Synergistic Effect of Ag and ZnO Nanoparticles on Polypyrrole-Incorporated Epoxy/2pack Coatings and Their Corrosion Performances in Chloride Solutions
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Mechanical Properties
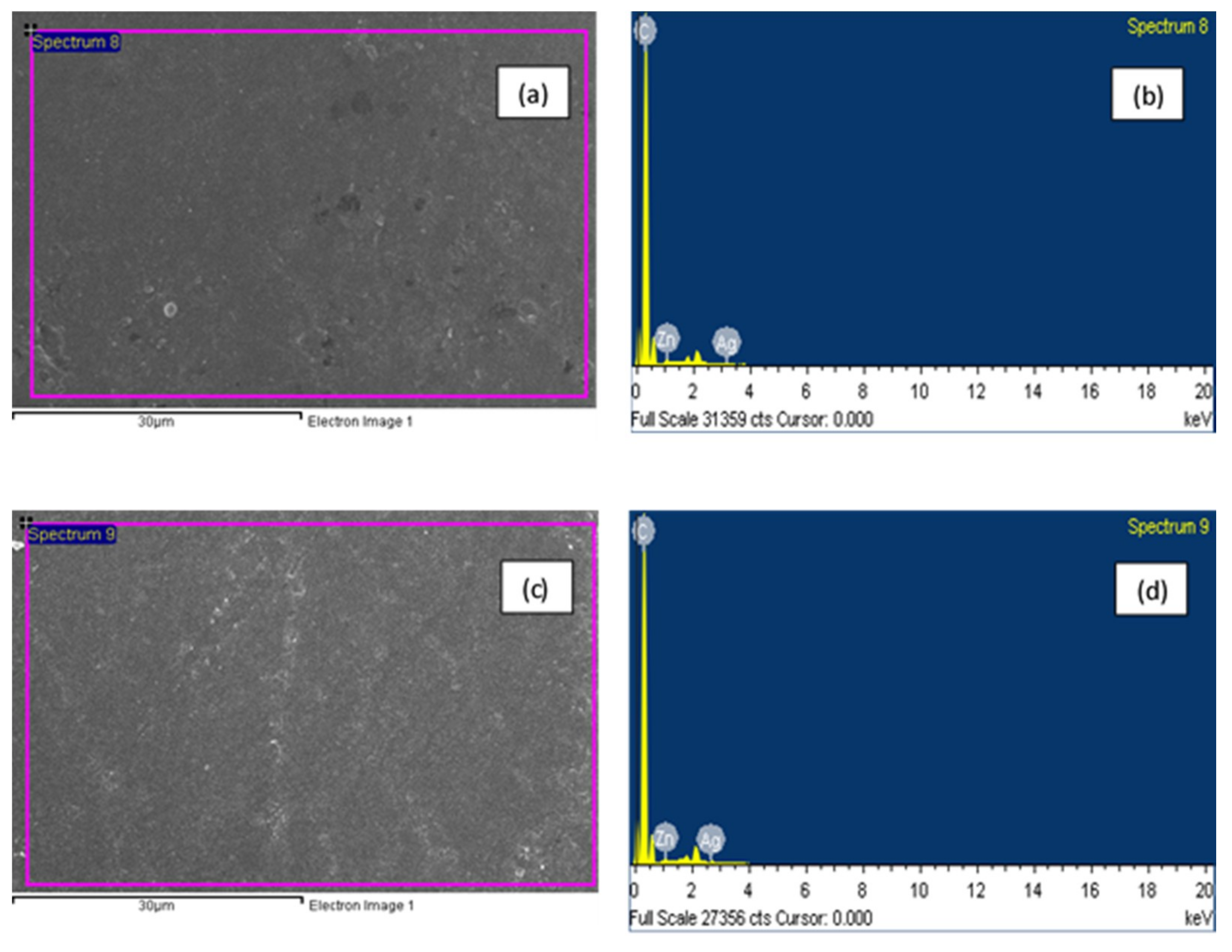
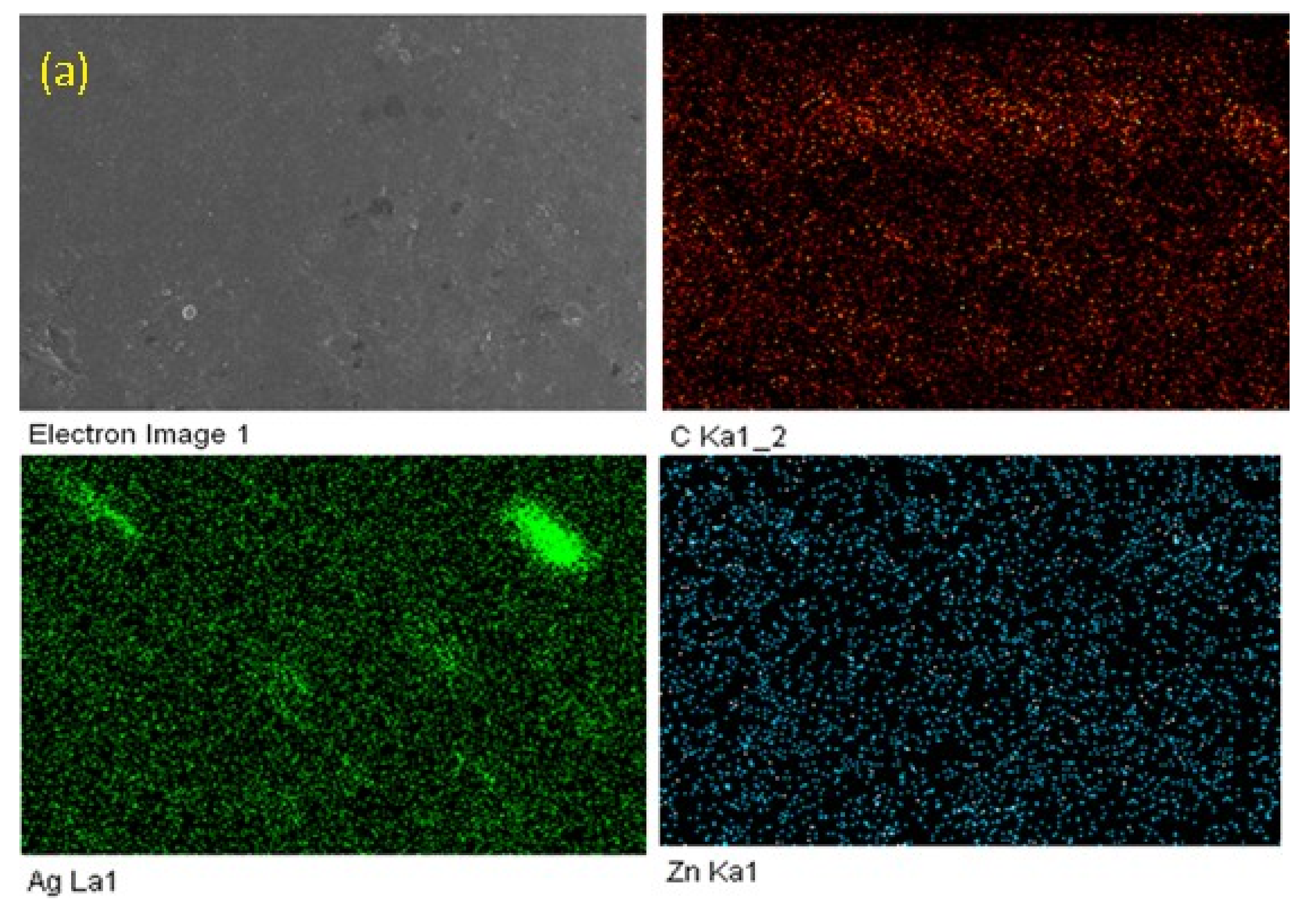
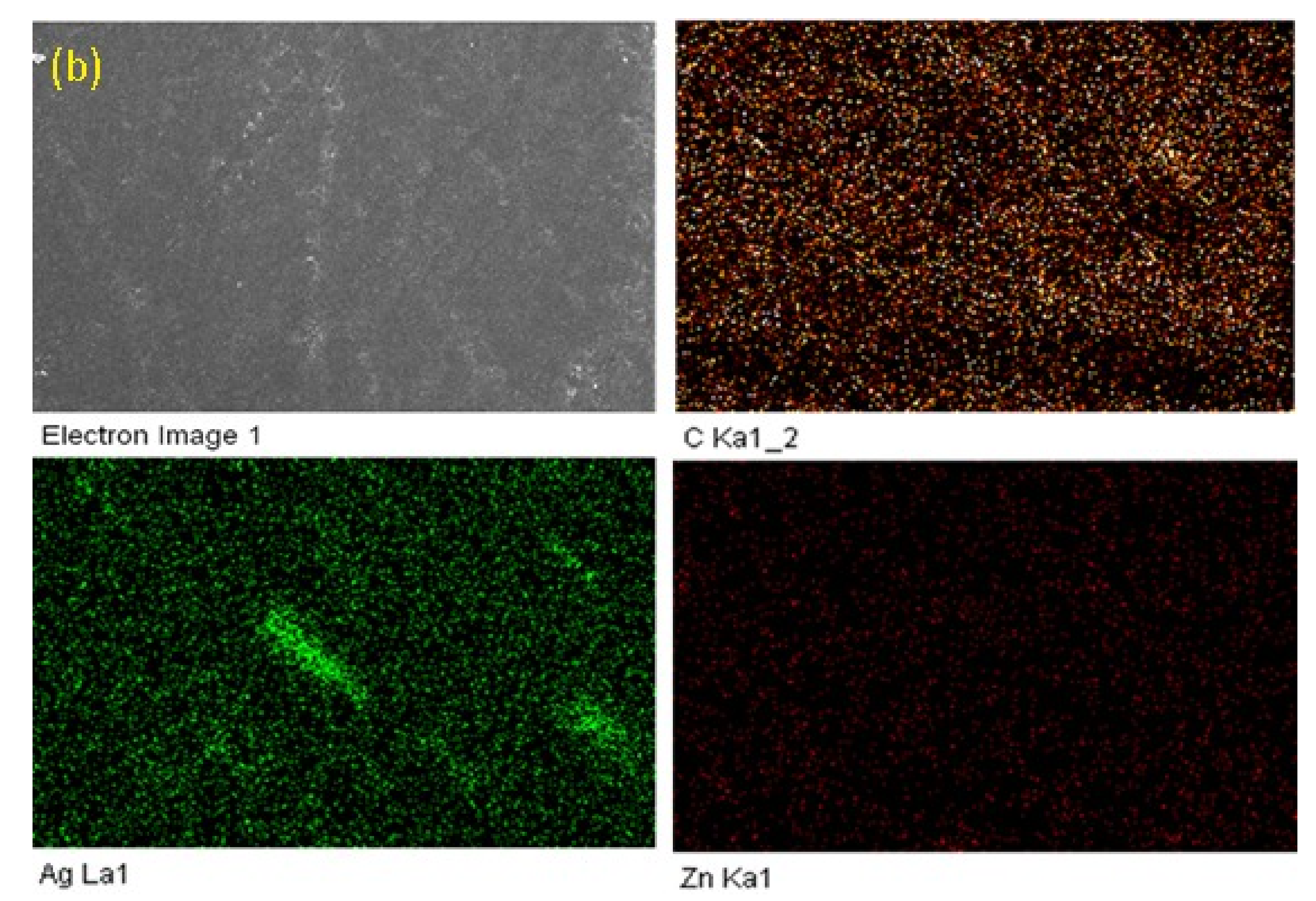
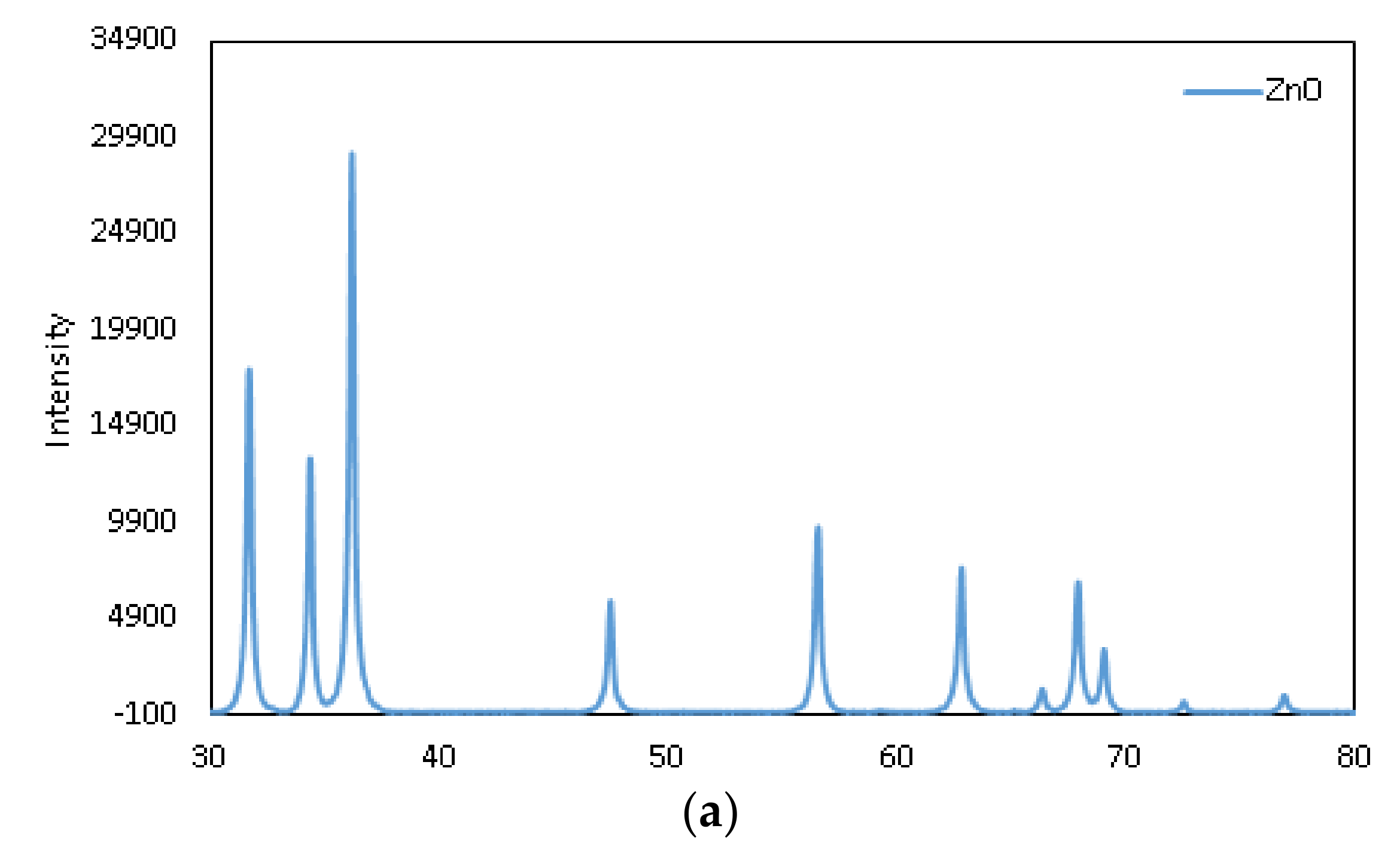
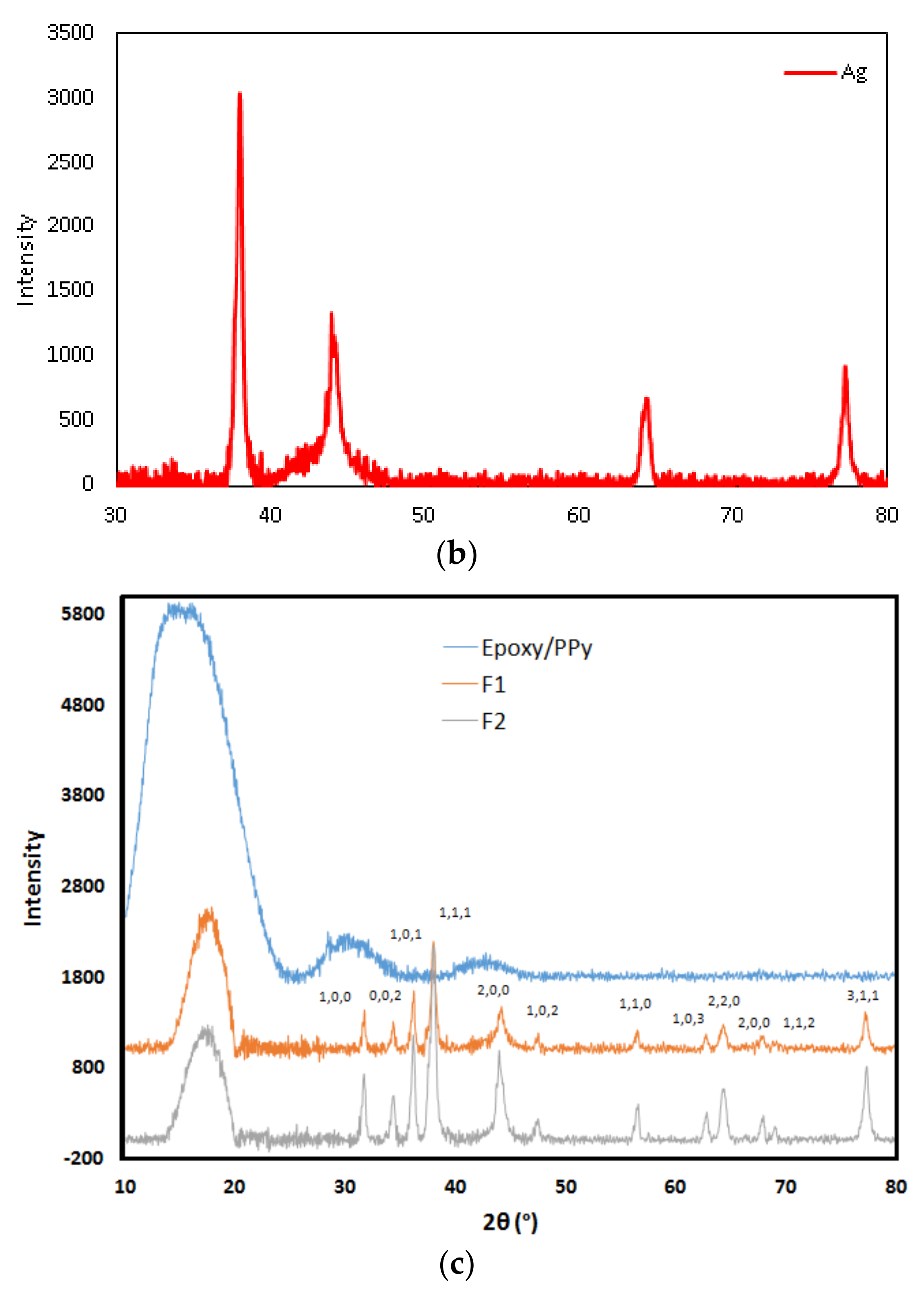
3.2. Surface Morphology and X-ray Differaction (XRD) Spectroscopy
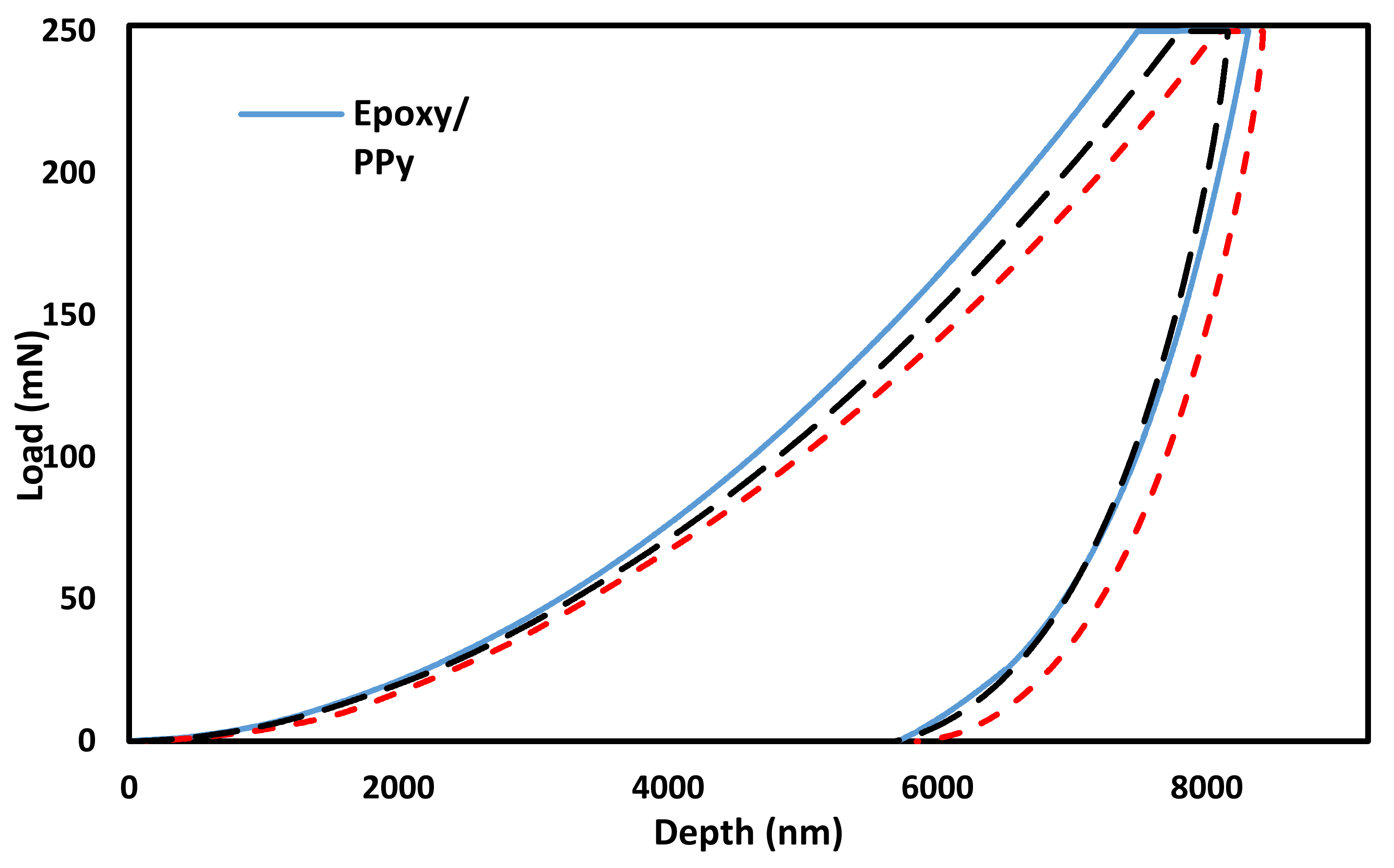
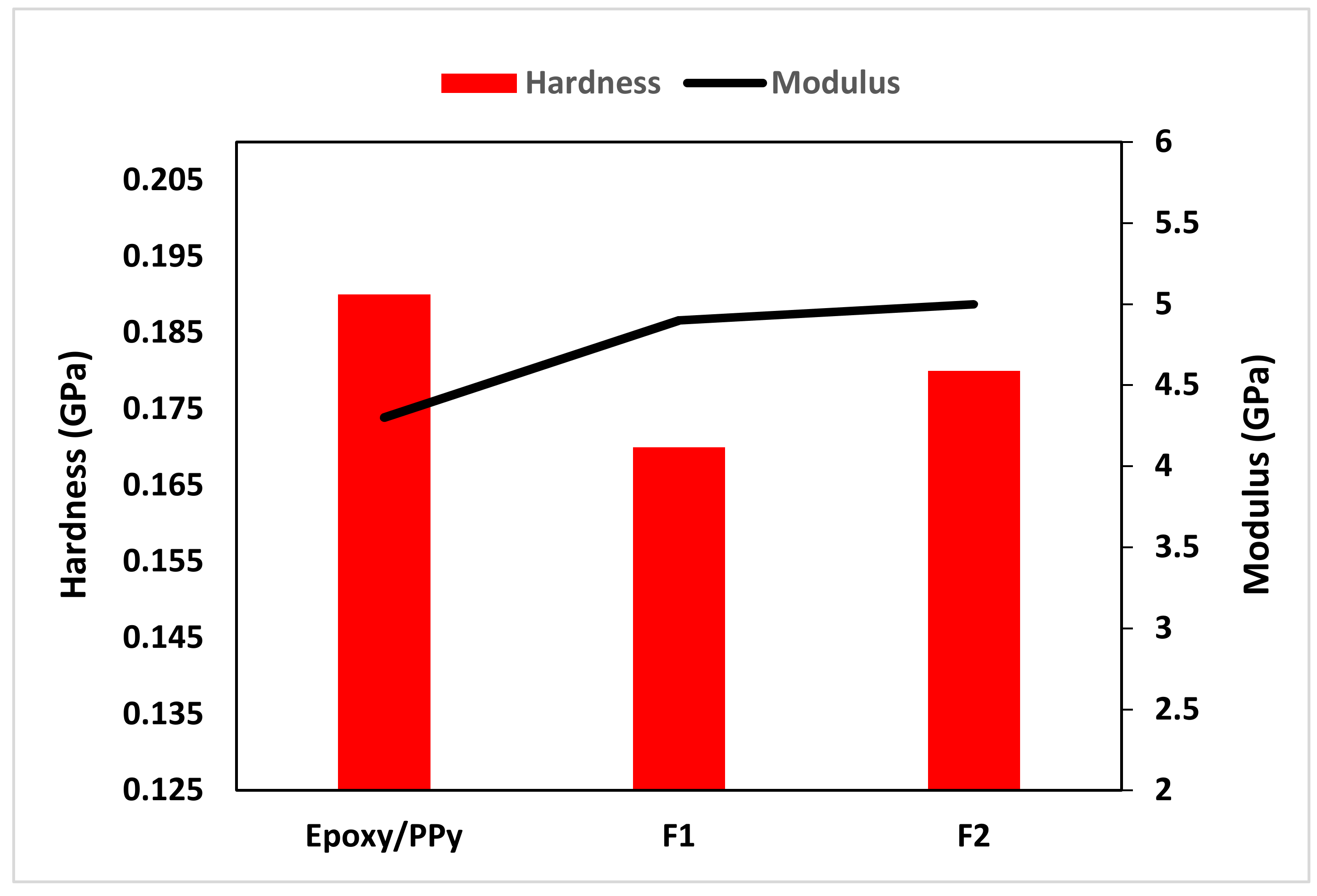
3.3. Nanoindentation Analysis
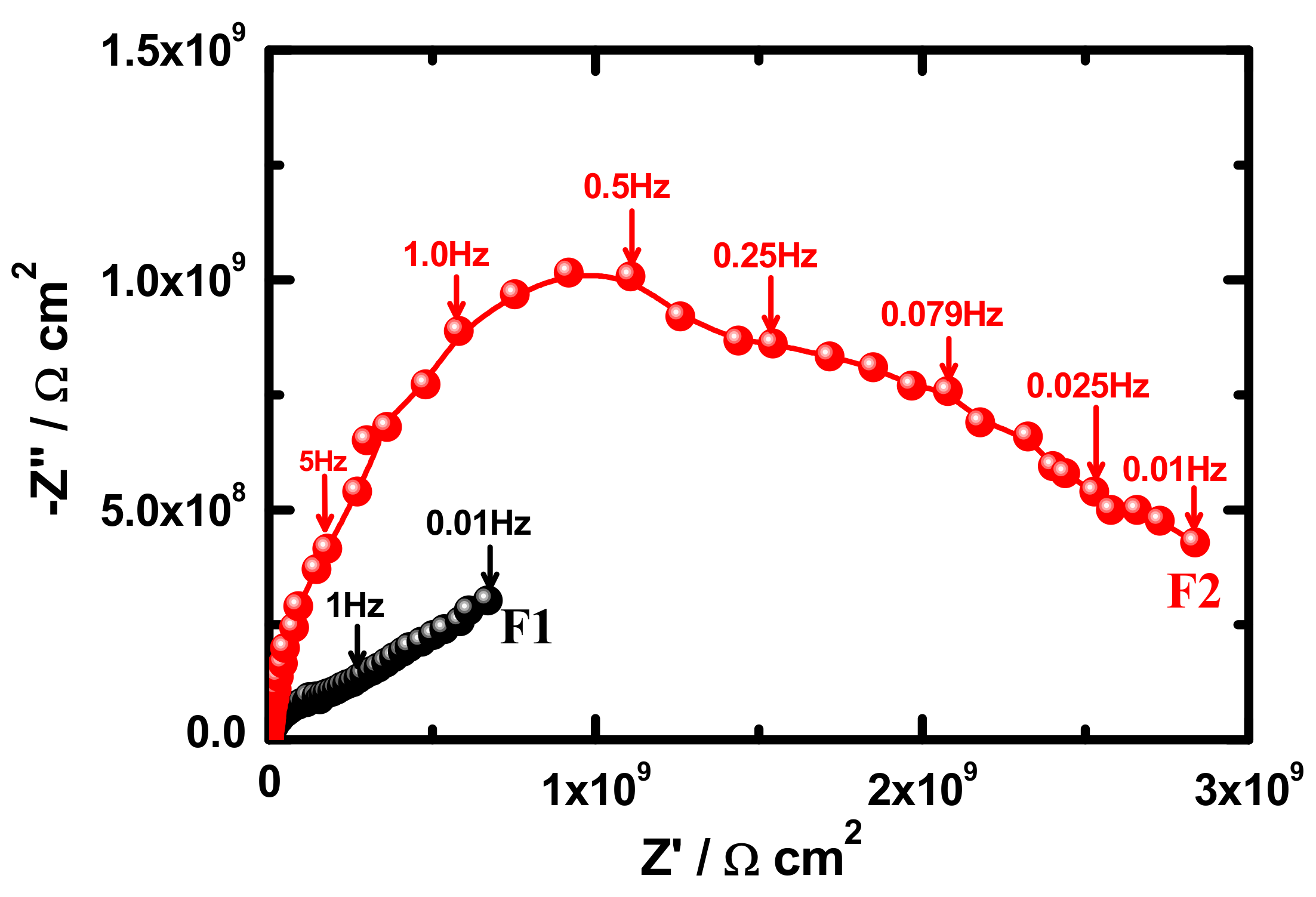
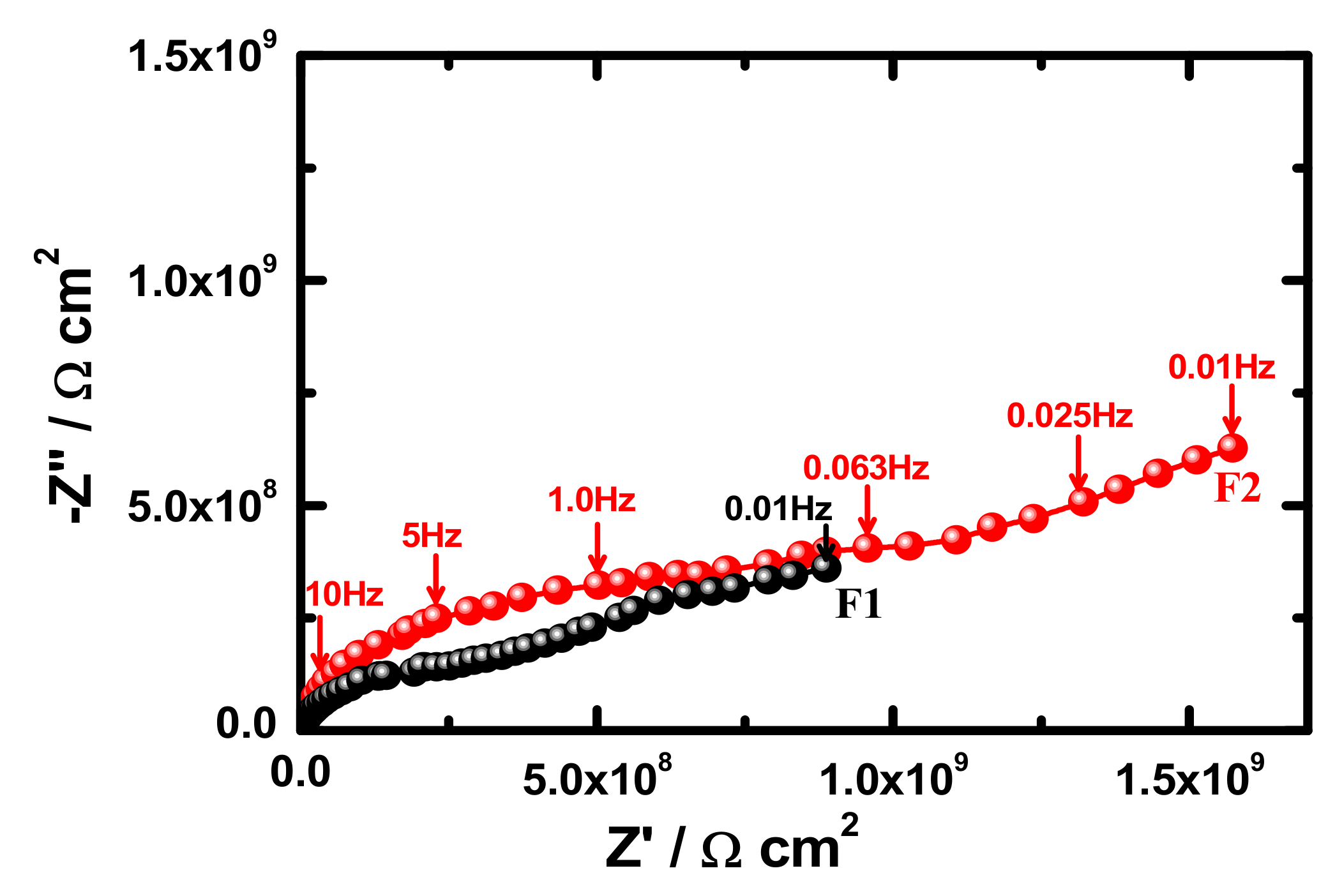
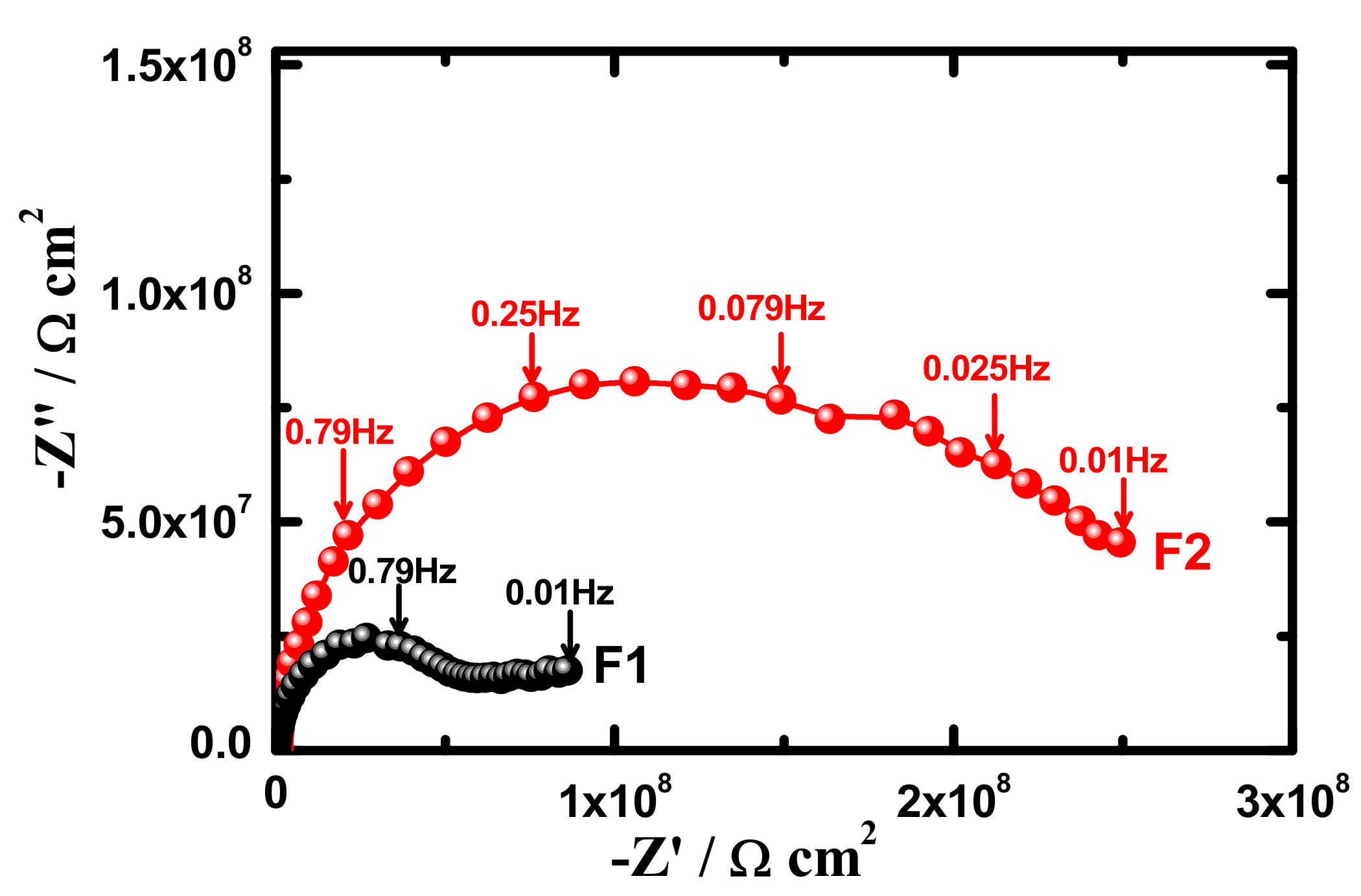
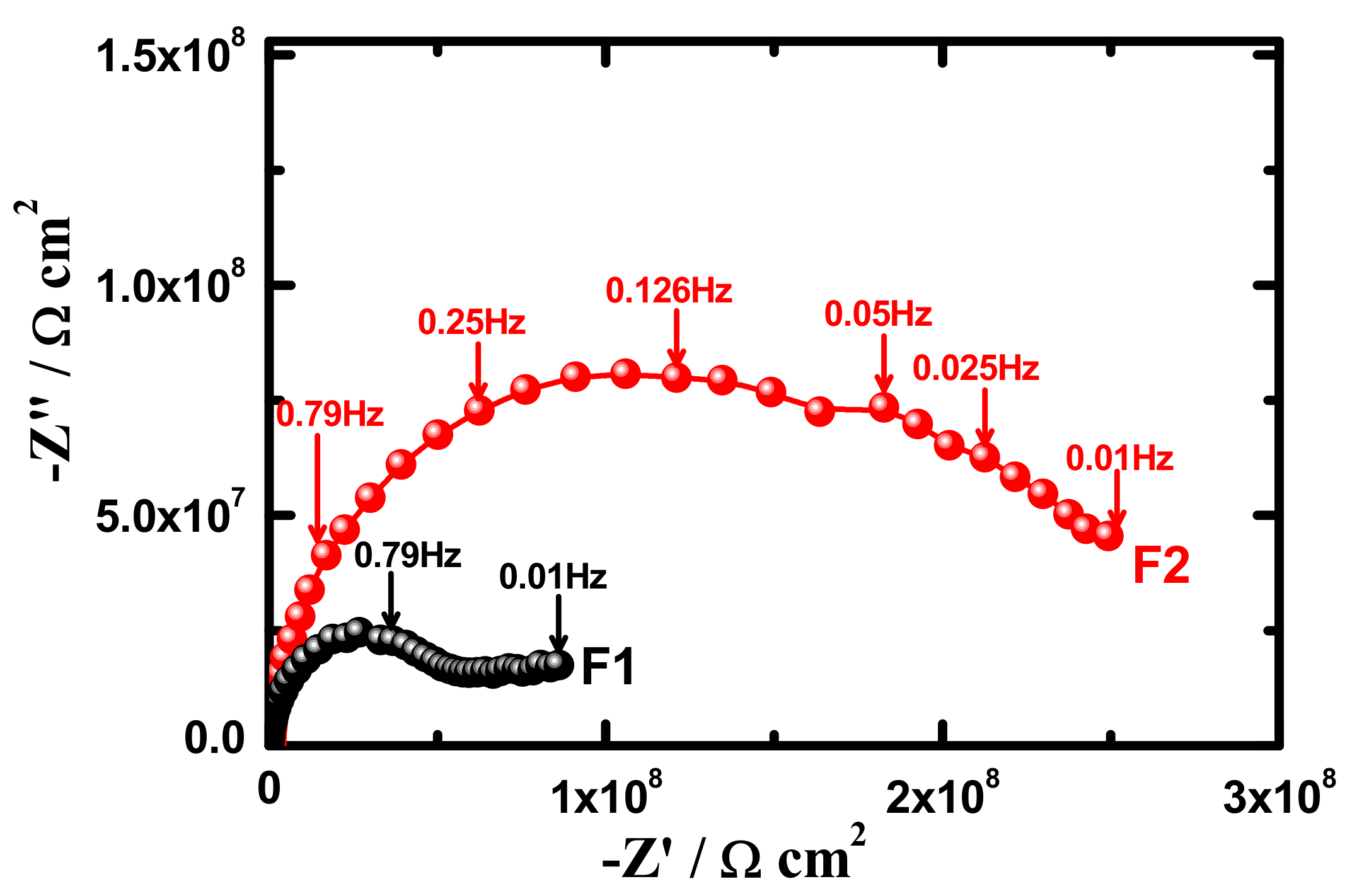
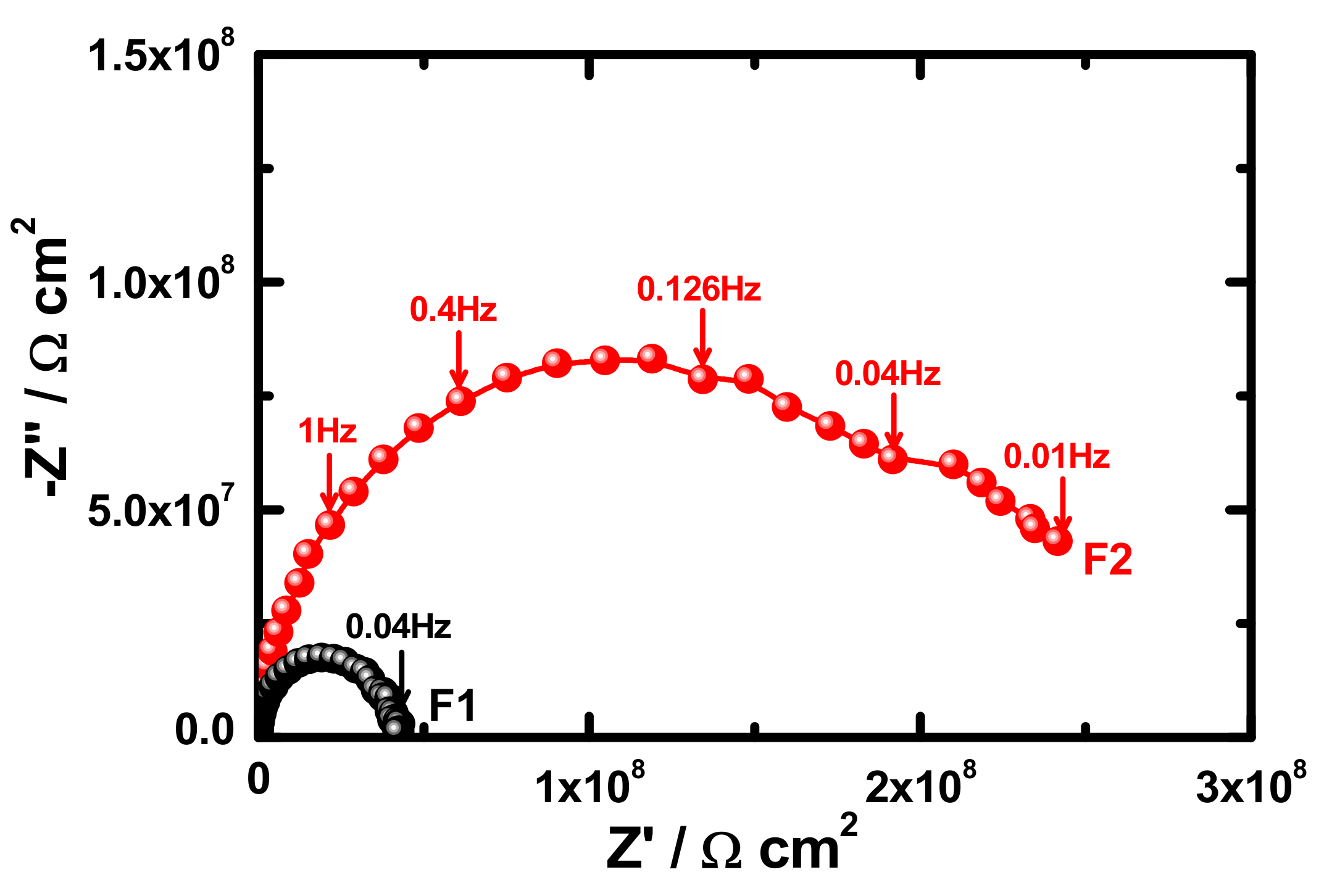
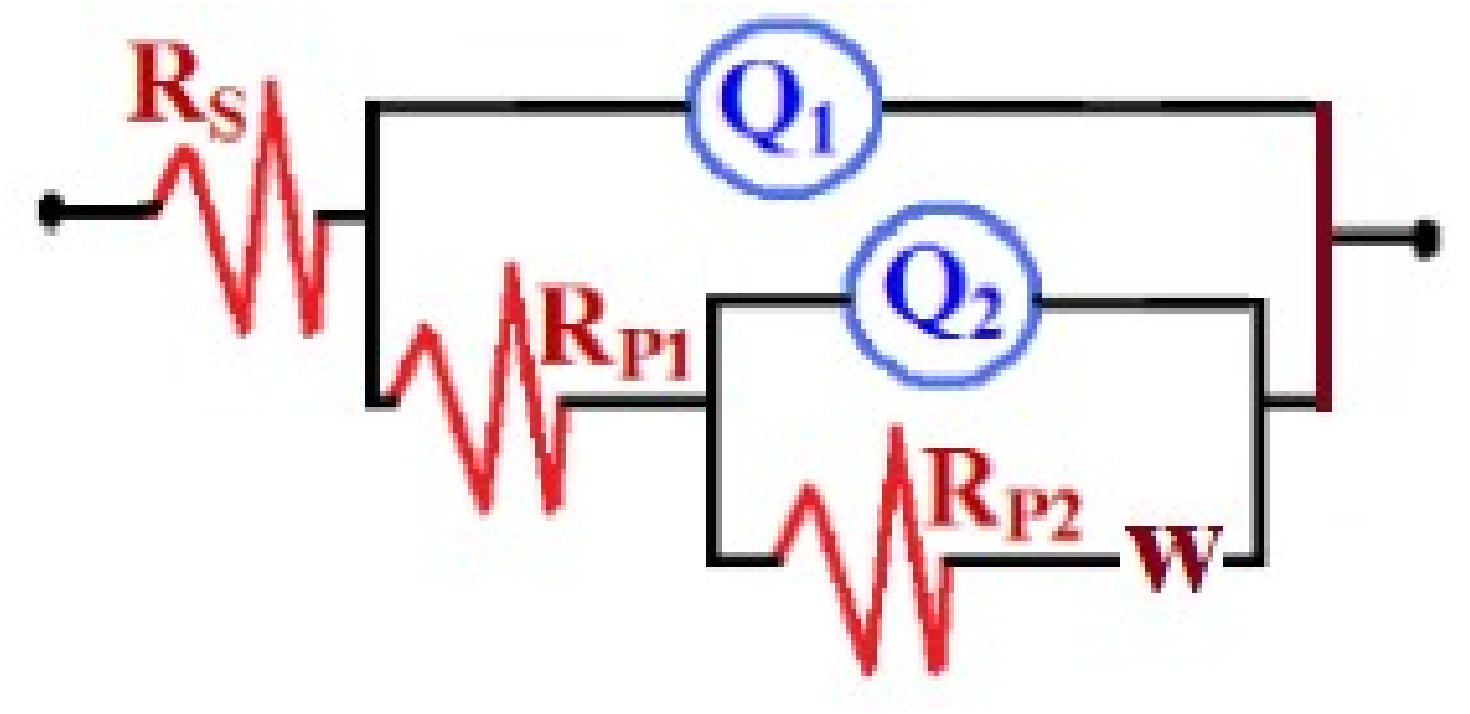
3.4. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armelin, E.; Pla, R.; Liesa, F.; Ramis, X.; Iribarren, J.I.; Alemán, C. Corrosion protection with polyaniline and polypyrrole as anticorrosive additives for epoxy paint. Corros. Sci. 2008, 50, 721–728. [Google Scholar] [CrossRef]
- Kugler, S.; Kowalczyk, K.; Spychaj, T. Transparent epoxy coatings with improved electrical, barrier and thermal features made of mechanically dispersed carbon nanotubes. Prog. Org. Coat. 2017, 111, 196–201. [Google Scholar] [CrossRef]
- Chang, C.-H.; Huang, T.-C.; Peng, C.-W.; Yeh, T.-C.; Lu, H.-I.; Hung, W.-I.; Weng, C.-J.; Yang, T.-I.; Yeh, J.-M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Greenham, N.C.; Moratti, S.C.; Bradley, D.D.C.; Friend, R.H.; Holmes, A.B. Efficient light-emitting diodes based on polymers with high electron affinities. Nature 1993, 365, 628–630. [Google Scholar] [CrossRef]
- Koul, S.; Chandra, R.; Dhawan, S.K. Conducting polyaniline composite for ESD and EMI at 101 GHz. Polymer 2000, 41, 9305–9310. [Google Scholar] [CrossRef]
- Glatthaar, M.; Niggemann, M.; Zimmermann, B.; Lewer, P.; Riede, M.; Hinsch, A.; Luther, J. Organic Solar Cells Using Inverted Layer Sequence. Thin Solid Film. 2005, 491, 298–300. [Google Scholar] [CrossRef]
- Mengoli, G.; Munari, M.T.; Bianco, P.; Musiani, M.M. Anodic synthesis of polyaniline coatings onto Fe sheets. J. Appl. Polym. Sci. 1981, 26, 4247–4257. [Google Scholar] [CrossRef]
- DeBerry, D.W. Modification of the Electrochemical and Corrosion Behavior of Stainless Steels with an Electroactive Coating. J. Electrochem. Soc. 1985, 132, 1022–1026. [Google Scholar] [CrossRef]
- Tallman, D.E.; Spinks, G.; Dominis, A.; Wallace, G.G. Electroactive Conducting Polymers for Corrosion Control Part 1. General Introduction and a Review of Non-ferrous Metals. J. Solid State Electrochem. 2002, 6, 73–84. [Google Scholar] [CrossRef]
- Spinks, G.M.; Dominis, A.J.; Wallace, G.G.; Tallman, D.E. Electroactive conducting polymers for corrosion control. J. Solid State Electrochem. 2002, 6, 85–100. [Google Scholar] [CrossRef]
- Lu, W.K.; Elsenbaumer, R.L.; Wessling, B. Corrosion protection of mild steel by coatings containing polyaniline. Synth. Met. 1995, 71, 2163–2166. [Google Scholar] [CrossRef]
- Dhawan, S.K.; Trivedi, D.C. Synthesis and characterization of poly(o-ethoxyaniline): A processable conducting polymer. J. Appl. Polym. Sci. 1995, 58, 815–826. [Google Scholar] [CrossRef]
- Daouadji, M.M.; Chelali, N. Influence of molecular weight of poly(ortho-ethoxyaniline) on the corrosion inhibition efficiency of mild steel in acidic media. J. Appl. Polym. Sci. 2004, 91, 1275–1284. [Google Scholar] [CrossRef]
- Alam, M.A.; Samad, U.A.; Sherif, E.-S.M.; Seikh, A.; Al-Zahrani, S.M.; Alharthi, N.H.; Alam, M. Synergistic effect of Ag and ZnO nanoparticles on polyaniline incorporated epoxy/2pack coatings for splash zone applications. J. Coat. Technol. Res. 2019, in press. [Google Scholar] [CrossRef]
- Ates, M.; Topkayaa, E. Nanocomposite film formations of polyaniline via TiO2, Ag and Zn, and their corrosion protection properties. Prog. Org. Coat. 2015, 82, 33–40. [Google Scholar] [CrossRef]
- Gomez, H.; Ram, M.K.; Alvi, F.; Stefanakos, E.; Kumar, A. Novel synthesis, characterization, and corrosion inhibition properties of nano-diamond-polyaniline films. J. Phys. Chem. C. 2010, 114, 18797–18804. [Google Scholar] [CrossRef]
- Alam, M.A.; Samad, U.A.; Sherif, E.-S.M.; Alothman, O.; Seikh, A.H.; Al-Zahrani, S.M. Effects of minor additions of polypyrrole on the thermal, mechanical and electrochemical properties of epoxy-2pack coatings. Int. J. Electrochem. Sci. 2017, 12, 74–89. [Google Scholar] [CrossRef]
- Karasinski, E.N.; Da Luz, M.G.; Lepienski, C.M.; Coelho, L.A.F. Nanostructured coating based on epoxy/metal oxides: Kinetic curing and mechanical properties. Thermochim. Acta. 2013, 569, 167–176. [Google Scholar] [CrossRef]
- Samad, U.A.; Alam, M.A.; Chafidz, A.; Al-Zahrani, S.M.; Alharthi, N.H. Enhancing mechanical properties of epoxy/polyaniline coating with addition of ZnO nanoparticles: Nanoindentation characterization. Prog. Org. Coat. 2018, 119, 109–115. [Google Scholar] [CrossRef]
- Samad, U.A.; Alam, M.A.; Sherif, E.-S.M.; Alothman, O.; Seikh, A.H.; Al-Zahrani, S.M. Manufacturing and characterization of corrosion resistant epoxy/2pack coatings incorporated with polyaniline conductive polymer. Int. J. Electrochem. Sci. 2015, 10, 5599–5613. [Google Scholar]
- Shen, L.; Wang, L.; Liu, T.; He, C. Nanoindentation and morphological studies of epoxy nanocomposites. Macromol. Mater. Eng. 2006, 291, 1358–1366. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Vyazovkin, S. Modification of the integral isoconversional method to account for variation in the activation energy. J. Comput. Chem. 2001, 22, 178–183. [Google Scholar] [CrossRef]
- Shi, X.; Nguyen, T.A.; Suo, Z.; Liu, Y.; Avci, R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf. Coat. Technol. 2009, 204, 237–245. [Google Scholar] [CrossRef]
- AlOtaibi, A.; Sherif, E.-S.M.; Zinelis, S.; Al-Jabbari, Y. Corrosion Behavior of Two cp Titanium Dental Implants Connected by Cobalt Chromium Metal Superstructure in Artificial Saliva and the Influence of Immersion Time. Inter. J. Electrochem. Sci. 2016, 11, 5877–5890. [Google Scholar] [CrossRef]
- Seikh, A.H.; Halfa, H.; Sherif, E.-S.M. Corrosion resistance performance of newly developed cobalt-free maraging steel over conventional maraging steel in acidic media. Adv. Mater. Proc. Technol. 2016, 2, 283–292. [Google Scholar] [CrossRef]
- Alharthi, N.; Sherif, E.-S.M.; Abdo, H.S.; El-Abedin, S.Z. Effect of nickel content on the corrosion resistance of iron-nickel alloys in concentrated hydrochloric acid pickling solutions. Adv. Mater. Sci. Eng. 2017, 2017. [Google Scholar] [CrossRef]
- Gopi, D.; Sherif, E.-S.M.; Surendiran, M.; Sakila, D.M.A.; Kavitha, L. Corrosion inhibition by benzotriazole derivatives and sodium dodecyl sulphate as corrosion inhibitors for copper in ground water at different temperatures. Surf. Interf. Anal. 2015, 47, 618–625. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; El-Danaf, E.A.; Abdo, H.S.; El-Abedin, S.Z.; Al-Khazraji, H. Effect of annealing temperature on the corrosion protection of hot swaged Ti-54M alloy in 2 M HCl pickling solutions. Metals 2017, 7, 29. [Google Scholar]
- Sherif, E.-S.M.; Abdo, H.S.; El Abedin, S.Z. Electrochemical corrosion behavior of Fe64/Ni36 and Fe55/Ni45 alloys in 4.0% sodium chloride solutions. Inter. J. Electrochem. Sci. 2017, 12, 1600–1611. [Google Scholar] [CrossRef]
- Tsai, P.Y.; Chen, T.E.; Lee, Y.L. Development and characterization of anticorrosion and antifriction properties for high performance polyurethane/graphene composite coatings. Coatings 2018, 8, 250. [Google Scholar] [CrossRef]
- Akbarian, M.; Olya, M.E.; Mahdavian, M.; Ataeefard, M. Effects of nanoparticulate silver on the corrosion protection performance of polyurethane coatings on mild steel in sodium chloride solution. Prog. Org. Coat. 2014, 77, 1233–1240. [Google Scholar] [CrossRef]
| Sample | Epoxy Resin | PPy Wt% | Xylene | MIBK | Dispersing Agent | Silane Wt.% | Nano Ag | Nano ZnO | Hardener D-3282 Wt.% |
|---|---|---|---|---|---|---|---|---|---|
| Epoxy-PPy | 83.34 | 0.95 | 10 | 10 | 1 | 1 | 0 | 0 | 16.66 |
| F1 | 83.34 | 0.95 | 10 | 10 | 1 | 1 | 0.2 | 0.2 | 16.66 |
| F2 | 83.34 | 0.95 | 10 | 10 | 1 | 1 | 0.4 | 0.4 | 16.66 |
| Samples | PPy | Nanoparticles | Dry Film Thickness (DFT) (µm) | Pendulum Hardness (Oscillations) | Scratch Resistance (Kg) | Impact Strength (Kg/cm) | |
|---|---|---|---|---|---|---|---|
| Ag | ZnO | ||||||
| Epoxy/PPy | 0.95% | 0 | 0 | 60–80 | 135 | 5 | 3.37 |
| F1 | 0.95% | 0.2% | 0.2% | 70–90 | 145 | 8 | 11.43 |
| F2 | 0.95% | 0.4% | 0.4% | 70–90 | 153 | 7 | 12.86 |
| Sample | Nano Particles | Elements Present | Weight % |
|---|---|---|---|
| F1 | Ag, Zn | C, Zn, Ag | 99.10, 0.59, 0.31, |
| F2 | Ag, Zn | C, Zn, Ag | 98.82, 0.85, 0.33, |
| Sample | Nanoparticle | Hardness (GPa) | Elastic Modulus (GPa) | |
|---|---|---|---|---|
| Ag | ZnO | |||
| Epoxy/PPy | 0.0 | 0.0 | 0.19 | 4.3 |
| F1 | 0.2% | 0.2% | 0.17 | 5.0 |
| F2 | 0.4% | 0.4% | 0.18 | 4.9 |
| Samples | RS/Ωcm2 | Q1 | RP1/kΩcm2 | Q1 | RP2/kΩcm2 | W/ΩS−1/2 | ||
|---|---|---|---|---|---|---|---|---|
| YQ1/Fcm−2 | n1 | YQ1/Fcm−2 | n2 | |||||
| F1 (1 h) | 95 | 1.29 × 10−10 | 0.94 | 8999 | 2.76 × 10−9 | 0.45 | 1596 | 1.93 × 10−9 |
| F2 (1 h) | 104 | 7.86 × 10−11 | 0.98 | 9794 | 1.08 × 10−9 | 0.43 | 2432 | 6.29 × 10−9 |
| F1 (7 days) | 96 | 1.19 × 10−10 | 0.95 | 6603 | 2.09 × 10−9 | 0.42 | 1568 | 3.29 × 10−9 |
| F2 (7 days) | 103 | 1.48 × 10−10 | 0.91 | 7732 | 6.15 × 10−9 | 0.43 | 2351 | 2.68 × 10−9 |
| F1 (15 days) | 87 | 1.57 × 10−9 | 0.97 | 5468 | 2.56 × 10−9 | 0.48 | 1497 | 7.11 × 10−9 |
| F2 (15 days) | 101 | 5.13 × 10−9 | 0.94 | 6336 | 3.10 × 10−9 | 0.44 | 2145 | 5.31 × 10−9 |
| F1 (21 days) | 85 | 1.59 × 10−9 | 0.97 | 4595 | 1.03 × 10−8 | 0.39 | 1335 | 5.17 × 10−9 |
| F2 (21 days) | 105 | 2.57 × 10−9 | 0.97 | 6161 | 6.04 × 10−9 | 0.44 | 1931 | 3.06 × 10−9 |
| F1 (30 days) | 85 | 2.02 × 10−9 | 0.98 | 3254 | 3.3 × 10−8 | 0.41 | 1096 | 4.68 × 10−9 |
| F2 (30 days) | 103 | 3.15 × 10−9 | 0.95 | 5465 | 2.29 × 10−8 | 0.45 | 1512 | 1.37 × 10−9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdus Samad, U.; Alam, M.A.; Sherif, E.-S.M.; Alam, M.; Shaikh, H.; Alharthi, N.H.; Al-Zahrani, S.M. Synergistic Effect of Ag and ZnO Nanoparticles on Polypyrrole-Incorporated Epoxy/2pack Coatings and Their Corrosion Performances in Chloride Solutions. Coatings 2019, 9, 287. https://doi.org/10.3390/coatings9050287
Abdus Samad U, Alam MA, Sherif E-SM, Alam M, Shaikh H, Alharthi NH, Al-Zahrani SM. Synergistic Effect of Ag and ZnO Nanoparticles on Polypyrrole-Incorporated Epoxy/2pack Coatings and Their Corrosion Performances in Chloride Solutions. Coatings. 2019; 9(5):287. https://doi.org/10.3390/coatings9050287
Chicago/Turabian StyleAbdus Samad, Ubair, Mohammad Asif Alam, El-Sayed M. Sherif, Manawwar Alam, Hamid Shaikh, Nabeel H. Alharthi, and Saeed M. Al-Zahrani. 2019. "Synergistic Effect of Ag and ZnO Nanoparticles on Polypyrrole-Incorporated Epoxy/2pack Coatings and Their Corrosion Performances in Chloride Solutions" Coatings 9, no. 5: 287. https://doi.org/10.3390/coatings9050287
APA StyleAbdus Samad, U., Alam, M. A., Sherif, E.-S. M., Alam, M., Shaikh, H., Alharthi, N. H., & Al-Zahrani, S. M. (2019). Synergistic Effect of Ag and ZnO Nanoparticles on Polypyrrole-Incorporated Epoxy/2pack Coatings and Their Corrosion Performances in Chloride Solutions. Coatings, 9(5), 287. https://doi.org/10.3390/coatings9050287







