Detection of Volatile Organic Compounds Using Surface Acoustic Wave Sensor Based on Nanoparticles Incorporated in Polymer
Abstract
:1. Introduction
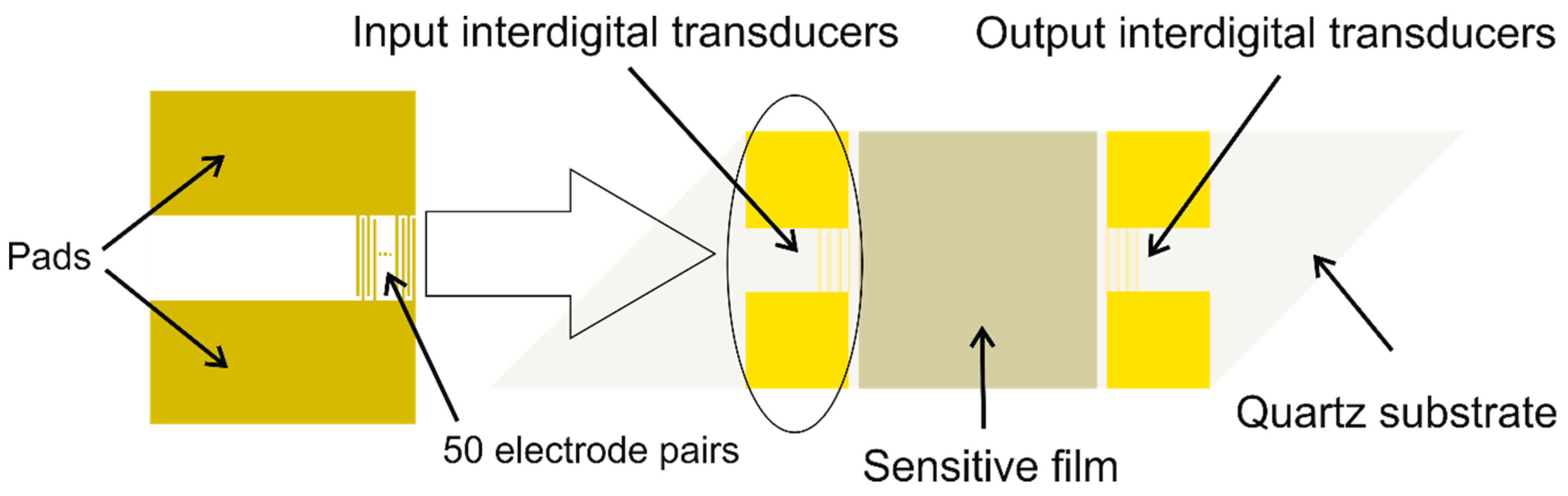
2. Materials and Methods
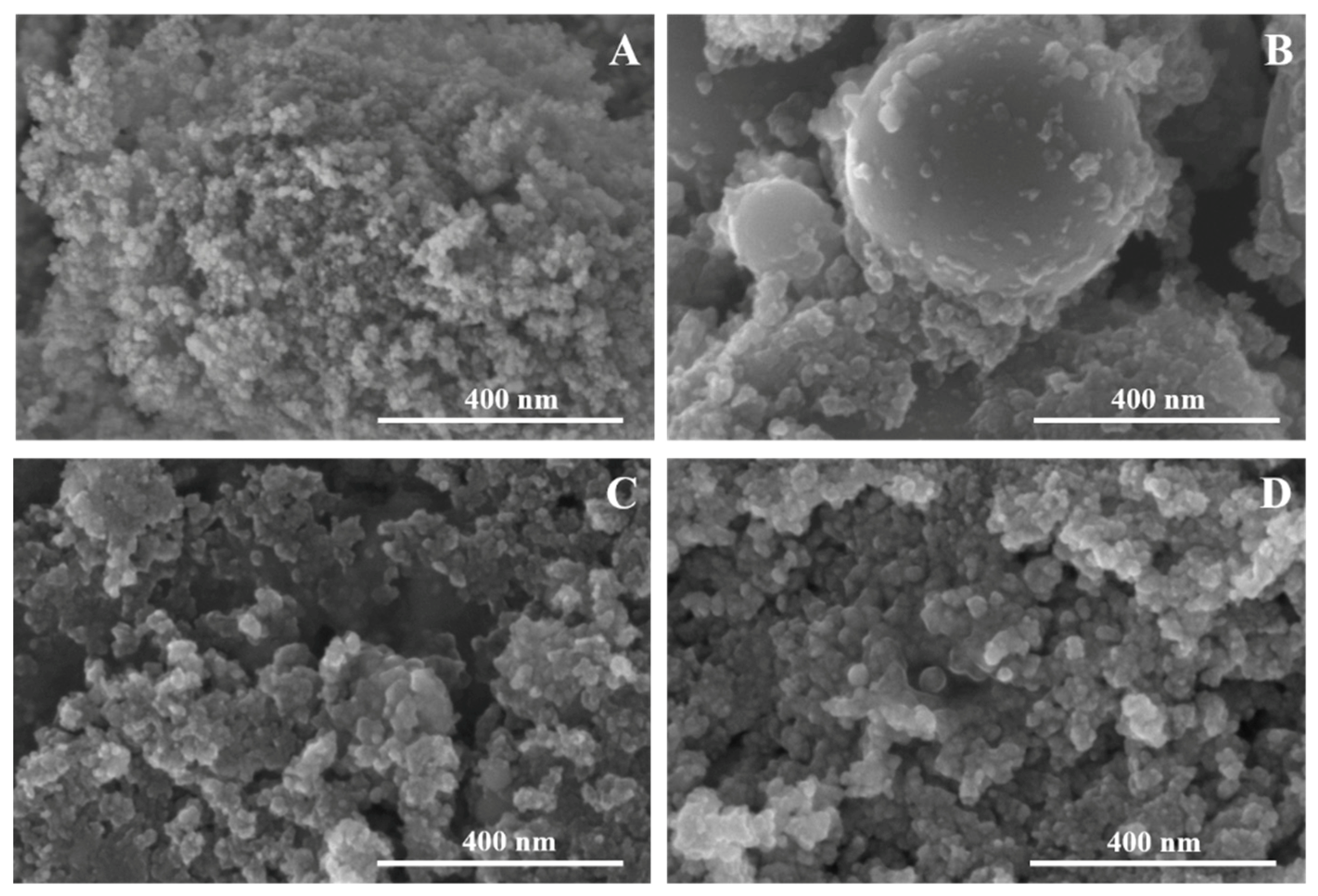
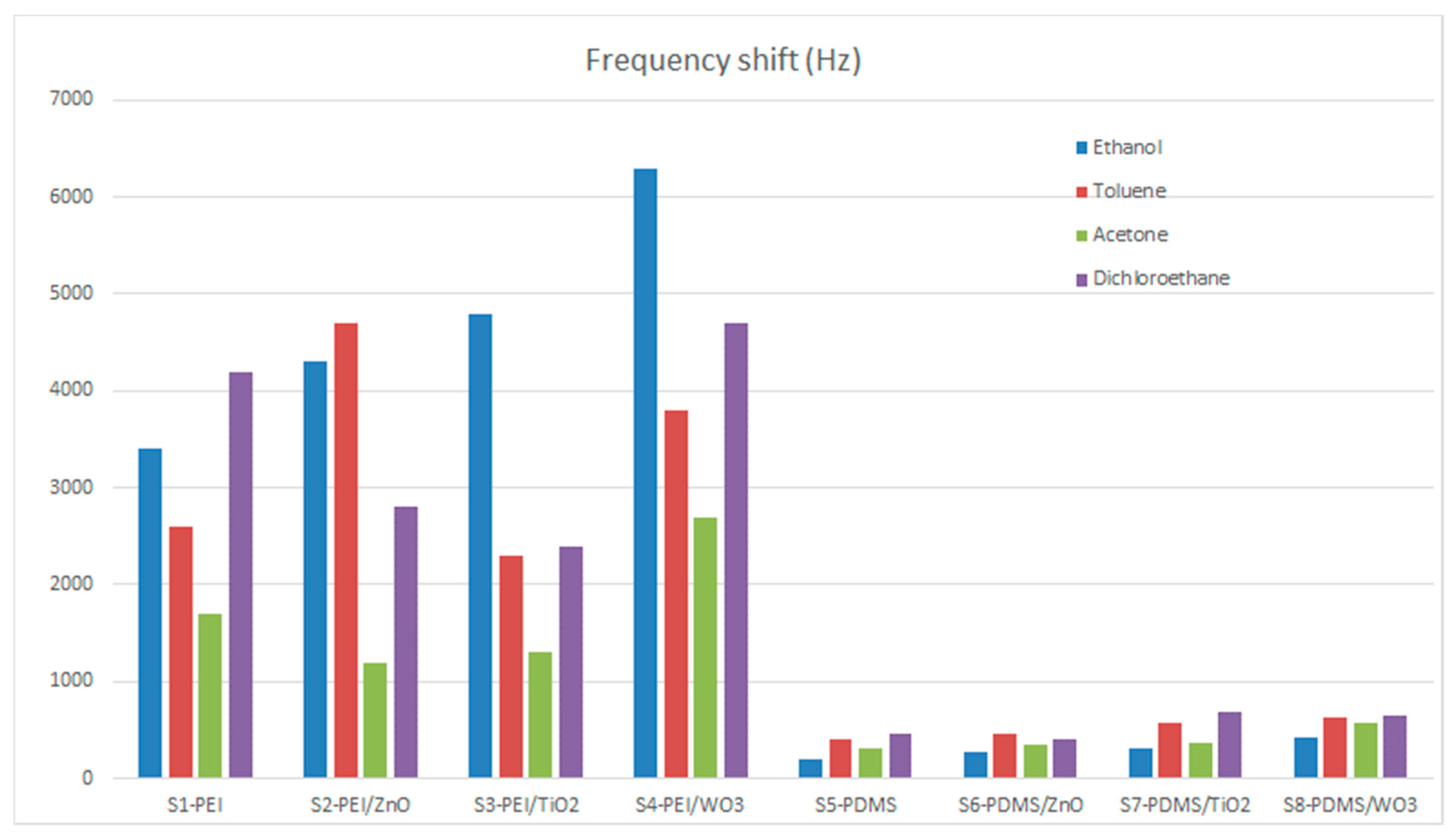
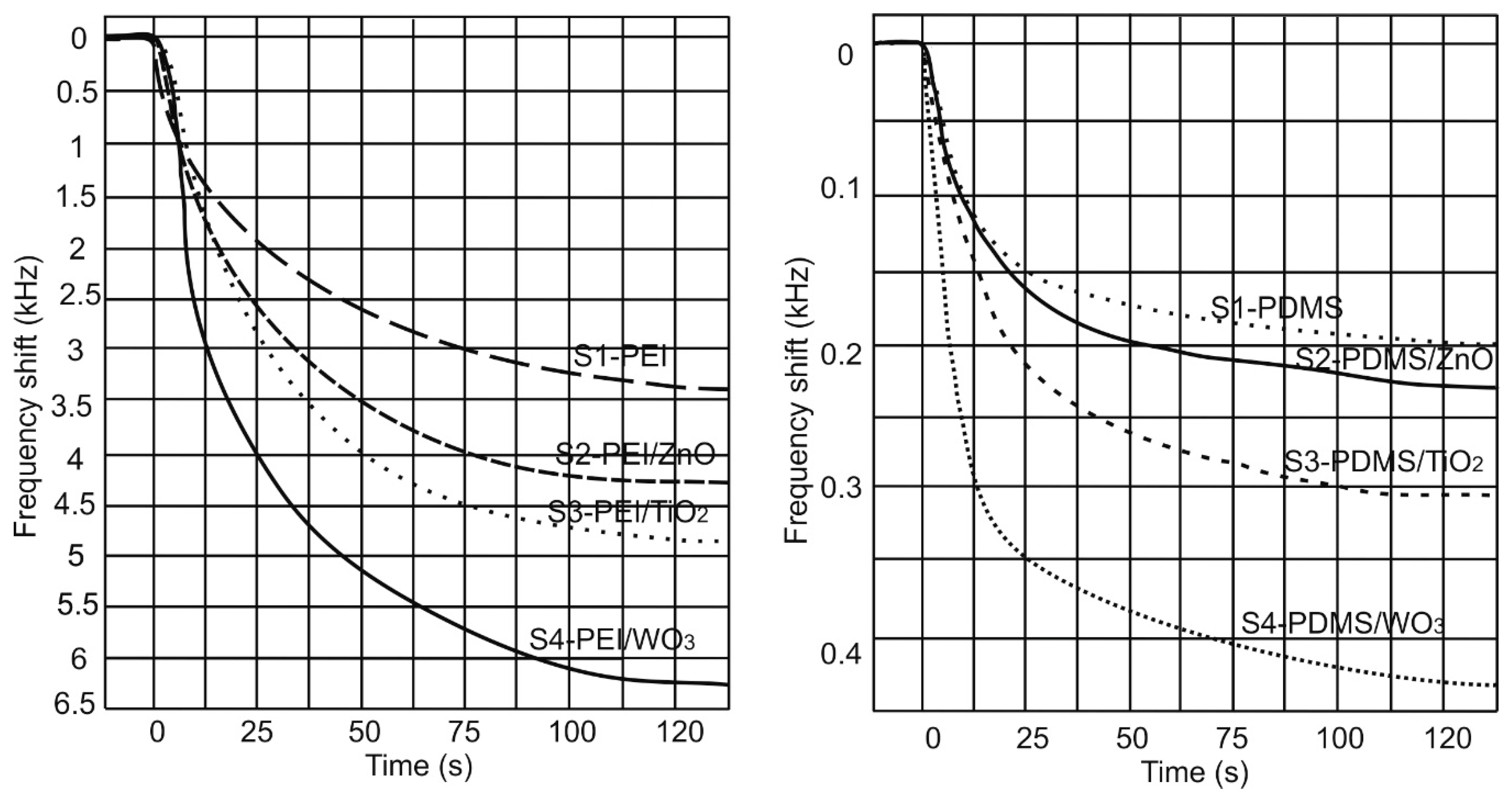
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, Q.; Wang, Q.; Li, Y.; Ning, P.; Tian, S. Influence of volatile organic compounds (VOCs) on pulmonary surfactant monolayers at air-water interface: Implication for the pulmonary health. Colloids Surf. A-Physicochem. Eng. Asp. 2019, 562, 402–408. [Google Scholar] [CrossRef]
- Duffy, E.; Morrin, A. Endogenous and microbial volatile organic compounds in cutaneous health and disease. Trac-Trends Anal. Chem. 2019, 111, 163–172. [Google Scholar] [CrossRef]
- Cincinelli, A.; Martellini, T. Indoor air quality and health. Int. J. Environ. Res. Public Health 2017, 14, 1286. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Tang, N.; Qu, H.; Liu, J.; Zhang, D.; Zhang, H.; Pang, W.; Duan, X. Detection of volatile organic compounds by self-assembled monolayer coated sensor array with concentration-independent fingerprints. Sci. Rep. 2016, 6, 23970. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, S.; Homayoonnia, S.; Homayoonnia, G. Comparative investigation of interdigitated and parallel-plate capacitive gas sensors based on Cu-BTC nanoparticles for selective detection of polar and apolar VOCs indoors. Sens. Actuator B–Chem. 2019, 278, 153–164. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, T.; Dong, Y.; Wang, X. A room temperature VOCs gas sensor based on a layer by layer multi-walled carbon nanotubes/poly-ethylene glycol composite. Sensors 2018, 18, 3113. [Google Scholar] [CrossRef]
- Szulczynski, B.; Gebicki, J. Currently commercially available chemical sensors employed for detection of volatile organic compounds in outdoor and indoor air. Environments 2017, 4, 21. [Google Scholar] [CrossRef]
- Sadaoka, Y.; Mori, M. Detection of VOC in air with a planar-type potentiometric gas sensor based on YSZ with a Pt electrode modified with TiO2. Sens. Actuator B–Chem. 2017, 248, 878–885. [Google Scholar] [CrossRef]
- Viespe, C.; Miu, D. Characteristics of surface acoustic wave sensors with nanoparticles embedded in polymer sensitive layers for VOC detection. Sensors 2018, 18, 2401. [Google Scholar] [CrossRef]
- Viespe, C. Surface acoustic wave sensors based on nanoporous films for hydrogen detection. Key Eng. Mater. 2014, 605, 331–334. [Google Scholar] [CrossRef]
- Devkota, J.; Ohodnicki, P.R.; Greve, D.W. SAW sensors for chemical vapors and gases. Sensors 2017, 17, 801. [Google Scholar] [CrossRef] [PubMed]
- Marcu, A.; Viespe, C. Surface acoustic wave sensors for Hydrogen and Deuterium detection. Sensors 2017, 17, 1417. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Choi, S.J.; Park, K.M.; Lee, S.S.; Choi, S.; Kim, I.D.; Park, C.O. The stability, sensitivity and response transients of ZnO, SnO2 and WO3 sensors under acetone, toluene and H2S environments. Sens. Actuator B–Chem. 2014, 197, 300–307. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures—Based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Viespe, C.; Miu, D. Surface acoustic wave sensor with Pd/ZnO bilayer structure for room temperature Hydrogen detection. Sensors 2017, 17, 1529. [Google Scholar] [CrossRef]
- Benetti, M.; Cannatà, D.; Verona, E.; Palla Papavlu, A.; Dinca, V.C.; Lippert, T.; Dinescu, M.; Di Pietrantonio, F. Highly selective surface acoustic wave e-nose implemented by laser direct writing. Sens. Actuator B–Chem. 2019, 283, 154–162. [Google Scholar] [CrossRef]
- Torino, S.; Conte, L.; Iodice, M.; Coppola, G.; Prien, R.D. PDMS membranes as sensing element in optical sensors for gas detection in water. Sens. Biosensing Res. 2017, 16, 74–78. [Google Scholar] [CrossRef]
- Kim, M.; Osone, S.; Kim, T.; Higashi, H.; Seto, T. Synthesis of nanoparticles by laser ablation: A review. KONA Powder Part. J. 2017, 34, 80–90. [Google Scholar] [CrossRef]
- De Vero, J.C.; Jasmin, A.C.; Dasallas, L.L.; Garcia, W.O.; Sarmago, R.V. Synthesis of iron oxide nanostructures via carbothermal reaction of Fe microspheres generated by infrared pulsed laser ablation. Coatings 2019, 9, 179. [Google Scholar] [CrossRef]
- Vidap, R.V.; Mathe, V.L.; Shahane, G.S. Structural, optical and gas sensing properties of spin coated nanocrystalline zinc oxide thin films. J. Mater. Sci.–Mater. Electron 2013, 24, 3170–3174. [Google Scholar] [CrossRef]
- Ballantine, D.S.; White, R.M.; Martin, S.J.; Rico, A.J.; Frye, G.C.; Zellers, E.T.; Wohltjen, H. Acoustic Wave Sensors: Theory, Design, and Physico-Chemical Applications; Academic Press: San Diego, CA, USA, 1997; pp. 31–35. [Google Scholar]
- Viespe, C.; Grigoriu, C. Surface acoustic wave sensors with carbon nanotubes and SiO2/Si nanoparticles based nanocomposites for VOC detection. Sens. Actuator B–Chem. 2010, 147, 43–47. [Google Scholar] [CrossRef]
- Zhao, Q.; He, Z.; Jiang, Y.; Yuan, Z.; Wu, H.; Su, C.; Tai, H. Enhanced acetone-sensing properties of PEI thin film by GO-NH2 functional groups modification at room temperature. Front. Mater. 2019, 5, 82. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Yang, J.; Guo, W.; Zhu, L.; Zheng, W. Temperature and acidity effects on WO3 nanostructures and gas-sensing properties of WO3 nanoplates. Mater. Res. Bull. 2014, 57, 260–267. [Google Scholar] [CrossRef]
- Wei, S.; Zhao, G.; Du, W.; Tian, Q. Synthesis and excellent acetone sensing properties of porous WO3 nanofibers. Vacuum 2016, 124, 32–39. [Google Scholar] [CrossRef]

| Sensor Name | Polymer | Solvent for Polymers | NP |
|---|---|---|---|
| S1 | PEI | Ethanol | − |
| S2 | PEI | Ethanol | ZnO |
| S3 | PEI | Ethanol | TiO2 |
| S4 | PEI | Ethanol | WO3 |
| S5 | PDMS | Toluene | − |
| S6 | PDMS | Toluene | ZnO |
| S7 | PDMS | Toluene | TiO2 |
| S8 | PDMS | Toluene | WO3 |
| SAW | Ra (nm) | Rq (nm) |
|---|---|---|
| S1-PEI | 41 | 52 |
| S2-PEI/ZnO | 23 | 30 |
| S3-PEI/TiO2 | 79 | 101 |
| S4-PEI/WO3 | 24 | 31 |
| S5-PDMS | 20 | 28 |
| S6-PDMS/ZnO | 16 | 23 |
| S7-PDMS/TiO2 | 40 | 50 |
| S8-PDMS/WO3 | 19 | 25 |
| SAW Sensors | Ethanol | Toluene | Acetone | Dichloroethane | Noise Level Hz | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity Δf/c Hz/ppm | LOD ppm | Sensitivity Δf/c Hz/ppm | LOD ppm | Sensitivity Δf/c Hz/ppm | LOD ppm | Sensitivity Δf/c Hz/ppm | LOD ppm | ||
| S1-PEI | 4.25 | 17 | 3.25 | 22 | 2.13 | 34 | 5.25 | 14 | 24 |
| S2-PEI/ZnO | 5.38 | 9 | 5.88 | 9 | 1.50 | 34 | 3.50 | 15 | 17 |
| S3-PEI/TiO2 | 6.00 | 19 | 2.88 | 39 | 1.63 | 68 | 3.00 | 37 | 37 |
| S4- PEI/WO3 | 7.88 | 6 | 4.75 | 11 | 3.38 | 15 | 5.88 | 9 | 17 |
| S5-PDMS | 0.25 | 240 | 0.50 | 120 | 0.38 | 160 | 0.56 | 107 | 20 |
| S6-PDMS/ZnO | 0.34 | 133 | 0.56 | 80 | 0.44 | 103 | 0.50 | 90 | 15 |
| S7-PDMS/TiO2 | 0.39 | 232 | 0.73 | 124 | 0.46 | 195 | 0.85 | 106 | 30 |
| S8-PDMS/WO3 | 0.54 | 84 | 0.78 | 58 | 0.71 | 63 | 0.81 | 55 | 15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantinoiu, I.; Viespe, C. Detection of Volatile Organic Compounds Using Surface Acoustic Wave Sensor Based on Nanoparticles Incorporated in Polymer. Coatings 2019, 9, 373. https://doi.org/10.3390/coatings9060373
Constantinoiu I, Viespe C. Detection of Volatile Organic Compounds Using Surface Acoustic Wave Sensor Based on Nanoparticles Incorporated in Polymer. Coatings. 2019; 9(6):373. https://doi.org/10.3390/coatings9060373
Chicago/Turabian StyleConstantinoiu, Izabela, and Cristian Viespe. 2019. "Detection of Volatile Organic Compounds Using Surface Acoustic Wave Sensor Based on Nanoparticles Incorporated in Polymer" Coatings 9, no. 6: 373. https://doi.org/10.3390/coatings9060373
APA StyleConstantinoiu, I., & Viespe, C. (2019). Detection of Volatile Organic Compounds Using Surface Acoustic Wave Sensor Based on Nanoparticles Incorporated in Polymer. Coatings, 9(6), 373. https://doi.org/10.3390/coatings9060373






