Pre-Coated Fe–Ni Film to Promote Low-Pressure Carburizing of 14Cr14Co13Mo4 Steel
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. LPC Parameters and Heat Treatment Conditions
2.3. Testing and Analysis Methods
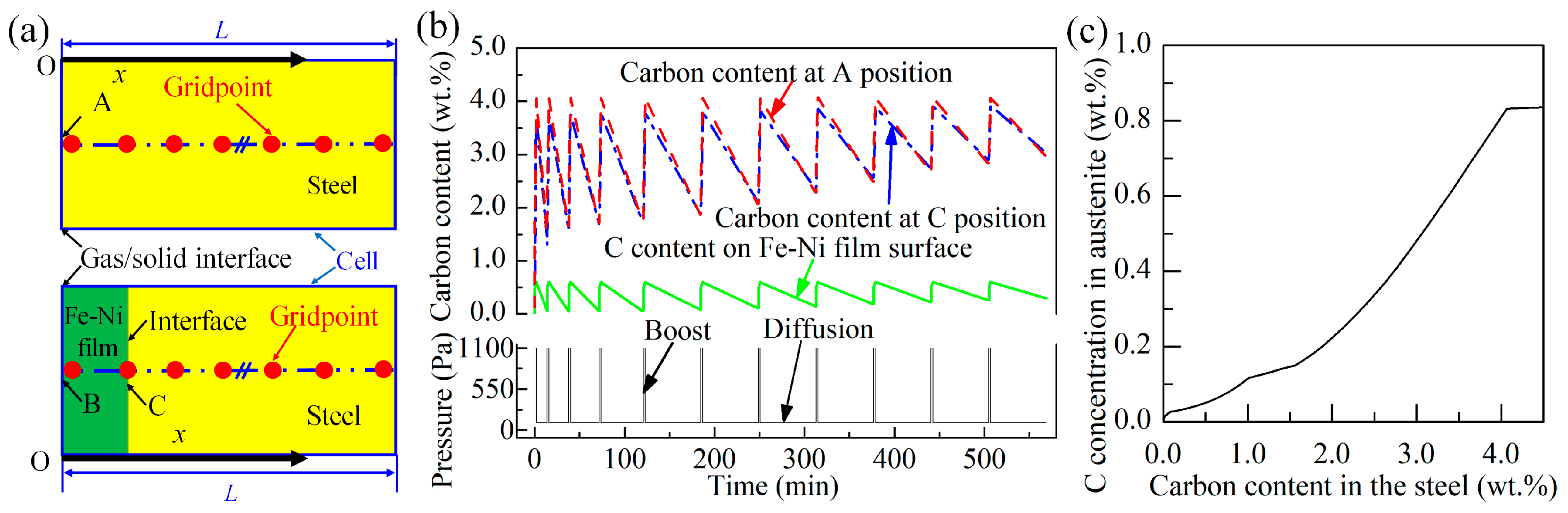
2.4. Experimental Design Principle
3. Results
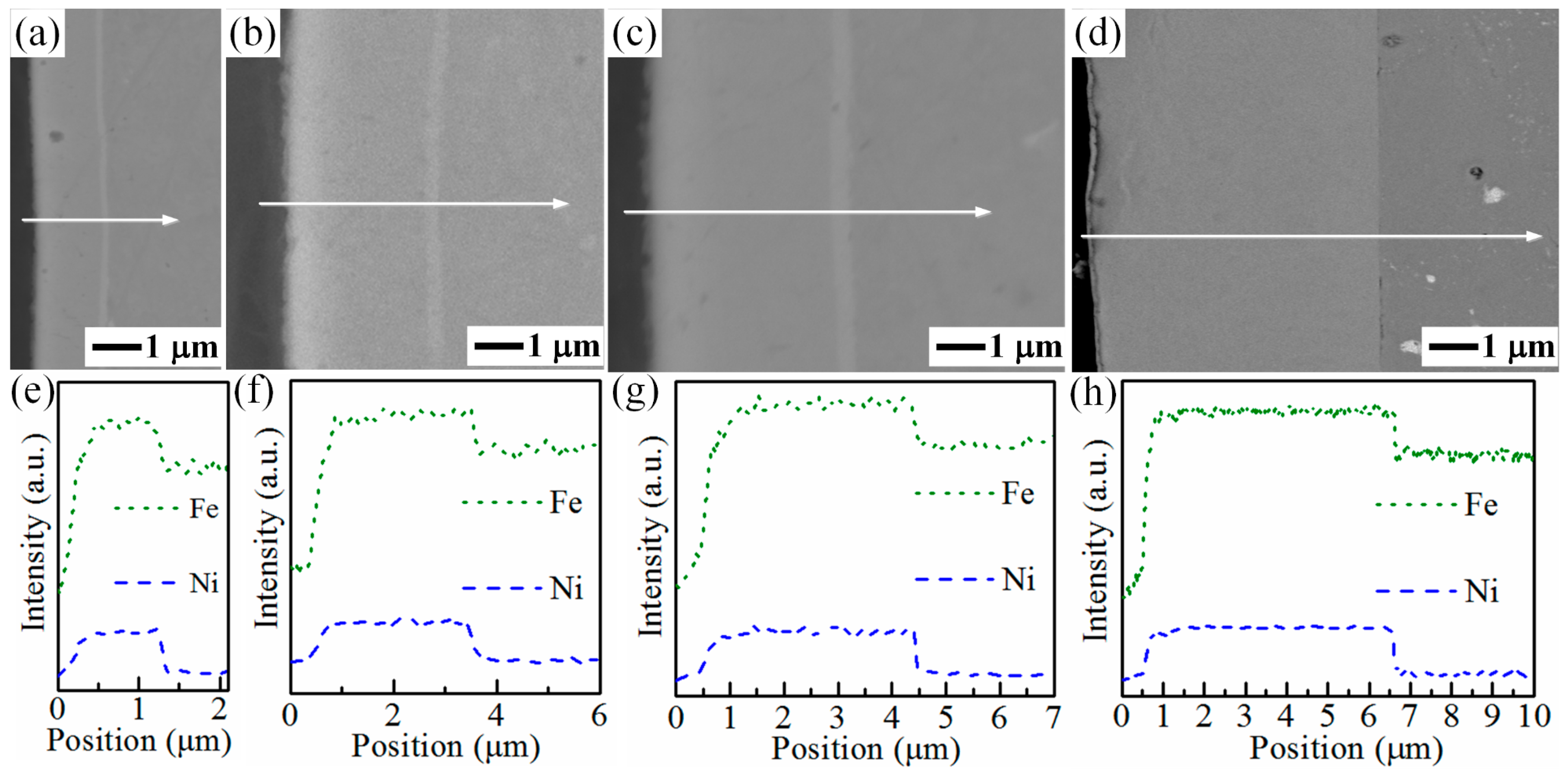
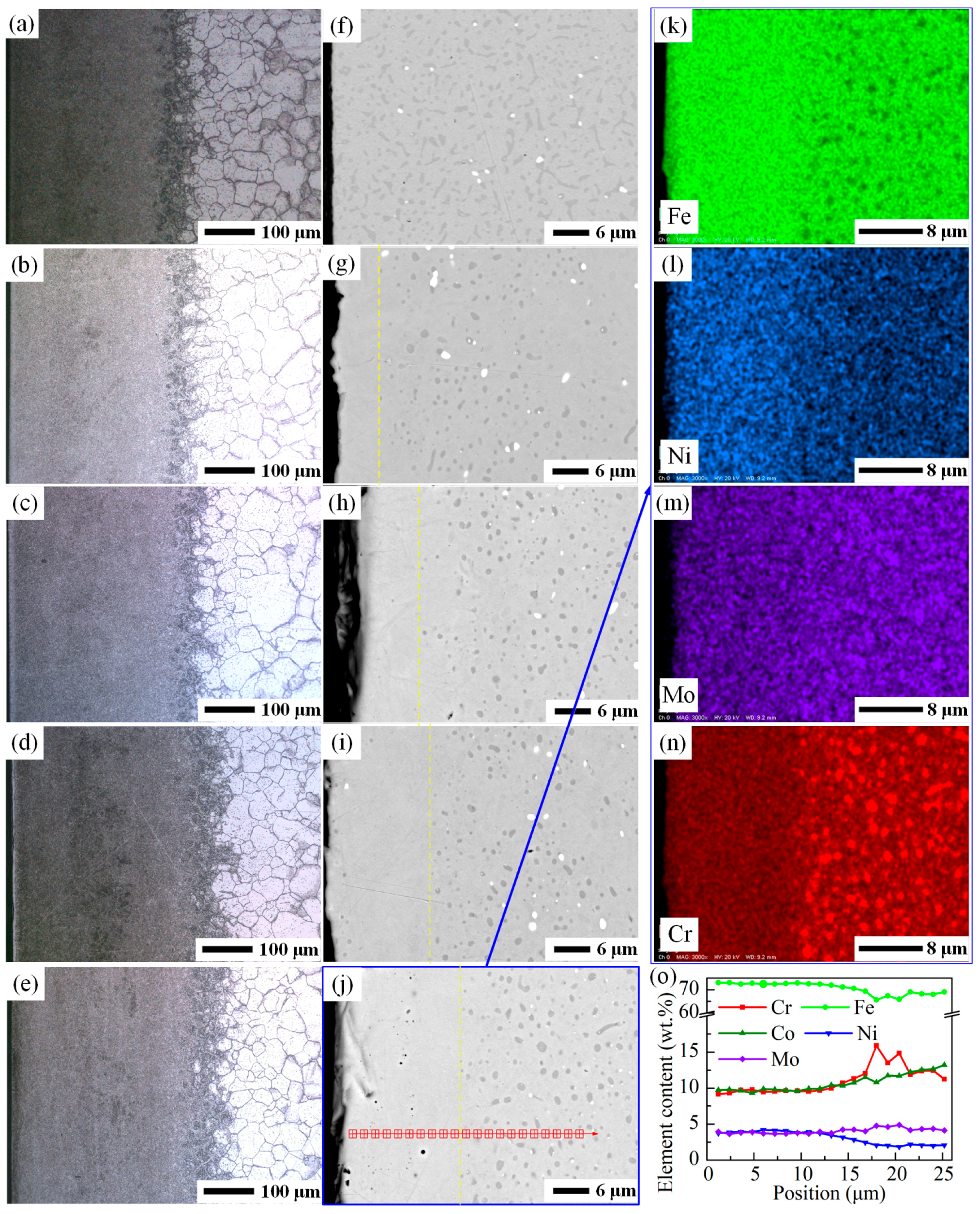
3.1. Characterization of the Fe–Ni Film
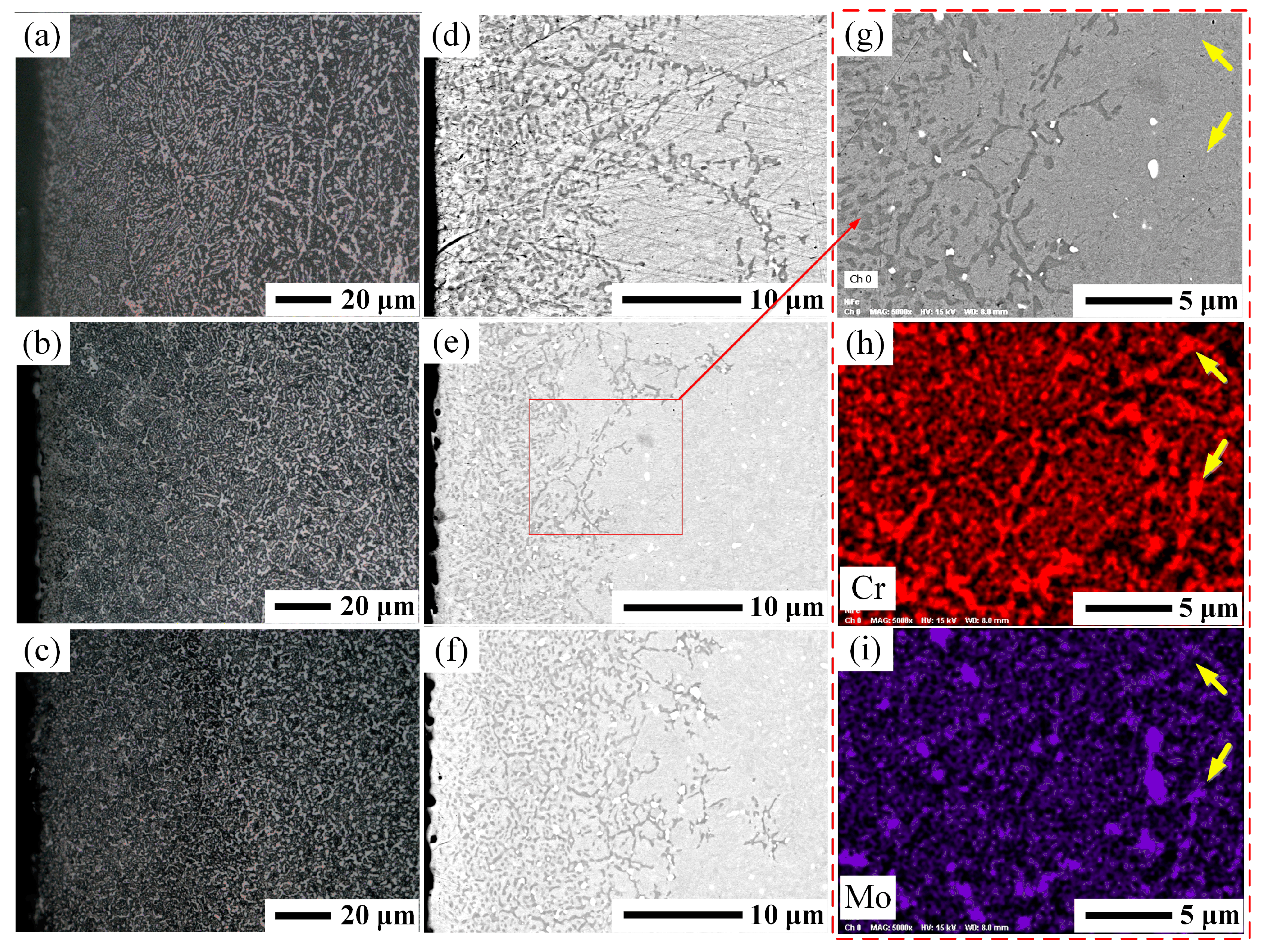
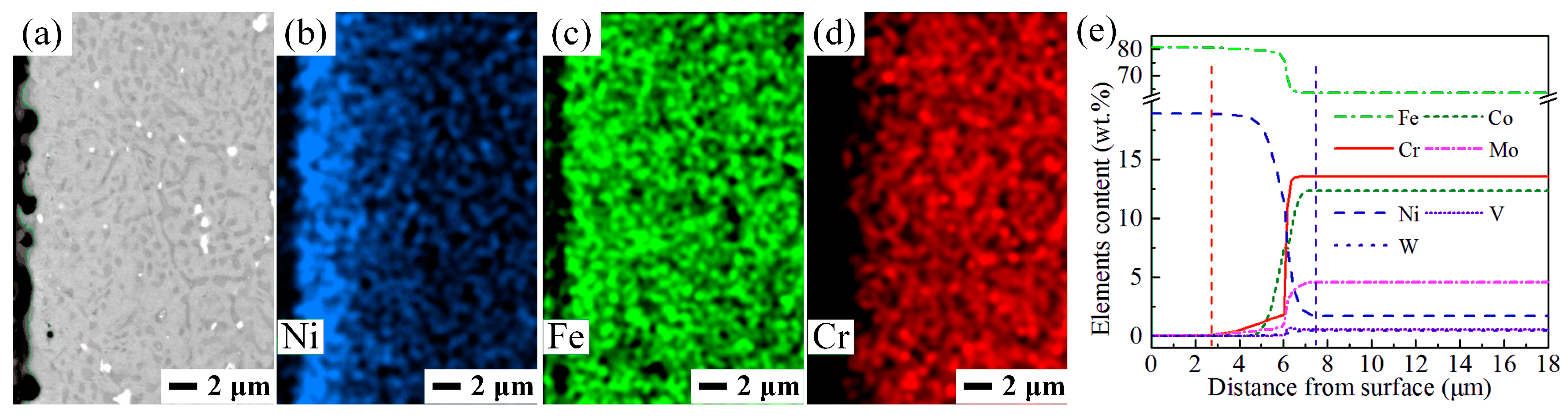
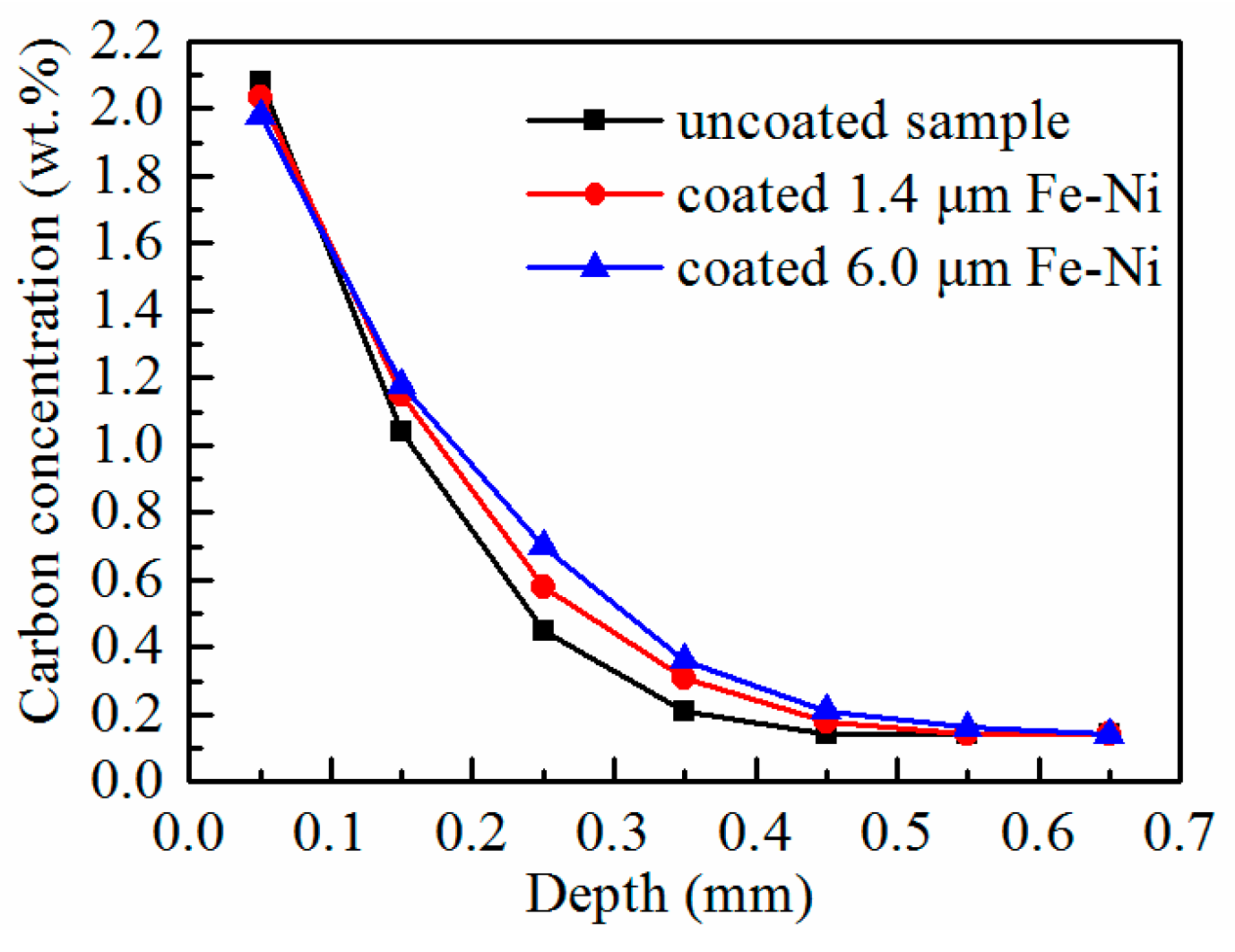
3.2. Characterization of the As-Carburized Layer
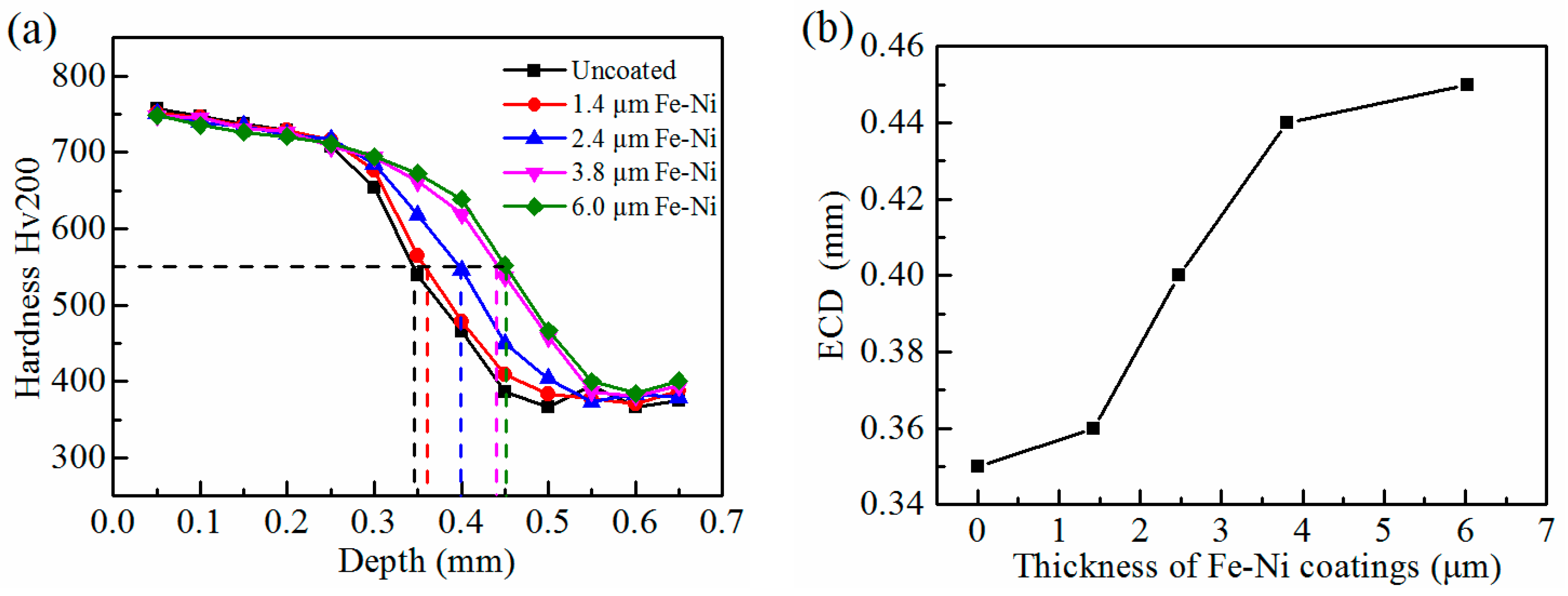
3.3. Characterization of the Carburized Layers after Hardening Treatment
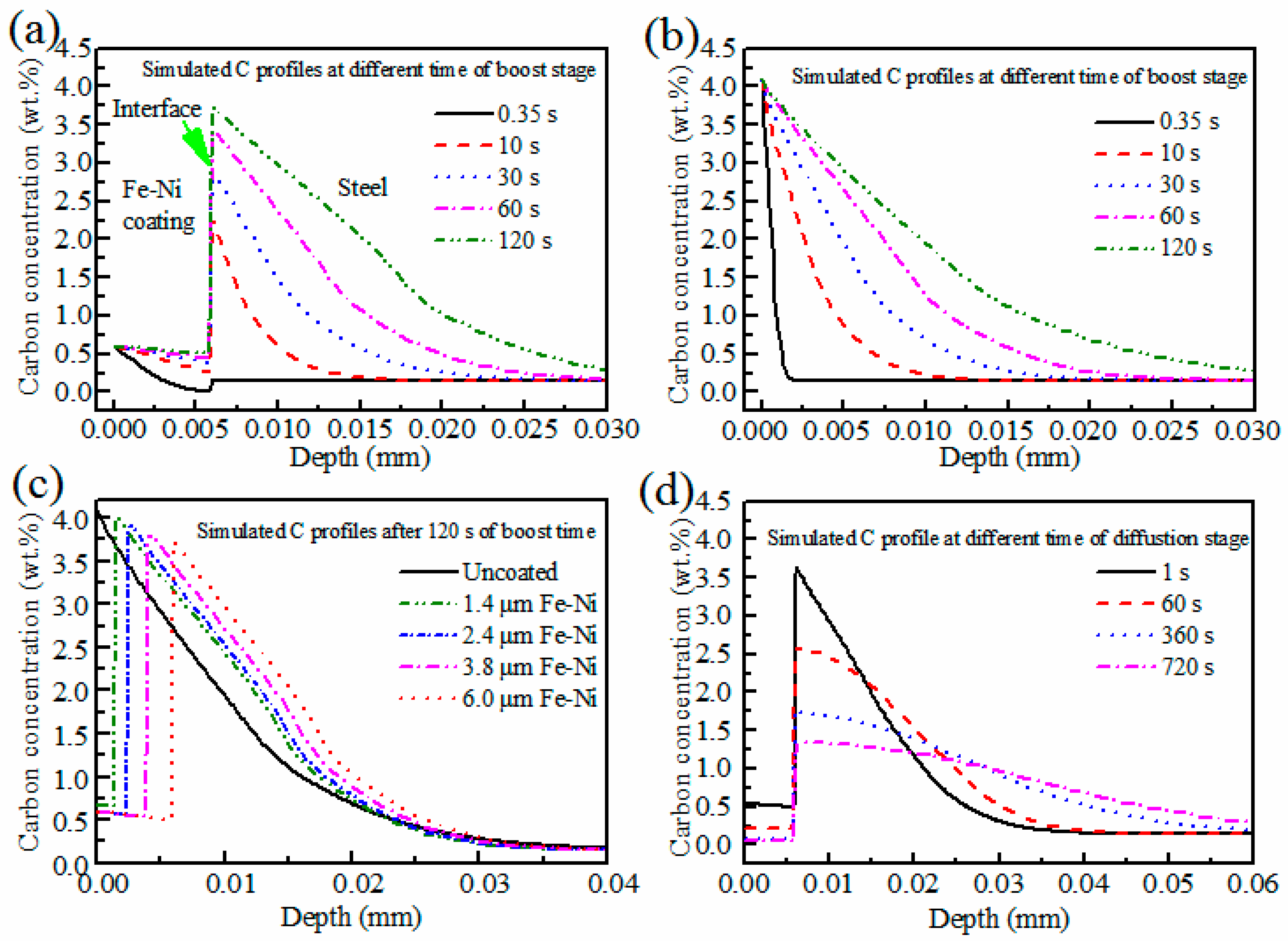
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhadeshia, H.K.D.H. Steels for bearings. Prog. Mater. Sci. 2012, 57, 268–435. [Google Scholar] [CrossRef]
- Trivedi, H.F.; Otto, F.; McCoy, B.; Bhattacharya, S.R.; Piazza, T. heat treatment process for martensitic stainless steel pyrowear 675 for improved corrosion resistance. In STP1580-EB Bearing Steel Technologies: 10th Volume, Advances in Steel Technologies for Rolling Bearings; Beswick, J., Ed.; ASTM International: West Conshohocken, PA, USA, 2015; pp. 465–484. [Google Scholar]
- Tomasello, C.M.; Burrrier, H.I.; Knepper, R.A.; Balliett, S.; Maloney, J.L. Progress in the evaluation of CSS-42LTM: A high performance bearing alloy. In STP1419-EB Bearing Steel Technology; Beswich, J.M., Ed.; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Ryzhov, N.M.; Smirnov, A.E.; Fakhurtdinov, R.S.; Mulyakaev, L.M.; Gromov, V.I. Special features of vacuum carburizing of heat-resistant steel in acetylene. Metal Sci. Heat Treat. 2004, 46, 230–235. [Google Scholar] [CrossRef]
- Wolowiec-Korecka, E.; Korecki, M.; Klimek, L.; Kula, P.; Brewka, A.; Buczek, M. Low-pressure carburizing in a large-chamber device for high-performance and precision thermal treatment of parts of mechanical gear. Adv. Sci. Technol. Res. J. 2017, 11, 253–258. [Google Scholar] [CrossRef]
- Yada, K.; Watanabe, O. Reactive flow simulation of vacuum carburizing by acetylene gas. Comput. Fluids 2013, 79, 65–76. [Google Scholar] [CrossRef]
- Rokicki, P.; Dychton, K. Acetylene flow rate as a crucial parameter of vacuum carburizing process of modern tool steels. Arch. Metall. Mater. 2016, 61, 2009–2012. [Google Scholar] [CrossRef]
- Kula, P.; Pietrasik, R.; Dybowski, K. Vacuum carburizing-process optimization. J. Mater. Process. Technol. 2005, 164–165, 876–881. [Google Scholar] [CrossRef]
- Gorockiewicz, R.; Łapiński, A. Structure of the carbon layer deposited on the steel surface after low pressure carburizing. Vacuum 2010, 85, 429–433. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, L.; Sisson, R.D., Jr. Modeling of carbon concentration profile development during both atmosphere and low pressure carburizing processes. J. Mater. Eng. Perform. 2013, 22, 1886–1891. [Google Scholar] [CrossRef]
- Gronkiewicz, R. The kinetics of low-pressure carburizing of alloy steels. Vacuum 2011, 86, 448–451. [Google Scholar] [CrossRef]
- Kula, P.; Dybowski, K.; Wolowiec, E.; Pietrasik, R. “Boost-diffusion” vacuum carburizing—Process optimization. Vacuum 2014, 99, 175–179. [Google Scholar] [CrossRef]
- Zajusz, M.; Tkacz-Śmiech, K.; Danielewski, M. Modeling of vacuum pulse carburizing of steel. Surf. Coat. Technol. 2014, 258, 646–651. [Google Scholar] [CrossRef]
- Yin, L.; Ma, X.; Tang, G.; Fu, Z.; Yang, S.; Wang, T.; Wang, L.; Li, L. Characterization of carburized 14Cr14Co13Mo4 stainless steel by low pressure carburizing. Surf. Coat. Technol. 2019, 358, 654–660. [Google Scholar] [CrossRef]
- Turpin, T.; Dulcy, J.; Gantois, M. Carbon diffusion and phase transformations during gas carburizing of high-alloyed stainless steels: Experimental study and theoretical modeling. Metall. Mater. Trans. A 2005, 36, 2751–2760. [Google Scholar] [CrossRef]
- Hetzner, D.W.; Van Geertruyden, W. Crystallography and metallography of carbides in high alloy steels. Mater. Charact. 2008, 59, 825–841. [Google Scholar] [CrossRef]
- Rowan, O.K.; Sisson, R.D., Jr. Effect of alloy composition on carburizing performance of steel. J. Phase Equilib. Diffus. 2009, 30, 235–241. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, M.; Xiao, J.; Dong, J.; Du, C. Investigations on carburizing mechanisms of Cr35Ni45Nb subjected to different service conditions in a high-temperature vacuum environment. J. Mater. Res. 2015, 30, 841–851. [Google Scholar] [CrossRef]
- Trillo, E.A.; Murr, L.E. A TEM investigation of M23C6 carbide precipitation behaviour on varying grain boundary misorientations in 304 stainless steels. J. Mater. Sci. 1998, 33, 1263–1271. [Google Scholar] [CrossRef]
- Kaya, A.A. Microstructure of HK40 alloy after high-temperature service in oxidizing/carburizing environment: II. Carburization and carbide transformations. Mater. Charact. 2002, 49, 23–34. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W. Microstructures, properties and high-temperature carburization resistances of HVOF thermal sprayed NiAl intermetallic-based alloy coatings. Surf. Coat. Technol. 2004, 183, 18–28. [Google Scholar] [CrossRef]
- Uribe, E.; Salas, O.; Melo-Máximo, D.V.; Hernández-Durán, P.E.; Oseguera, J.; Torres, R.D. Evolution of PVD Al oxide coatings in carburizing atmospheres at high temperature. Surf. Coat. Technol. 2015, 284, 2–8. [Google Scholar] [CrossRef]
- Melo-Máximo, D.V.; Murillo, A.E.; Salas, O.; Melo-Máximo, L.; Oseguera, J. Behavior of uncoated and coated pure Fe in metal dusting conditions. Surf. Coat. Technol. 2016, 308, 10–18. [Google Scholar] [CrossRef]
- Li, M.; Li, N.; Zhang, J.; Zhou, C. Inhibiting effect of Ni–Re interlayer between Ni–Al coating and steel substrate on interdiffusion and carburization. Surf. Coat. Technol. 2018, 337, 68–74. [Google Scholar] [CrossRef]
- Crafts, W.; Lamont, J.L. Secondary hardening of tempered martensitic alloy steel. Trans. AIME 1949, 180, 471. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, L.; Wang, T.; Ma, X.; Fu, Z.; Hao, G.; Li, L.; Wang, L. Pre-Coated Fe–Ni Film to Promote Low-Pressure Carburizing of 14Cr14Co13Mo4 Steel. Coatings 2019, 9, 304. https://doi.org/10.3390/coatings9050304
Yin L, Wang T, Ma X, Fu Z, Hao G, Li L, Wang L. Pre-Coated Fe–Ni Film to Promote Low-Pressure Carburizing of 14Cr14Co13Mo4 Steel. Coatings. 2019; 9(5):304. https://doi.org/10.3390/coatings9050304
Chicago/Turabian StyleYin, Longcheng, Tingjian Wang, Xinxin Ma, Zhongyuan Fu, Guodong Hao, Liuhe Li, and Liqin Wang. 2019. "Pre-Coated Fe–Ni Film to Promote Low-Pressure Carburizing of 14Cr14Co13Mo4 Steel" Coatings 9, no. 5: 304. https://doi.org/10.3390/coatings9050304
APA StyleYin, L., Wang, T., Ma, X., Fu, Z., Hao, G., Li, L., & Wang, L. (2019). Pre-Coated Fe–Ni Film to Promote Low-Pressure Carburizing of 14Cr14Co13Mo4 Steel. Coatings, 9(5), 304. https://doi.org/10.3390/coatings9050304




