Effect of Surface Pre-Treatments on the Formation and Degradation Behaviour of a Calcium Phosphate Coating on Pure Magnesium
Abstract
1. Introduction
2. Experimental
2.1. Materials and Pre-Treatments
2.2. Deposition of the CaPh Coating
2.3. Characterization
2.4. Electrochemical Tests
2.5. Immersion Test
3. Results and Discussion
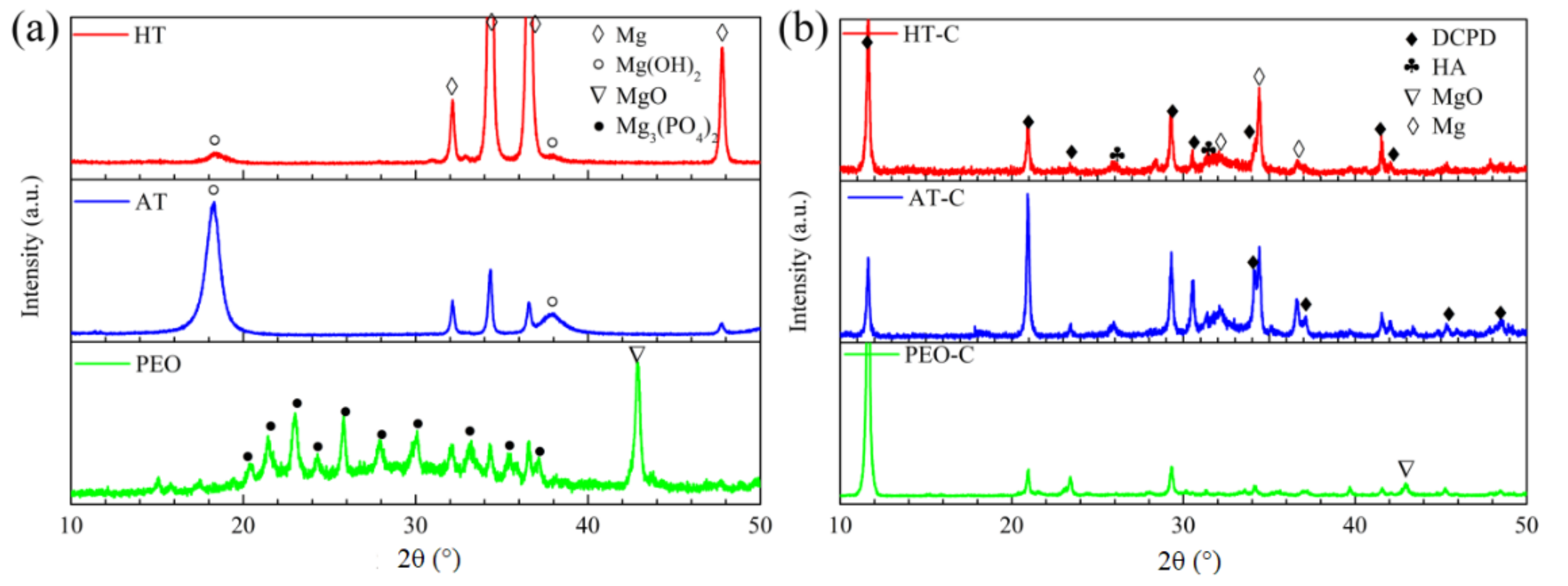
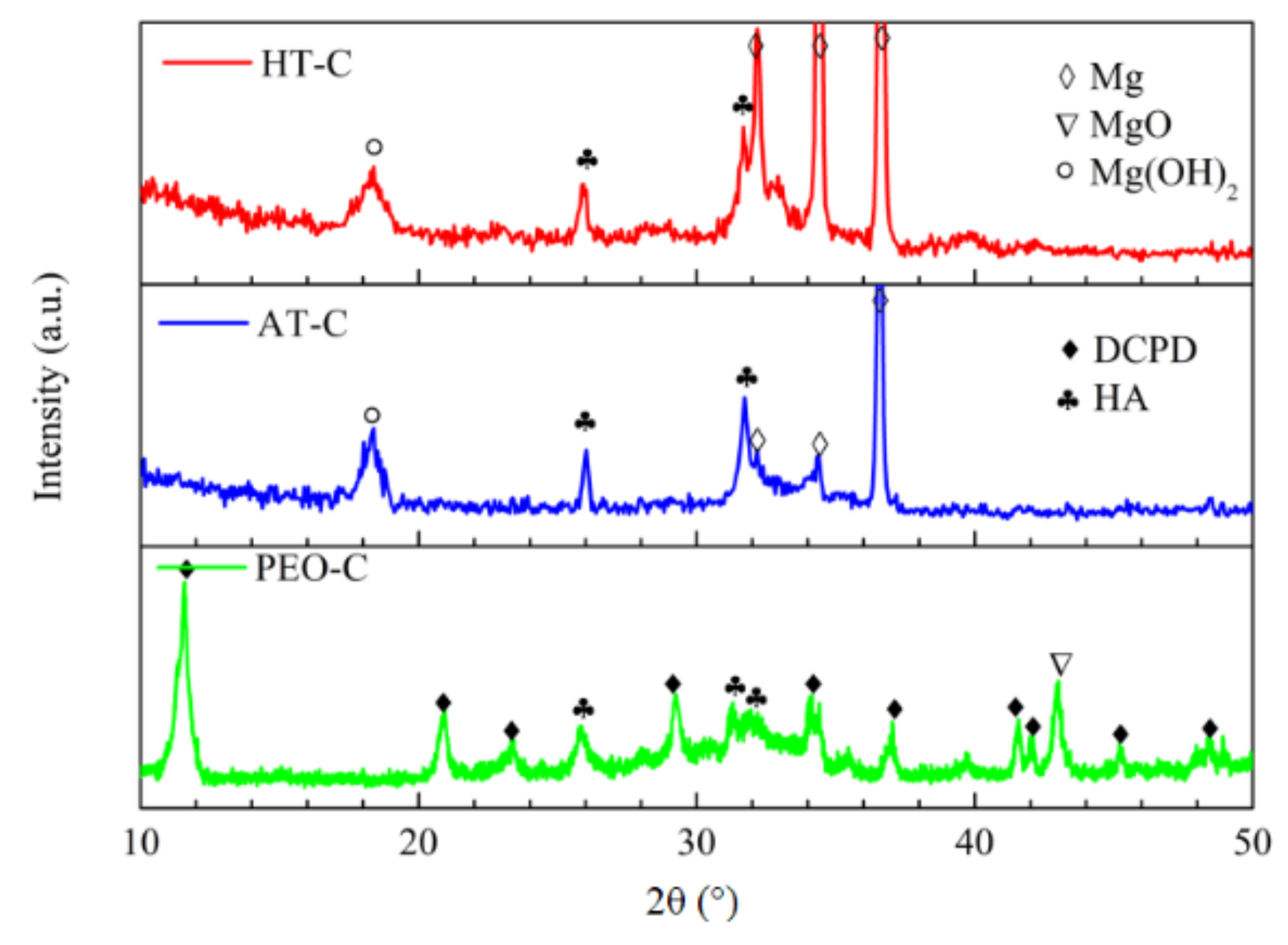
3.1. Phase Composition of the Pre-Treatments and CaPh-Coated Samples
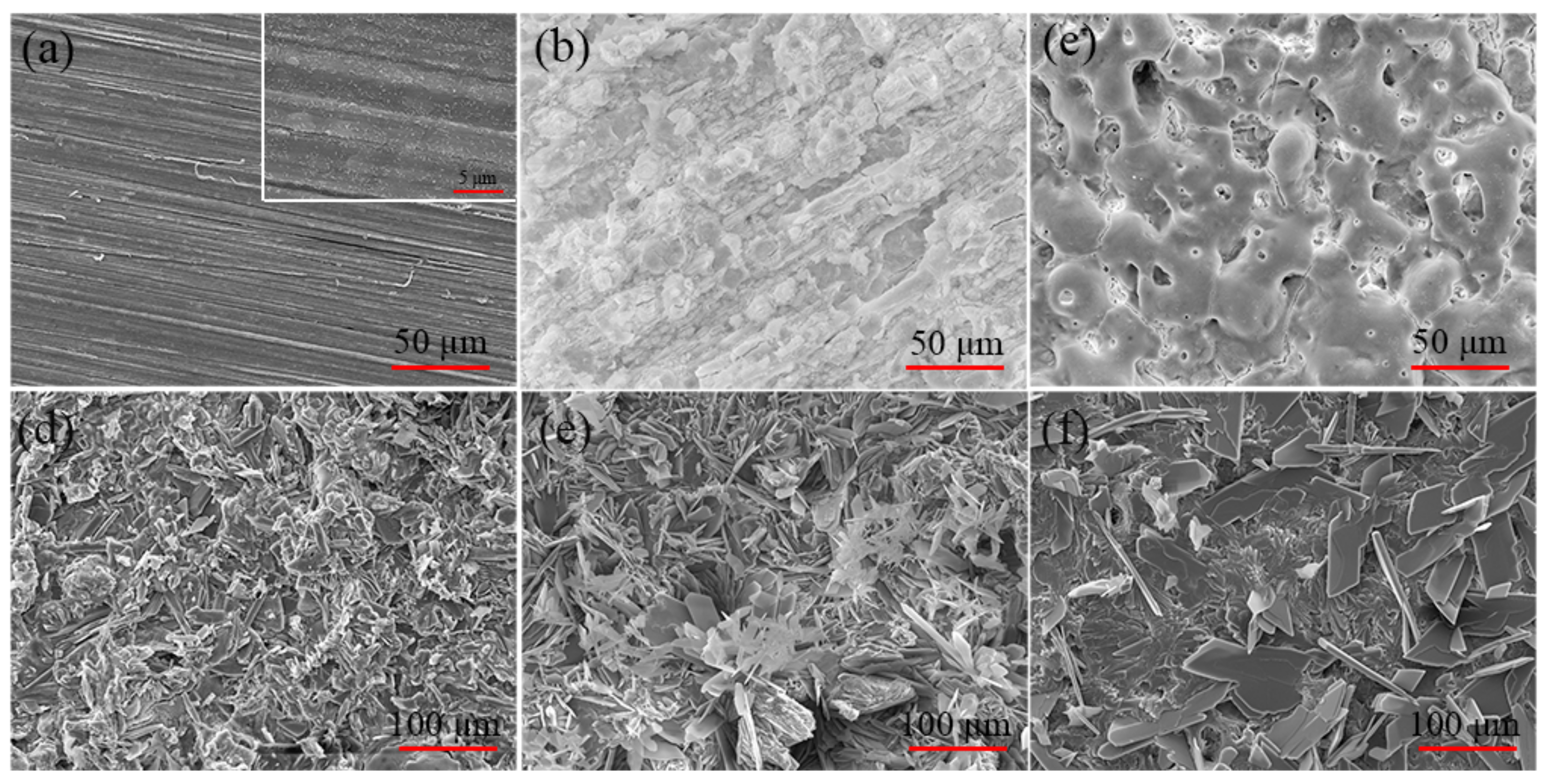
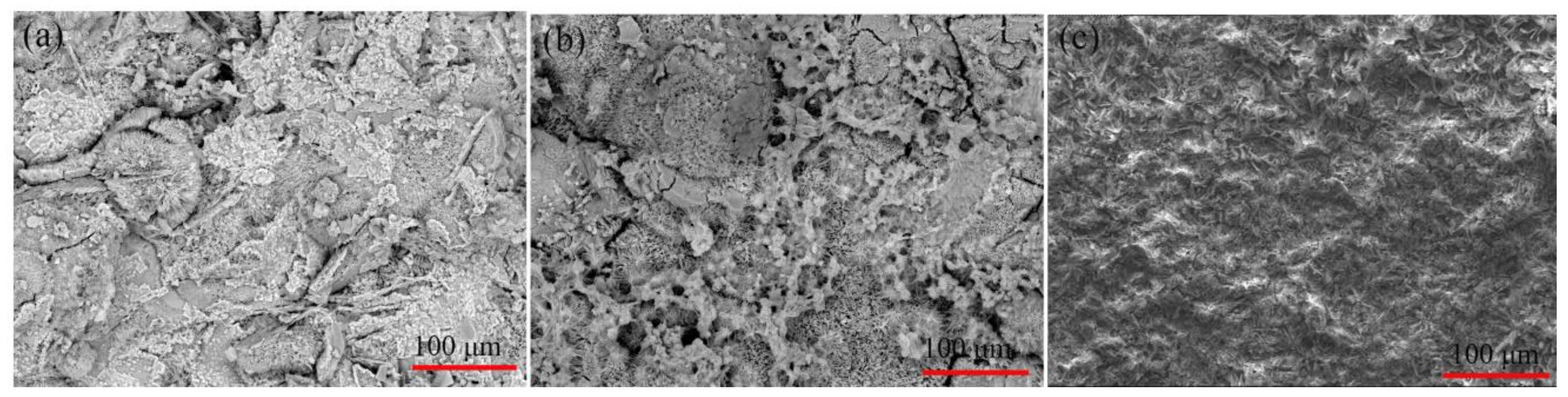
3.2. Morphologies of the Pre-Treatments and CaPh-Coated Samples
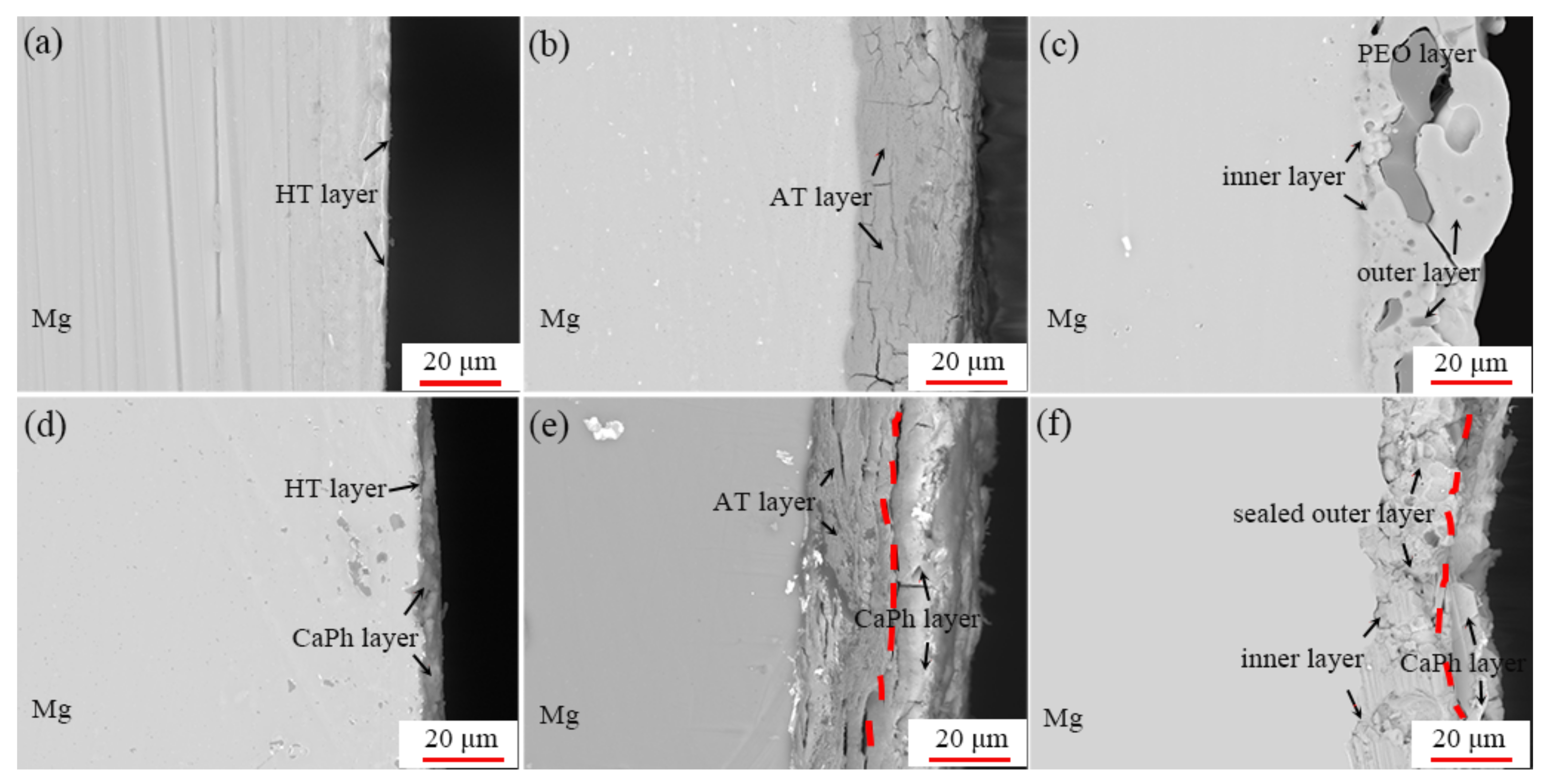
3.3. Formation Mechanism of the CaPh Coatings on the Pre-Treated Substrates
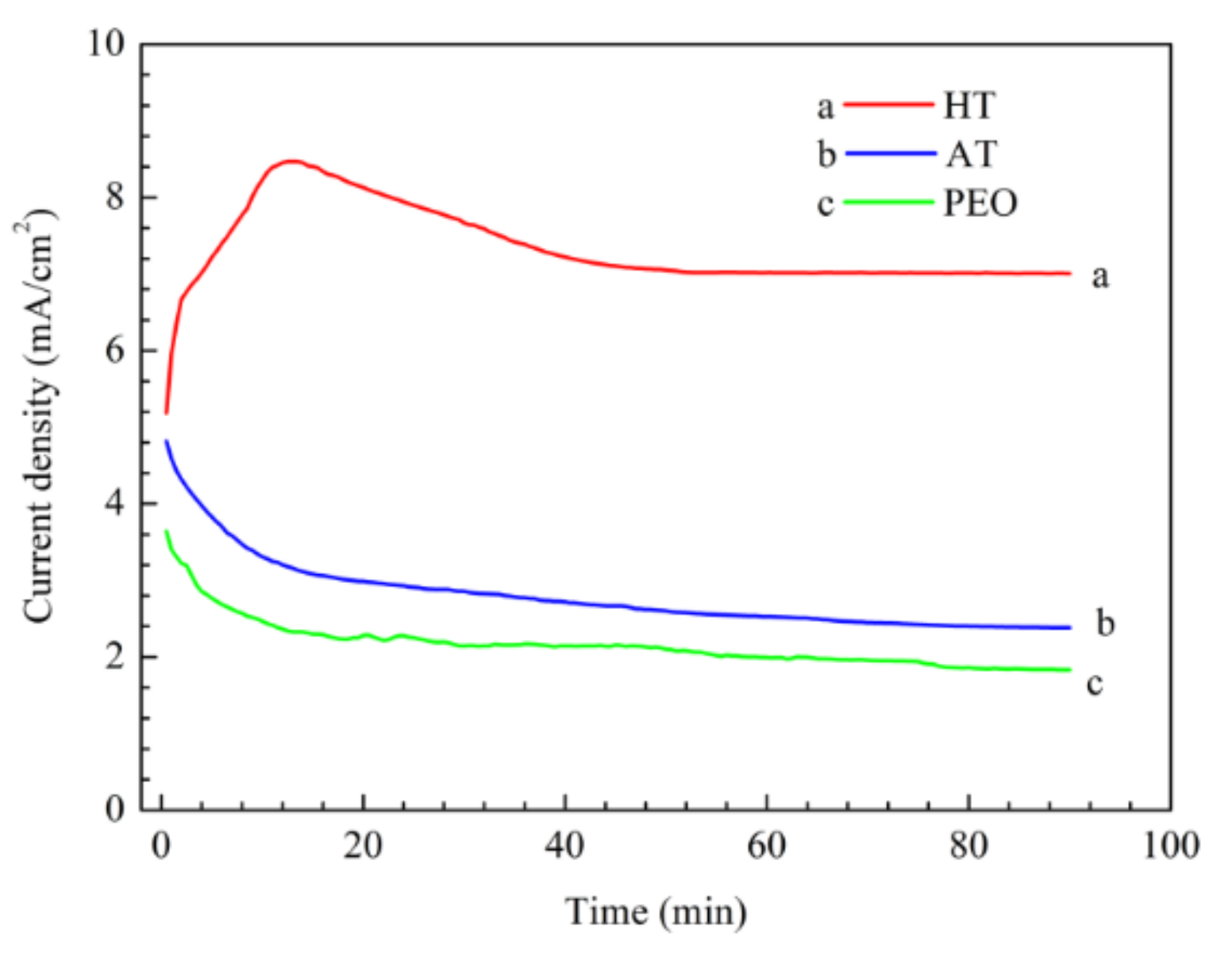
3.4. Corrosion Behaviour of the Pre-Treatments and CaPh-Coated Samples
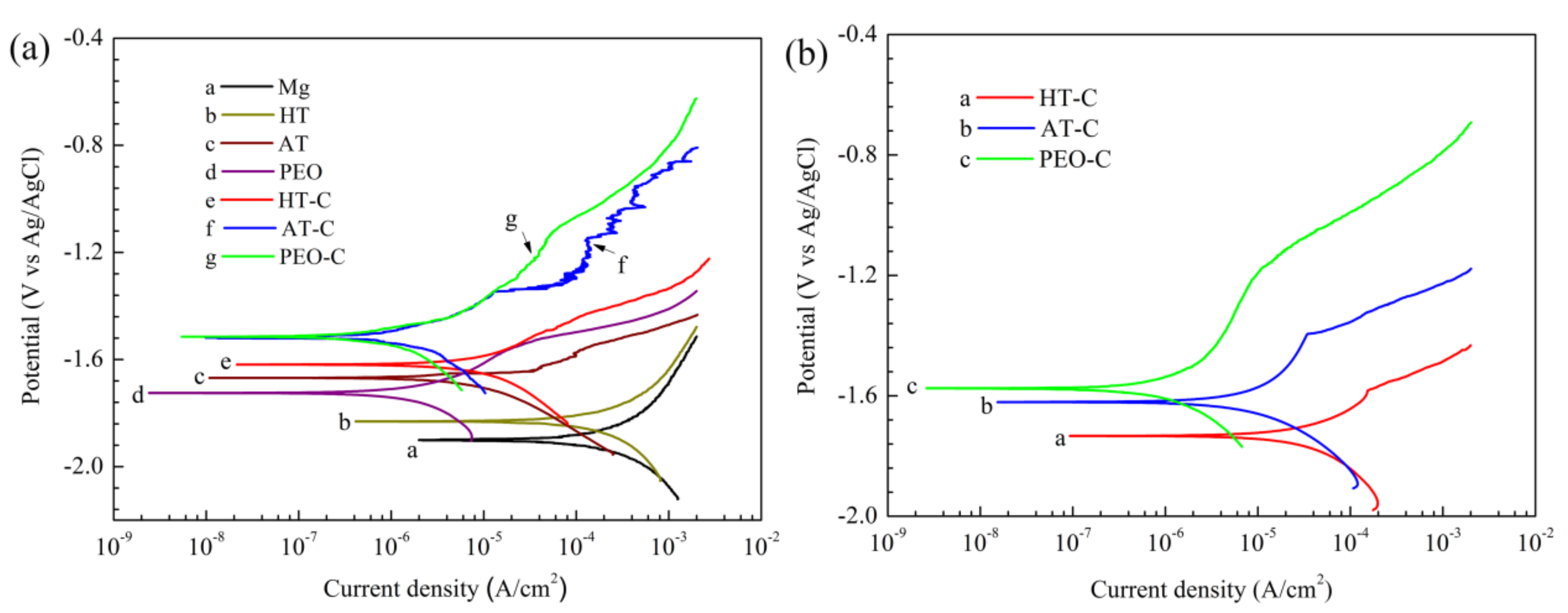
3.4.1. Potentiodynamic Polarization Curves
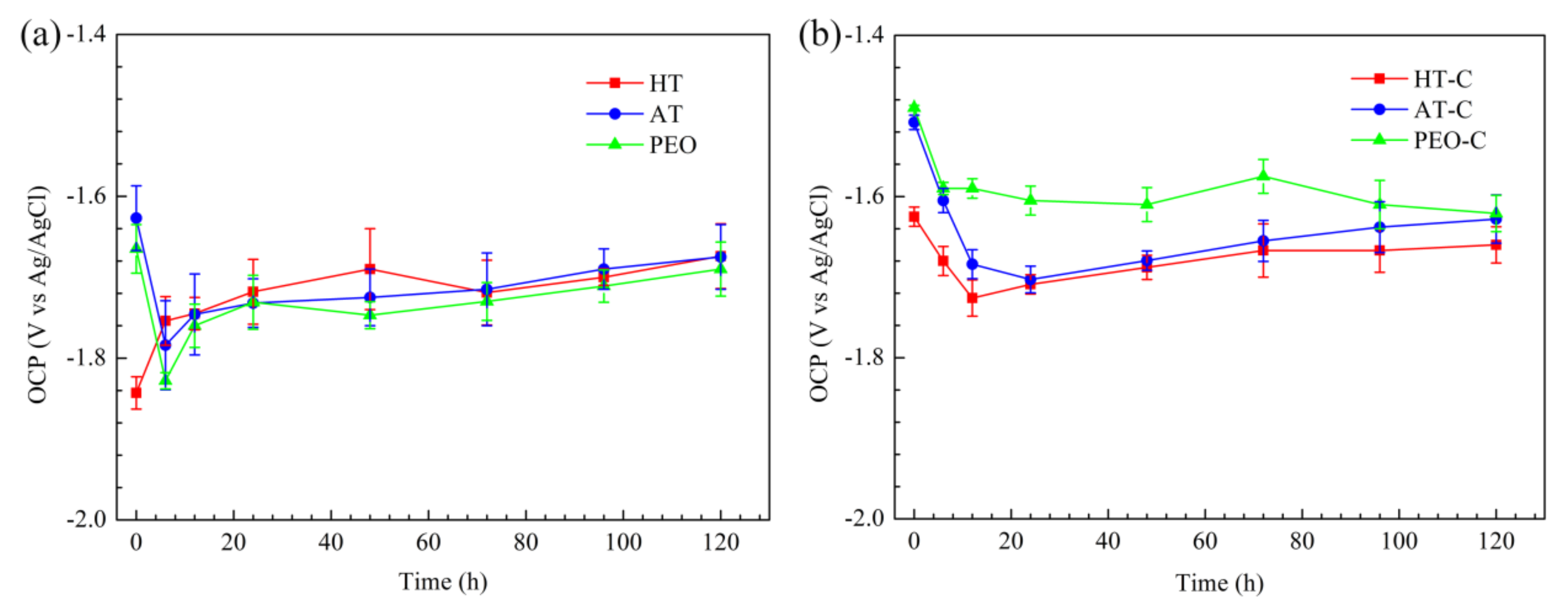
3.4.2. OCP
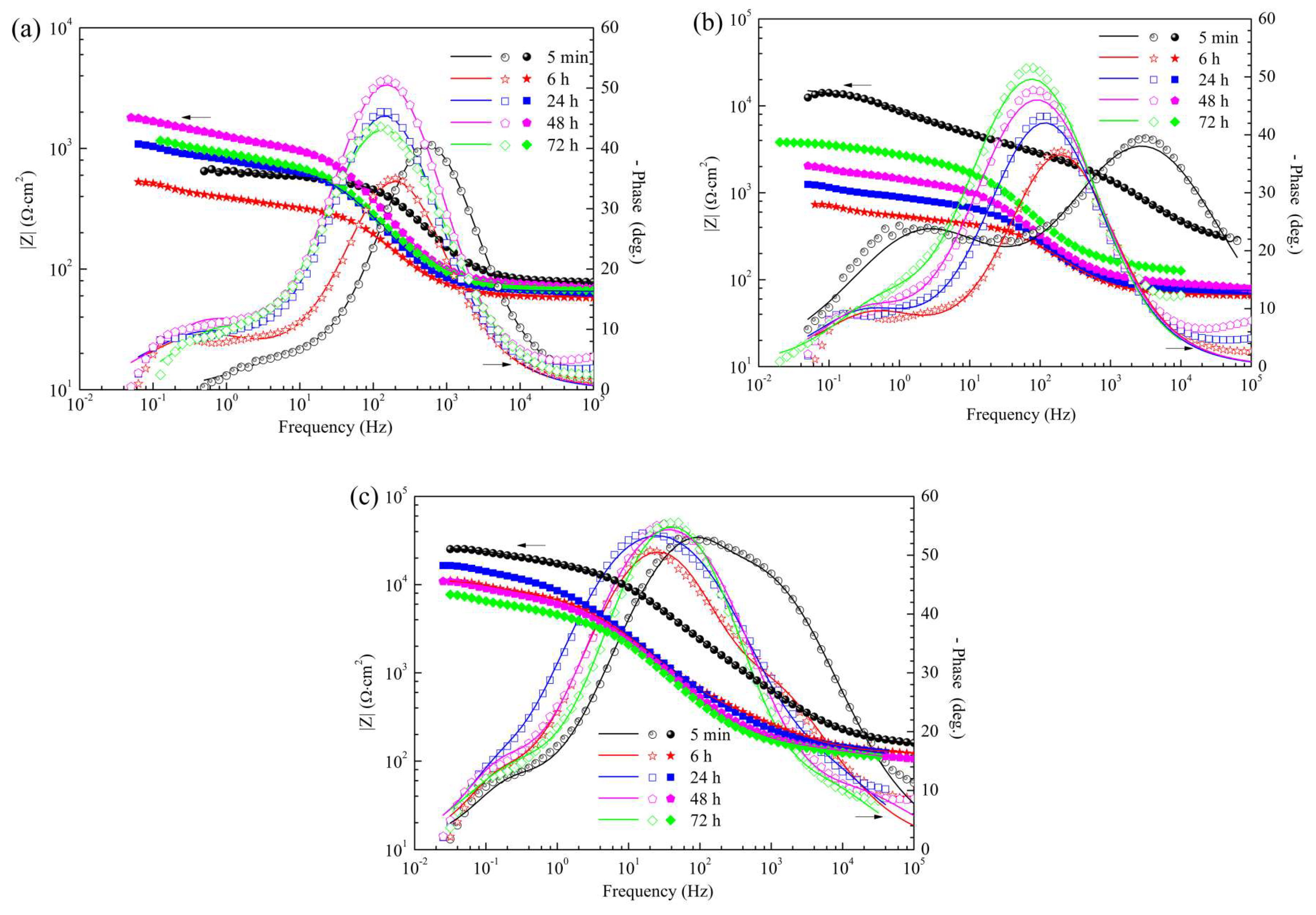
3.4.3. EIS Spectra of the Pre-Treated Samples
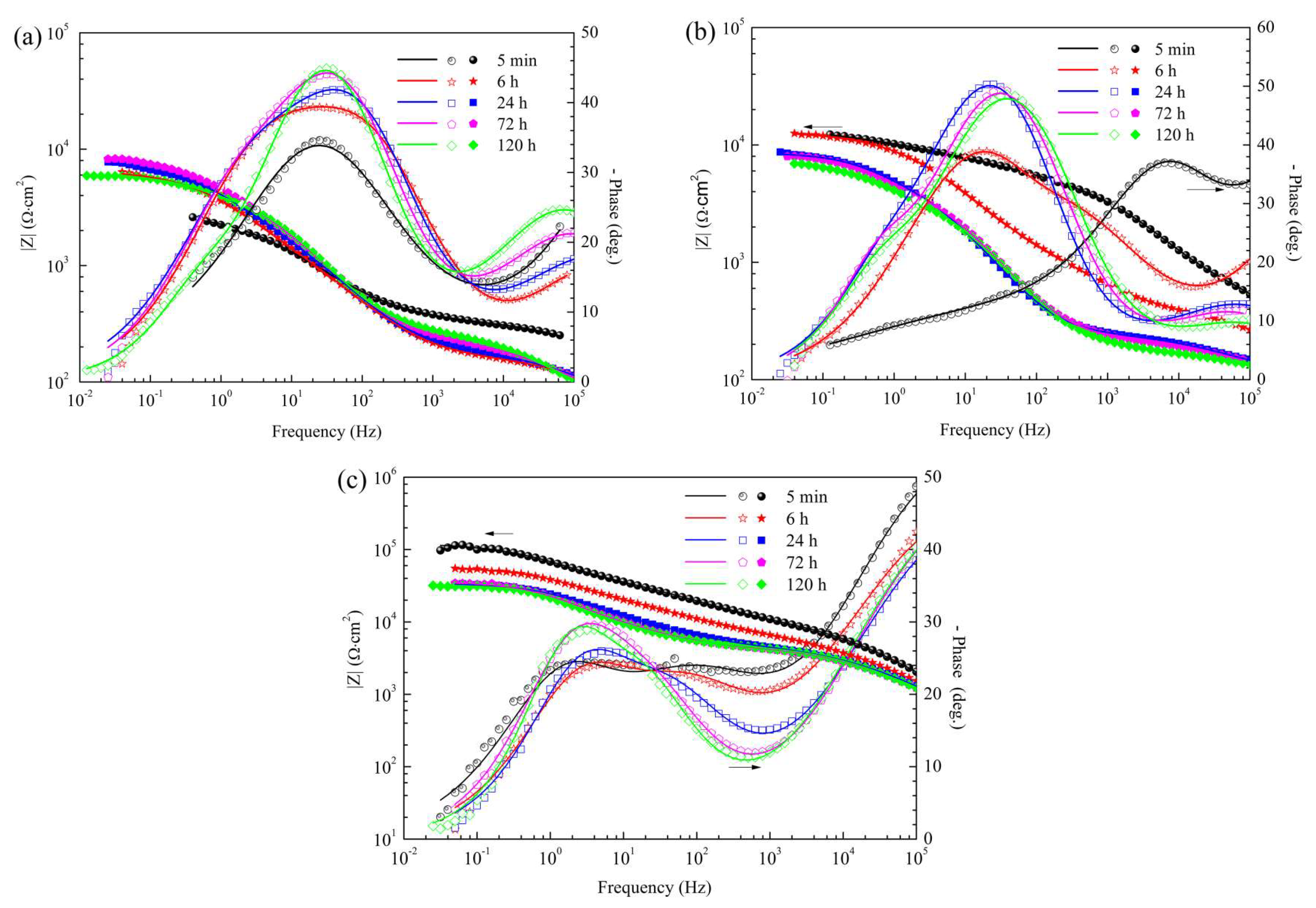
3.4.4. EIS Spectra of the CaPh-Coated Samples
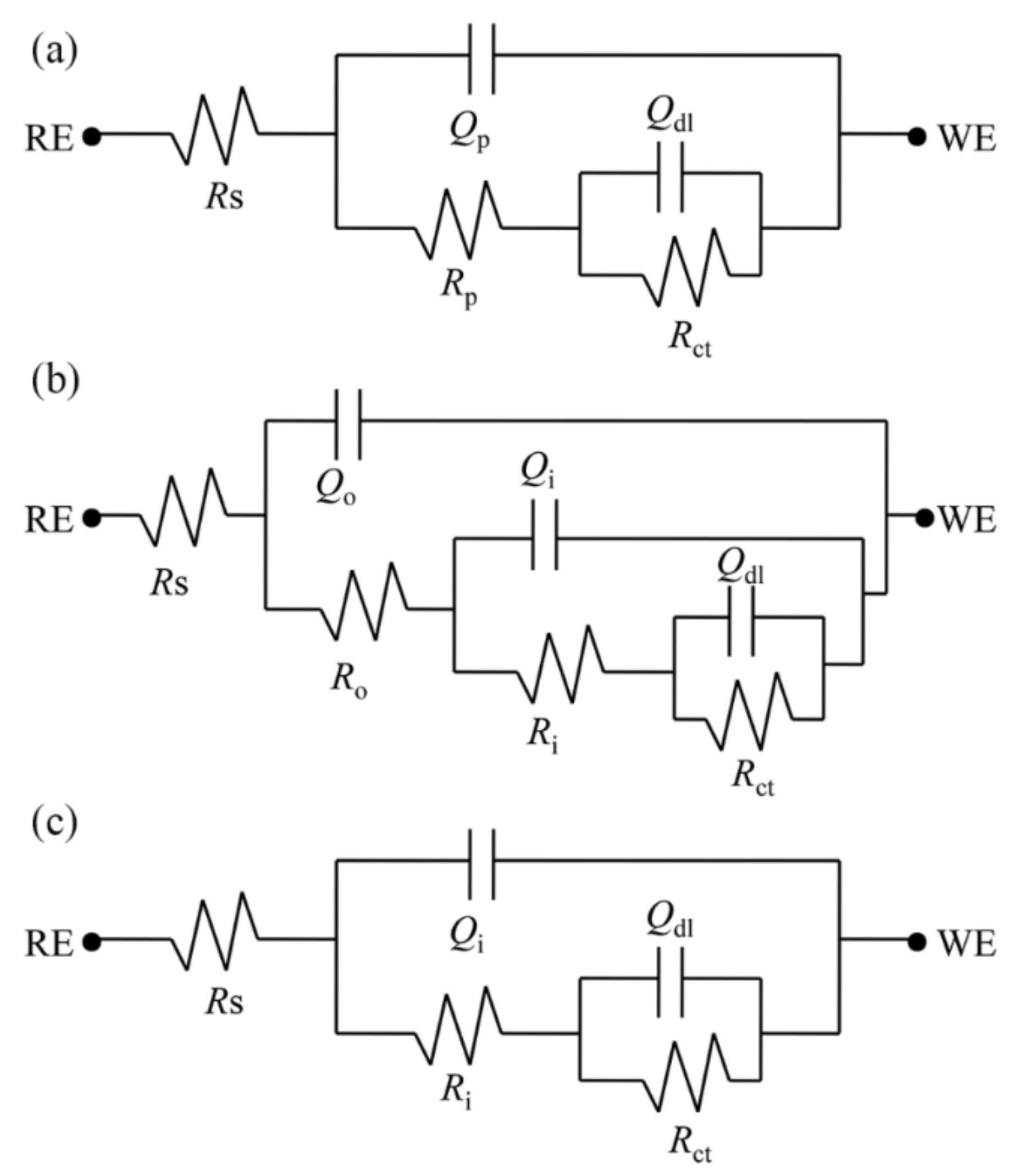
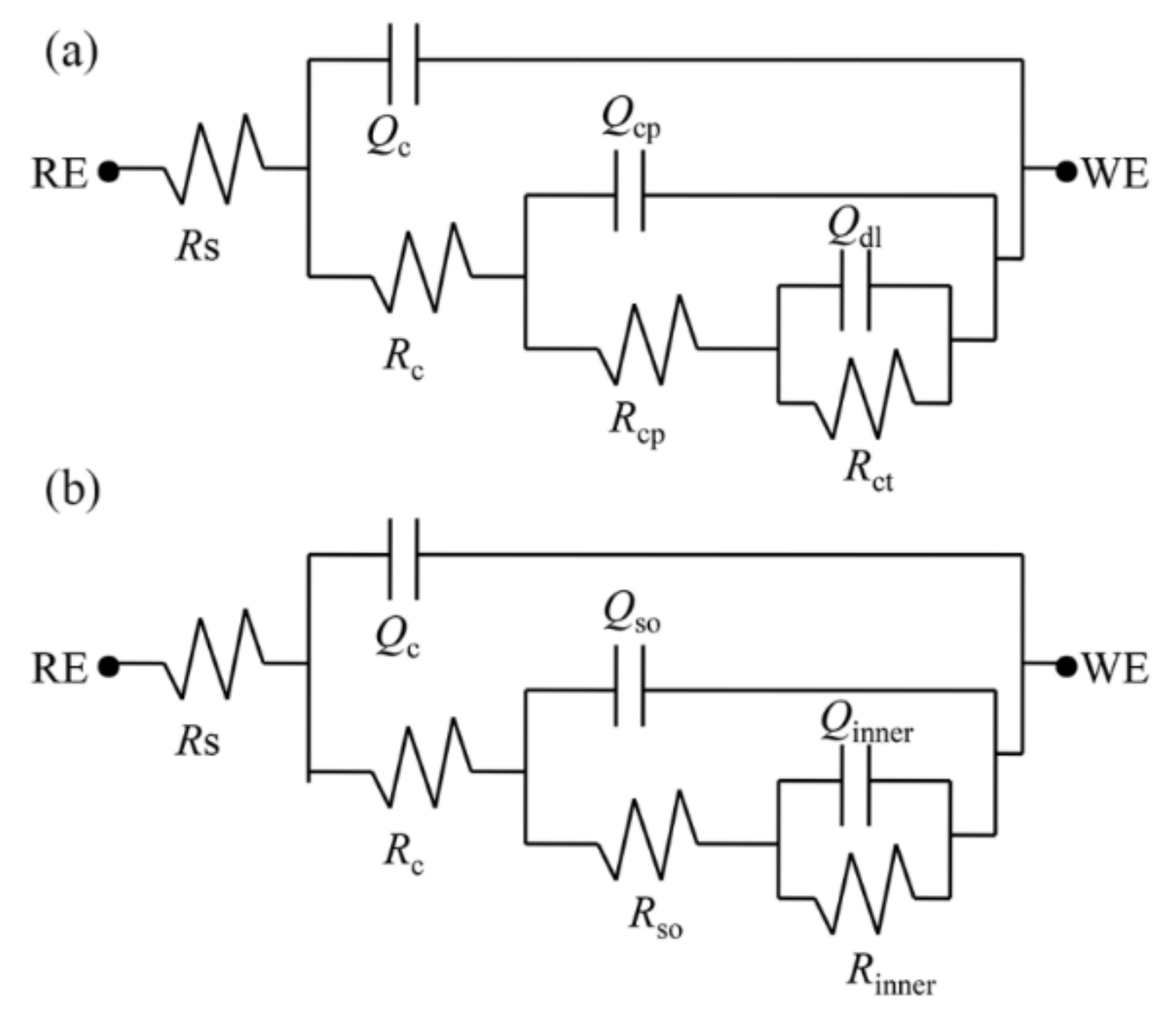
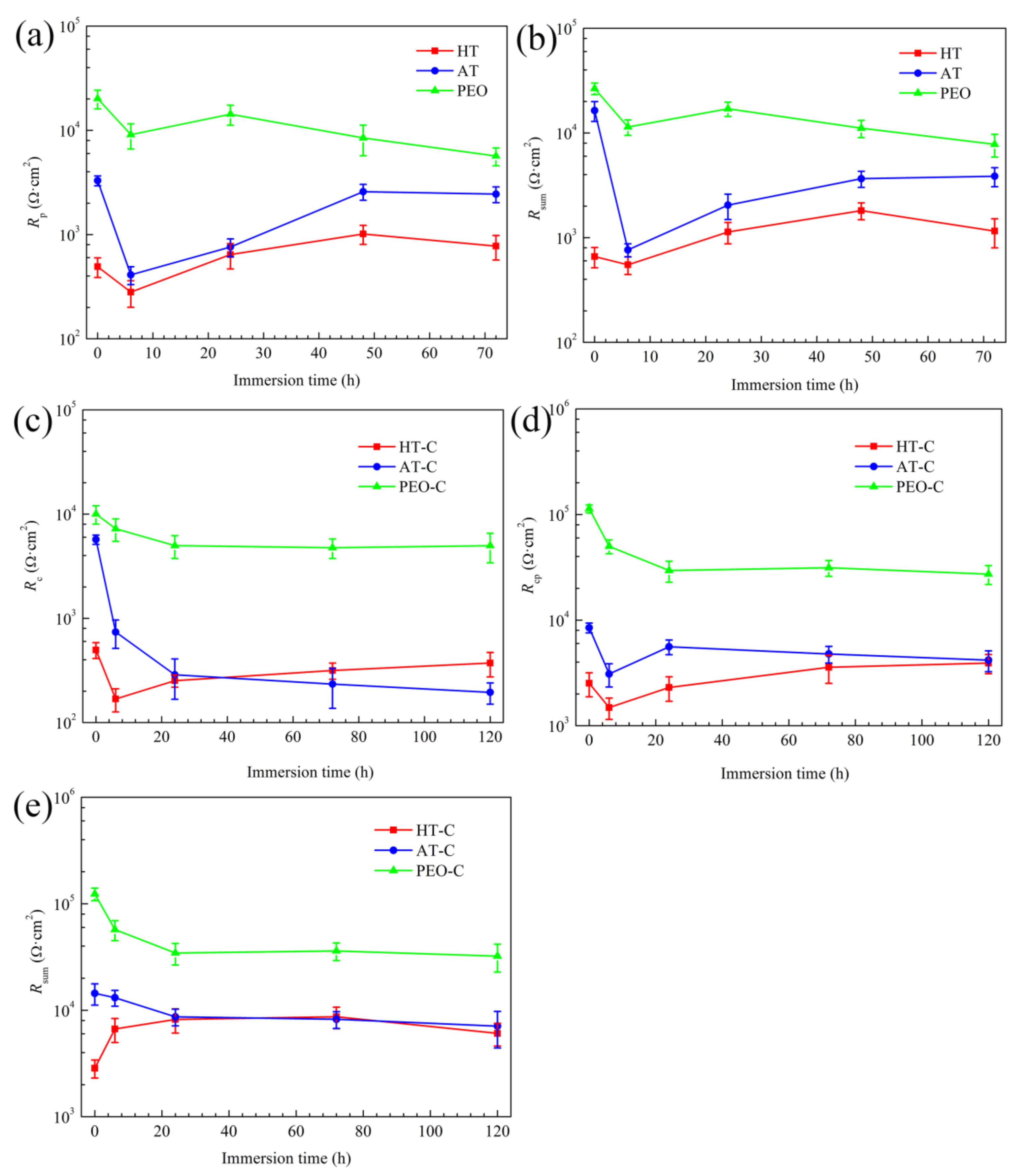
3.4.5. Evolution of the EIS Fitting Parameters
3.5. Immersion Tests
3.6. Corrosion Behaviour of Pre-Treated and CaPh-Coated Samples in SBF
4. Conclusions
- When a surface treatment is performed on pure magnesium, different surface treatment leads to different structures on the magnesium surface. After anodizing and hydrothermal treatment, Mg(OH)2 is formed on the magnesium surface, and MgO and Mg3(PO4)2 are the phase compositions of the PEO layer.
- The surface pre-treatment of pure magnesium prior to the CaPh coating influences the thickness, morphologies and phase composition as well as the formation mechanism of CaPh coatings due to the different morphologies and composition of the pre-treated layers.
- DCPD and HA are together deposited on the HT-C and AT-C samples, while only DCPD is detected on the PEO-C sample. During the SBF immersion, DCPD on the HT-C and AT-C samples disappears, whereas DCPD is present on the PEO-C sample all along. In any case, the coating surface can improve HA’s ability to precipitate in SBF. The pre-treatments play an important role in the corrosion resistance of the duplex coating systems. The best corrosion resistance is obtained for the PEO/CaPh combination because of the superior barrier properties of the PEO layer.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, B.; Wang, Y.; Geng, L.; Jiao, X. Preparation and characterization of a new biomedical Mg–Zn–Ca alloy. Mater. Des. 2012, 34, 58–64. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Mani, M.; Jaganathan, S.; Huang, Y.; Wang, C. Microwave-assisted dip coating of aloe vera on metallocene polyethylene incorporated with Nano-Rods of hydroxyapaptite for bone tissue engineering. Coatings 2017, 7, 182. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-S.; Zeng, R.-C.; Ding, Y.-F.; Li, R.W.; Easton, M.; Cole, I.; Birbilis, N.; Chen, X.-B. Recent advances in biodegradation controls over mg alloys for bone fracture management: A review. J. Mater. Sci. Technol. 2019, 35, 535–544. [Google Scholar] [CrossRef]
- Ong, T.H.D.; Yu, N.; Meenashisundaram, G.K.; Schaller, B.; Gupta, M. Insight into cytotoxicity of Mg nanocomposites using MTT assay technique. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 647–652. [Google Scholar] [CrossRef]
- Mueller, W.D.; Lucia Nascimento, M.; Lorenzo de Mele, M.F. Critical discussion of the results from different corrosion studies of Mg and Mg alloys for biomaterial applications. Acta Biomater. 2010, 6, 1749–1755. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg–Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef]
- Zhang, B.; Hou, Y.; Wang, X.; Wang, Y.; Geng, L. Mechanical properties, degradation performance and cytotoxicity of Mg–Zn–Ca biomedical alloys with different compositions. Mater. Sci. Eng. C 2011, 31, 1667–1673. [Google Scholar] [CrossRef]
- Lin, D.-J.; Hung, F.-Y.; Yeh, M.-L.; Lee, H.-P.; Lui, T.-S. Development of a novel micro-textured surface using duplex surface modification for biomedical Mg alloy applications. Mater. Lett. 2017, 206, 9–12. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Razavi, M.; Vashaee, D.; Tayebi, L. Surface modification of biodegradable porous Mg bone scaffold using polycaprolactone/bioactive glass composite. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 436–444. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Tolnai, D.; Subroto, T.; Kainer, K.U.; Zhang, T.; Wang, F.; Zheludkevich, M.L. 3D reconstruction of plasma electrolytic oxidation coatings on Mg alloy via synchrotron radiation tomography. Corros. Sci. 2018, 139, 395–402. [Google Scholar] [CrossRef]
- Shadanbaz, S.; Dias, G.J. Calcium phosphate coatings on magnesium alloys for biomedical applications: A review. Acta Biomater. 2012, 8, 20–30. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1261–1274. [Google Scholar] [CrossRef]
- Prakash, C.; Singh, S.; Pabla, B.S.; Uddin, M.S. Synthesis, characterization, corrosion and bioactivity investigation of nano-HA coating deposited on biodegradable Mg–Zn–Mn alloy. Surf. Coat. Technol. 2018, 346, 9–18. [Google Scholar] [CrossRef]
- Singh, S.S.; Roy, A.; Lee, B.E.; Ohodnicki, J.; Loghmanian, A.; Banerjee, I.; Kumta, P.N. A study of strontium doped calcium phosphate coatings on AZ31. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 357–365. [Google Scholar] [CrossRef]
- Waterman, J.; Pietak, A.; Birbilis, N.; Woodfield, T.; Dias, G.; Staiger, M.P. Corrosion resistance of biomimetic calcium phosphate coatings on magnesium due to varying pretreatment time. Mater. Sci. Eng. B 2011, 176, 1756–1760. [Google Scholar] [CrossRef]
- Cui, L.-Y.; Wei, G.-B.; Han, Z.-Z.; Zeng, R.-C.; Wang, L.; Zou, Y.-H.; Li, S.-Q.; Xu, D.-K.; Guan, S.-K. In vitro corrosion resistance and antibacterial performance of novel tin dioxide-doped calcium phosphate coating on degradable Mg–1Li–1Ca alloy. J. Mater. Sci. Technol. 2019, 35, 254–265. [Google Scholar] [CrossRef]
- Navarro da Rocha, D.; Cruz, L.R.d.O.; de Campos, J.B.; Marçal, R.L.S.B.; Mijares, D.Q.; Coelho, P.G.; Prado da Silva, M.H. Mg substituted apatite coating from alkali conversion of acidic calcium phosphate. Mater. Sci. Eng. C 2017, 70, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Lamaka, S.V.; Montemor, M.F.; Galio, A.F.; Zheludkevich, M.L.; Trindade, C.; Dick, L.F.; Ferreira, M.G.S. Novel hybrid sol-gel coatings for corrosion protection of AZ31B magnesium alloy. Electrochim. Acta 2008, 53, 4773–4783. [Google Scholar] [CrossRef]
- Wu, G.; Zeng, X.; Yuan, G. Growth and corrosion of aluminum PVD-coating on AZ31 magnesium alloy. Mater. Lett. 2008, 62, 4325–4327. [Google Scholar] [CrossRef]
- Chang, L.; Tian, L.; Liu, W.; Duan, X. Formation of dicalcium phosphate dihydrate on magnesium alloy by micro-arc oxidation coupled with hydrothermal treatment. Corros. Sci. 2013, 72, 118–124. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Bian, K.; Zhu, P. Preparation and characterization of nanostructured dibasic calcium phosphate coating on magnesium alloy wire. Mater. Lett. 2017, 209, 323–326. [Google Scholar] [CrossRef]
- Wang, M.-J.; Chao, S.-C.; Yen, S.-K. Electrolytic calcium phosphate/zirconia composite coating on AZ91D magnesium alloy for enhancing corrosion resistance and bioactivity. Corros. Sci. 2016, 104, 47–60. [Google Scholar] [CrossRef]
- Fathyunes, L.; Khalil-Allafi, J. The effect of graphene oxide on surface features, biological performance and bio-stability of calcium phosphate coating applied by pulse electrochemical deposition. Appl. Surf. Sci. 2018, 437, 122–135. [Google Scholar] [CrossRef]
- Wu, P.-P.; Zhang, Z.-Z.; Xu, F.-J.; Deng, K.-K.; Nie, K.-B.; Gao, R. Effect of duty cycle on preparation and corrosion behavior of electrodeposited calcium phosphate coatings on AZ91. Appl. Surf. Sci. 2017, 426, 418–426. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Han, E.H. A novel biodegradable nicotinic acid/calcium phosphate composite coating on Mg–3Zn alloy. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zeng, R.; Liu, C.; Gao, J. Comparison of calcium phosphate coatings on Mg–Al and Mg–Ca alloys and their corrosion behavior in hank’s solution. Surf. Coat. Technol. 2010, 204, 3636–3640. [Google Scholar]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. Effect of [Ca2+] and [PO43−] levels on the formation of calcium phosphate conversion coatings on die-cast magnesium alloy AZ91D. Corros. Sci. 2012, 55, 226–232. [Google Scholar] [CrossRef]
- Li, X.; Weng, Z.; Yuan, W.; Luo, X.; Wong, H.M.; Liu, X.; Wu, S.; Yeung, K.W.K.; Zheng, Y.; Chu, P.K. Corrosion resistance of dicalcium phosphate dihydrate/poly(lactic-co-glycolic acid) hybrid coating on AZ31 magnesium alloy. Corros. Sci. 2016, 102, 209–221. [Google Scholar] [CrossRef]
- Cui, W.; Beniash, E.; Gawalt, E.; Xu, Z.; Sfeir, C. Biomimetic coating of magnesium alloy for enhanced corrosion resistance and calcium phosphate deposition. Acta Biomater. 2013, 9, 8650–8659. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yerokhin, A.; Matthews, A. Mechanical behaviour of cp-magnesium with duplex hydroxyapatite and PEO coatings. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 190–200. [Google Scholar] [CrossRef]
- Liu, X.; He, D.; Zhou, Z.; Wang, G.; Wang, Z.; Wu, X.; Tan, Z. Characteristics of (002) oriented hydroxyapatite coatings deposited by atmospheric plasma spraying. Coatings 2018, 8, 258. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, X.; Dang, X.; Hao, J.; Lai, J.; Wang, K. Preparation and properties of composite MAO/ECD coatings on magnesium alloy. Colloids Surf. B Biointerfaces 2013, 102, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Idris, M.H.; Abdul-Kadir, M.R. Synthesis and in vitro degradation evaluation of the nano-HA/MgF2 and DCPD/MgF2 composite coating on biodegradable Mg–Ca–Zn alloy. Surf. Coat. Technol. 2013, 222, 79–89. [Google Scholar] [CrossRef]
- Niu, B.; Shi, P.; Shanshan, E.; Wei, D.; Li, Q.; Chen, Y. Preparation and characterization of HA sol-gel coating on MAO coated AZ31 alloy. Surf. Coat. Technol. 2016, 286, 42–48. [Google Scholar] [CrossRef]
- Liu, G.; Tang, S.; Li, D.; Hu, J. Self-adjustment of calcium phosphate coating on micro-arc oxidized magnesium and its influence on the corrosion behaviour in simulated body fluids. Corros. Sci. 2014, 79, 206–214. [Google Scholar] [CrossRef]
- Hiromoto, S.; Shishido, T.; Yamamoto, A.; Maruyama, N.; Somekawa, H.; Mukai, T. Precipitation control of calcium phosphate on pure magnesium by anodization. Corros. Sci. 2008, 50, 2906–2913. [Google Scholar] [CrossRef]
- Tian, P.; Liu, X.; Ding, C. In vitro degradation behavior and cytocompatibility of biodegradable AZ31 alloy with PEO/HT composite coating. Colloids Surf. B Biointerfaces 2015, 128, 44–54. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Daroonparvar, M.; Saebnoori, E.; Chami, A. In vitro degradation behavior, antibacterial activity and cytotoxicity of TiO2–MAO/ZnHA composite coating on Mg alloy for orthopedic implants. Surf. Coat. Technol. 2018, 334, 450–460. [Google Scholar] [CrossRef]
- Gao, Y.; Yerokhin, A.; Matthews, A. Deposition and evaluation of duplex hydroxyapatite and plasma electrolytic oxidation coatings on magnesium. Surf. Coat. Technol. 2015, 269, 170–182. [Google Scholar] [CrossRef]
- Shi, Y.; Qi, M.; Chen, Y.; Shi, P. MAO-DCPD composite coating on Mg alloy for degradable implant applications. Mater. Lett. 2011, 65, 2201–2204. [Google Scholar] [CrossRef]
- Friedemann, A.E.R.; Thiel, K.; Haßlinger, U.; Ritter, M.; Gesing, T.M.; Plagemann, P. Investigations into the structure of PEO-layers for understanding of layer formation. Appl. Surf. Sci. 2018, 443, 467–474. [Google Scholar] [CrossRef]
- Ng, W.F.; Wong, M.H.; Cheng, F.T. Stearic acid coating on magnesium for enhancing corrosion resistance in hanks’ solution. Surf. Coat. Technol. 2010, 204, 1823–1830. [Google Scholar] [CrossRef]
- Haghshenas, M. Mechanical characteristics of biodegradable magnesium matrix composites: A review. J. Magnes. Alloys 2017, 5, 189–201. [Google Scholar] [CrossRef]
- Khalajabadi, S.Z.; Abdul Kadir, M.R.; Izman, S.; Marvibaigi, M. The effect of mgo on the biodegradation, physical properties and biocompatibility of a Mg/HA/MgO nanocomposite manufactured by powder metallurgy method. J. Alloys Compd. 2016, 655, 266–280. [Google Scholar] [CrossRef]
- Husak, Y.; Solodovnyk, O.; Yanovska, A.; Kozik, Y.; Liubchak, I.; Ivchenko, V.; Mishchenko, O.; Zinchenko, Y.; Kuznetsov, V.; Pogorielov, M. Degradation and in vivo response of hydroxyapatite-coated Mg alloy. Coatings 2018, 8, 375. [Google Scholar] [CrossRef]
- Zhang, Y.; Blawert, C.; Tang, S.; Hu, J.; Mohedano, M.; Zheludkevich, M.L.; Kainer, K.U. Influence of surface pre-treatment on the deposition and corrosion properties of hydrophobic coatings on a magnesium alloy. Corros. Sci. 2016, 112, 483–494. [Google Scholar] [CrossRef]
- Chen, S.; Guan, S.; Li, W.; Wang, H.; Chen, J.; Wang, Y.; Wang, H. In vivo degradation and bone response of a composite coating on Mg–Zn–Ca alloy prepared by microarc oxidation and electrochemical deposition. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 533–543. [Google Scholar] [CrossRef]
- Zhao, L.; Cui, C.; Wang, Q.; Bu, S. Growth characteristics and corrosion resistance of micro-arc oxidation coating on pure magnesium for biomedical applications. Corros. Sci. 2010, 52, 2228–2234. [Google Scholar] [CrossRef]
- Tang, H.; Gao, Y. Preparation and characterization of hydroxyapatite containing coating on AZ31 magnesium alloy by micro-arc oxidation. J. Alloys Compd. 2016, 688, 699–708. [Google Scholar] [CrossRef]
- Anawati; Asoh, H.; Ono, S. Enhanced uniformity of apatite coating on a PEO film formed on AZ31 Mg alloy by an alkali pretreatment. Surf. Coat. Technol. 2015, 272, 182–189. [Google Scholar] [CrossRef]

| Element | Al | Cu | Fe | Mn | Zn | Ca | Ni | Si | P | Mg |
|---|---|---|---|---|---|---|---|---|---|---|
| wt % | 0.00105 | 0.00028 | 0.0042 | 0.00225 | 0.0021 | <0.0001 | <0.0002 | 0.0019 | <0.0001 | Balance |
| Pre-Treatments | Electrolyte | Parameters |
|---|---|---|
| Hydrothermal (HT) | – | Humidity 90% 90 °C 24 h |
| Anodizing (AT) | NaOH 40 g C2H6O2 50 mL Na2CO3 10 g | Direct current 0.1 A 40 °C 60 min Cathode:graphite |
| Plasma electrolytic oxidation (PEO) | KOH 1 g Na3PO4 20 g | 450 V 20 °C 10 min 100 Hz ton = 1 ms toff = 9 ms Cathode:steel |
| Samples | Ecorr (VKCl) | icorr (A/cm2) |
|---|---|---|
| HT | −1.82 ± 0.01 | (9.85 ± 0.56) × 10−5 |
| AT | −1.67 ± 0.02 | (4.95 ± 0.75) × 10−6 |
| PEO | −1.72 ± 0.02 | (1.25 ± 0.55) × 10−6 |
| HT-C | −1.62 ± 0.01 | (7.86 ± 0.51) × 10−6 |
| AT-C | −1.51 ± 0.02 | (9.65 ± 1.38) × 10−7 |
| PEO-C | −1.51 ± 0.01 | (5.09 ± 0.89) × 10−7 |
| HT-C (120 h) | −1.73 ± 0.03 | (1.58 ± 0.66) × 10−5 |
| AT-C (120 h) | −1.62 ± 0.03 | (6.20 ± 0.15) × 10−6 |
| PEO-C (120 h) | −1.57 ± 0.02 | (6.92 ± 0.10) × 10−7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Blawert, C.; Tang, S.; Yang, J.; Hu, J.; Zheludkevich, M.L. Effect of Surface Pre-Treatments on the Formation and Degradation Behaviour of a Calcium Phosphate Coating on Pure Magnesium. Coatings 2019, 9, 259. https://doi.org/10.3390/coatings9040259
Han J, Blawert C, Tang S, Yang J, Hu J, Zheludkevich ML. Effect of Surface Pre-Treatments on the Formation and Degradation Behaviour of a Calcium Phosphate Coating on Pure Magnesium. Coatings. 2019; 9(4):259. https://doi.org/10.3390/coatings9040259
Chicago/Turabian StyleHan, Jiaping, Carsten Blawert, Shawei Tang, Junjie Yang, Jin Hu, and Mikhail L. Zheludkevich. 2019. "Effect of Surface Pre-Treatments on the Formation and Degradation Behaviour of a Calcium Phosphate Coating on Pure Magnesium" Coatings 9, no. 4: 259. https://doi.org/10.3390/coatings9040259
APA StyleHan, J., Blawert, C., Tang, S., Yang, J., Hu, J., & Zheludkevich, M. L. (2019). Effect of Surface Pre-Treatments on the Formation and Degradation Behaviour of a Calcium Phosphate Coating on Pure Magnesium. Coatings, 9(4), 259. https://doi.org/10.3390/coatings9040259







