S.; Homlaead, S.; Voravuthikunchai, S.P.; Yingyongnarongkul, B.-E.; Dej-Adisai, S.; Subhadhirasakul, S. Antibacterial substances from Albizia myriophylla wood against cariogenic Streptococcus mutans. Arch. Pharmacal Res. 2013, 36, 723–730. [Google Scholar] [CrossRef]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial Polymers in Solution and on Surfaces: Overview and functional principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef]
- Kandelbauer, A.; Widsten, P. Antibacterial melamine resin surfaces for wood-based furniture and flooring. Prog. Org. Coat. 2009, 65, 305–313. [Google Scholar] [CrossRef]
- Gysels, K.; Delalieux, F.; Deutsch, F.; Van Grieken, R.; Camuffo, D.; Bernardi, A.; Sturaro, G.; Busse, H.-J.; Wieser, M. Indoor environment and conservation in the Royal Museum of Fine Arts, Antwerp, Belgium. J. Cult. Heritage 2004, 5, 221–230. [Google Scholar] [CrossRef]
- Abrishami, S.H.; Tall, B.D.; Bruursema, T.J.; Epstein, P.S.; Shah, D.B. Bacterial adherence and viability on cutting board surfaces. J. Food Saf. 1994, 14, 153–172. [Google Scholar] [CrossRef]
- Alvarez-Paino, M.; Munoz-Bonilla, A.; Fernandez-Garcia, M. Antimicrobial polymers in the nano-world. Nanomaterials 2017, 7, 48. [Google Scholar] [CrossRef]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Bonilla, A.; Fernandez-Garcia, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.-J. Anti-bacterial performance of colloidal silver-treated laminate wood flooring. Int. Biodeterior. Biodegrad. 2006, 57, 155–162. [Google Scholar] [CrossRef]
- Munoz-Bonilla, A.; Fernandez-Garcia, M. The roadmap of antimicrobial polymeric materials in macromolecular nanotechnology. Eur. Polym. J. 2015, 65, 46–62. [Google Scholar] [CrossRef]
- Seo, J.; Jeon, J.; Lee, J.-H.; Kim, S. Thermal performance analysis according to wood flooring structure for energy conservation in radiant floor heating systems. Energy Build. 2011, 43, 2039–2042. [Google Scholar] [CrossRef]
- Braun, M.; Sun, Y. Antimicrobial polymers containing melamine derivatives. I. Preparation and characterization of chloromelamine-based cellulose. J. Polym. Sci. Part A 2004, 42, 3818–3827. [Google Scholar] [CrossRef]
- Kuplennik, N.; Tchoudakov, R.; Zelas, Z.B.-B.; Sadovski, A.; Fishman, A.; Narkis, M. Antimicrobial packaging based on linear low-density polyethylene compounded with potassium sorbate. LWT-Food Sci. Technol. 2015, 62, 278–286. [Google Scholar] [CrossRef]
- Chakra, C.; Raob, K.; Rajendar, V. Nanocomposites of ZnO and TiO2 have enhanced antimicrobial and antibacterial properties than their disjoint counterparts. Dig. J. Nanomater. Biostruct. 2017, 12, 185–193. [Google Scholar]
- Youssef, A.; Abdel-Aziz, M.; El-Sayed, E.; Abdel-Aziz, M.; El-Hakim, A.A.; Kamel, S.; Turky, G. Morphological, electrical & antibacterial properties of trilayered Cs/PAA/PPy bionanocomposites hydrogel based on Fe3O4-NPs. Carbohydr. Polym. 2018, 196, 483–493. [Google Scholar] [PubMed]
- ISO 105-J03:2009 Textiles—Tests for Colour Fastness—Part J03: Calculation of Colour Differences; International Organization for Standardization: Geneva, Switzerland, 2009.
- QB/T 2591-2003 Antimicrobial Plastics—Test for Antimicrobial Activity and Efficacy; National Development and Reform Commission: Beijing, China, 2003.
- GB 18580-2001 The Indoor Decorating and Refurbishing Material-Limit of Formaldehyde Emission of Wood-Based Panels and Finishing Products; Standardisation Administration of China: Beijing, China, 2001.
- HJ571-2010 Technical Requirement for Environmental Labeling Products—Wood Based Panels and Finishing Products; Ministry of Environmental Protection of China: Beijing, China, 2010.
- ISO 10993-5:1999 Biological Evaluation of Medical Devices. Part 5. Tests for Cytotoxicity: In Vitro Methods; International Organization for Standardization: Geneva, Switzerland, 1999.
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Ferraris, M.; Perero, S.; Ferraris, S.; Miola, M.; Vernè, E.; Skoglund, S.; Blomberg, E.; Wallinder, I.O. Antibacterial silver nanocluster/silica composite coatings on stainless steel. Appl. Surf. Sci. 2017, 396, 1546–1555. [Google Scholar] [CrossRef]
- Liu, C.; Liu, J.; Ning, X.; Chen, S.; Liu, Z.; Jiang, S.; Miao, D. The effect of polydopamine on an Ag-coated polypropylene nonwoven fabric. Polymers 2019, 11, 627. [Google Scholar] [CrossRef]
- Geng, A.; Meng, L.; Han, J.; Zhong, Q.; Li, M.; Han, S.; Mei, C.; Xu, L.; Tan, L.; Gan, L. Highly efficient visible-light photocatalyst based on cellulose derived carbon nanofiber/BiOBr composites. Cellulose 2018, 25, 4133–4144. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloy. Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Roumeli, E.; Papadopoulou, E.; Pavlidou, E.; Vourlias, G.; Bikiaris, D.; Paraskevopoulos, K.; Chrissafis, K. Synthesis, characterization and thermal analysis of urea–formaldehyde/nanoSiO2 resins. Thermochim. Acta 2012, 527, 33–39. [Google Scholar] [CrossRef]
- Jin, Y.; Cheng, X.; Zheng, Z. Preparation and characterization of phenol–formaldehyde adhesives modified with enzymatic hydrolysis lignin. Bioresour. Technol. 2010, 101, 2046–2048. [Google Scholar] [CrossRef] [PubMed]
| Dispersing Agent | Subsidence Value/Condition | Appearance |
|---|---|---|
| Nil | 2.5 mm | Normal color |
| PAA | Precipitates appear | Grey in color |
| Na6P6O18 | 1 mm | Uniformly dispersed |
| Na-CMC | Na-CMC insoluble | Nanoparticle/Na-CMC separated |
| SDBS | 1.25 mm | Bubble appears, slight color change in solution |
| PAAS | Solution thickened | Viscosity increased, slight color change in solution |
| Sample | 490 nm | 570 nm | 630 nm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Abs. | RGR/% | Grade | Abs. | RGR/% | Grade | Abs. | RGR/% | Grade | |
| Pristine cedar substrate without melamine coating | 0.1090 | 32.09 | 4 | 0.5287 | 25.82 | 4 | 0.4643 | 33.27 | 3 |
| Cedar floor with melamine coating (no antibacterial agent) | 0.2221 | 68.45 | 2 | 0.9921 | 60.43 | 2 | 0.4619 | 33.10 | 3 |
| Prepared antibacterial floor | 0.2052 | 63.25 | 2 | 0.8943 | 54.45 | 2 | 0.4618 | 32.89 | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, C.; Zhang, Y.; Cui, J.; Gan, L. The Antibacterial Properties and Safety of a Nanoparticle-Coated Parquet Floor. Coatings 2019, 9, 403. https://doi.org/10.3390/coatings9060403
Jia C, Zhang Y, Cui J, Gan L. The Antibacterial Properties and Safety of a Nanoparticle-Coated Parquet Floor. Coatings. 2019; 9(6):403. https://doi.org/10.3390/coatings9060403
Chicago/Turabian StyleJia, Chong, Yang Zhang, Juqing Cui, and Lu Gan. 2019. "The Antibacterial Properties and Safety of a Nanoparticle-Coated Parquet Floor" Coatings 9, no. 6: 403. https://doi.org/10.3390/coatings9060403
APA StyleJia, C., Zhang, Y., Cui, J., & Gan, L. (2019). The Antibacterial Properties and Safety of a Nanoparticle-Coated Parquet Floor. Coatings, 9(6), 403. https://doi.org/10.3390/coatings9060403