Photovoltaic Characteristics of Multiwalled Carbon Nanotube Counter-Electrode Materials for Dye-Sensitized Solar Cells Produced by Chemical Treatment and Addition of Dispersant
Abstract
1. Introduction
2. Experimental Details
2.1. Preparation of Materials
2.2. Surface Modification of MWCNTs
2.3. Counter Electrode Fabrication
2.4. TiO2 Working Electrode and DSSC Fabrication
2.5. Characterization
3. Results and Discussion
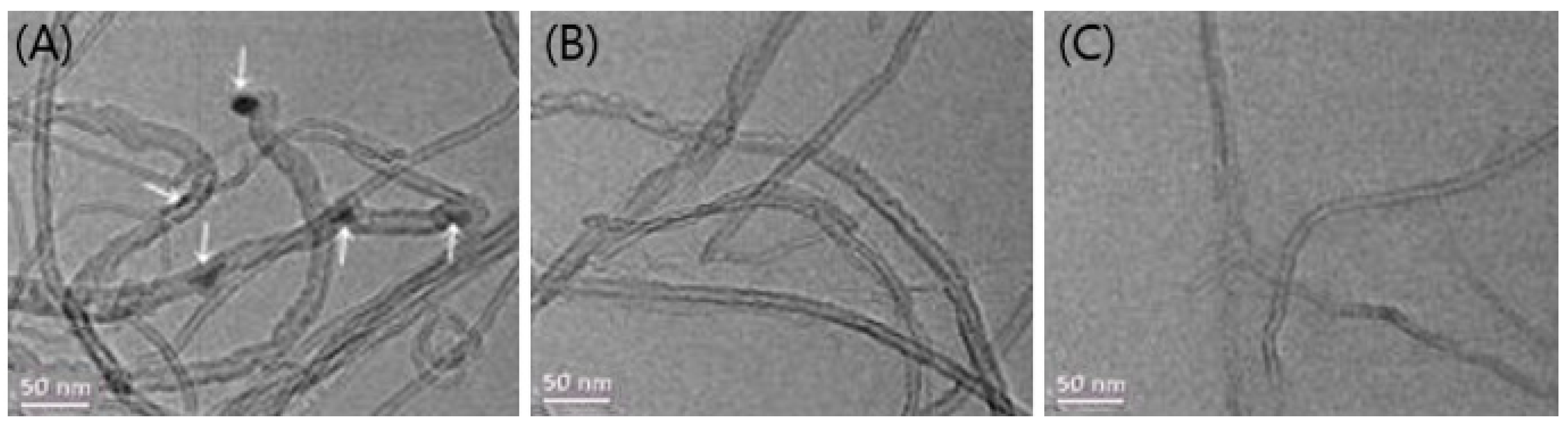
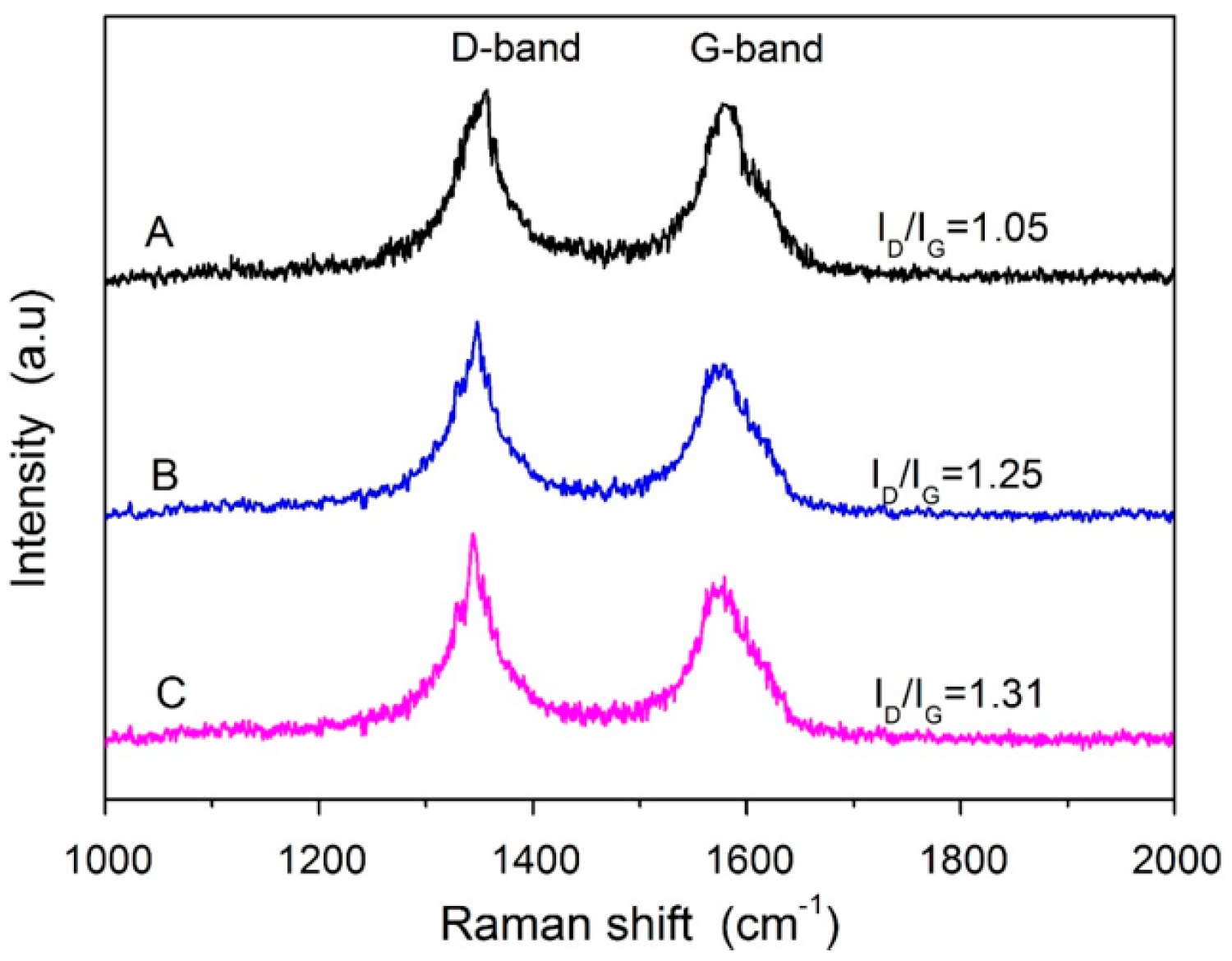
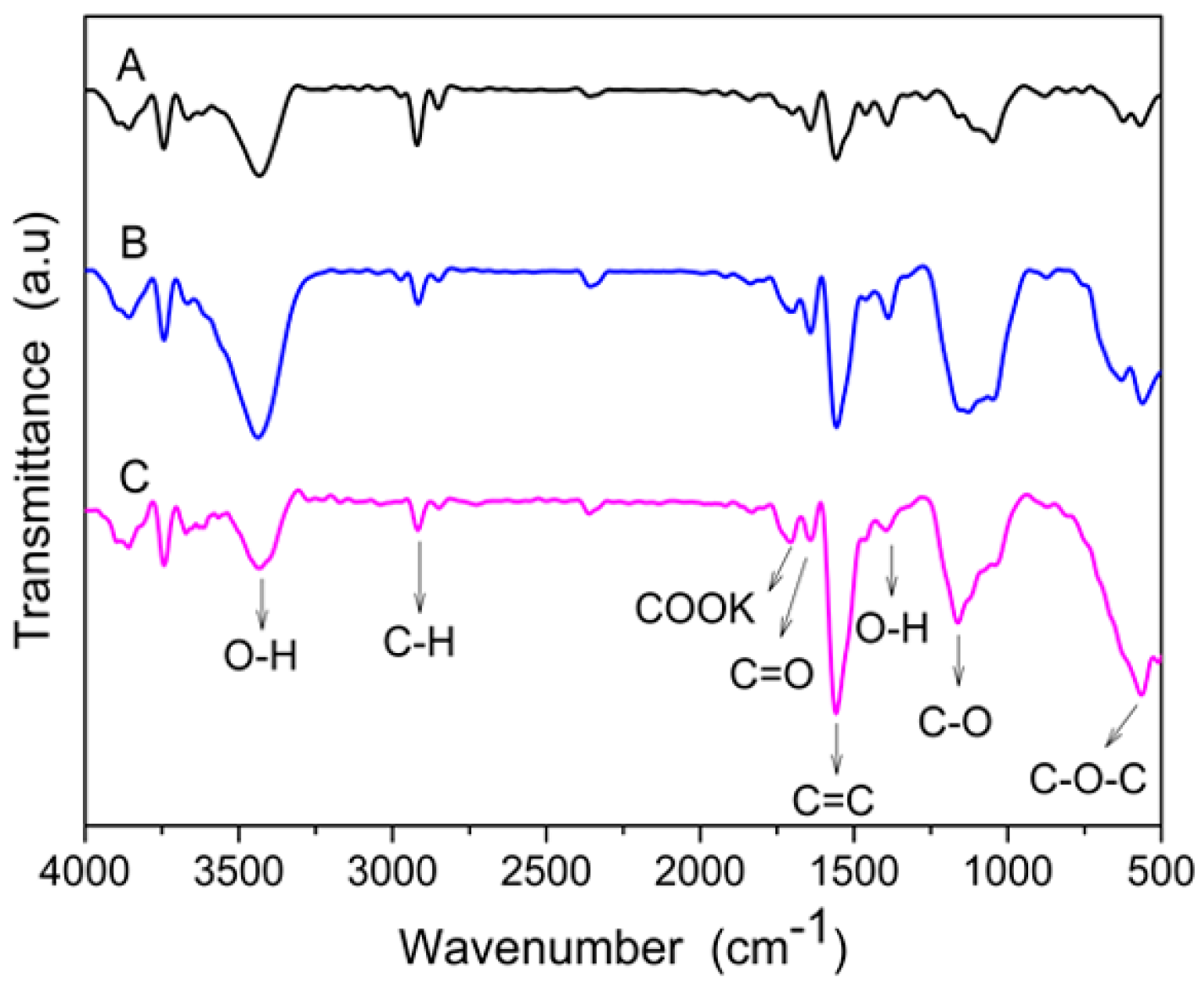
3.1. Morphological Surface Analysis of MWCNTs
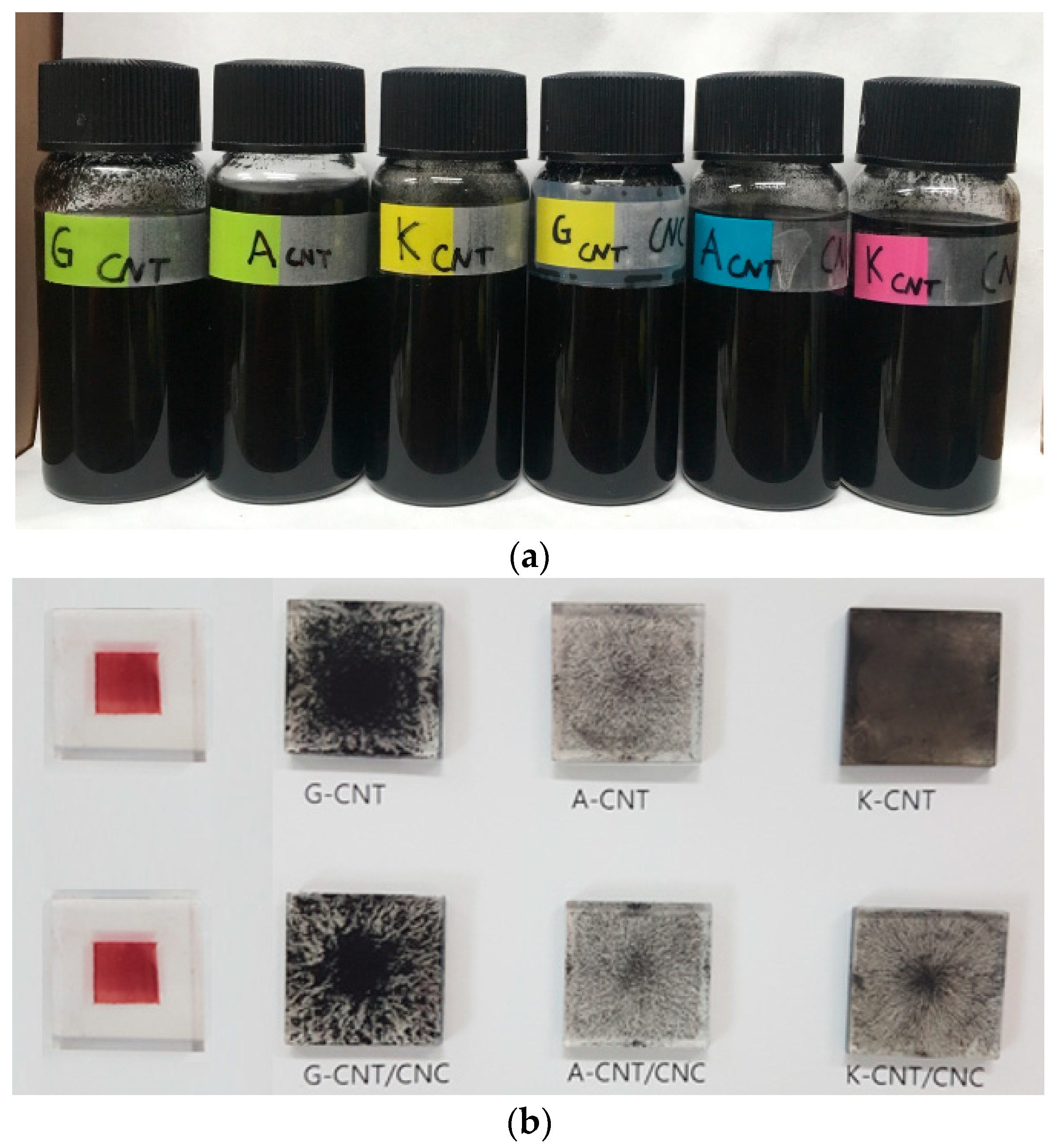
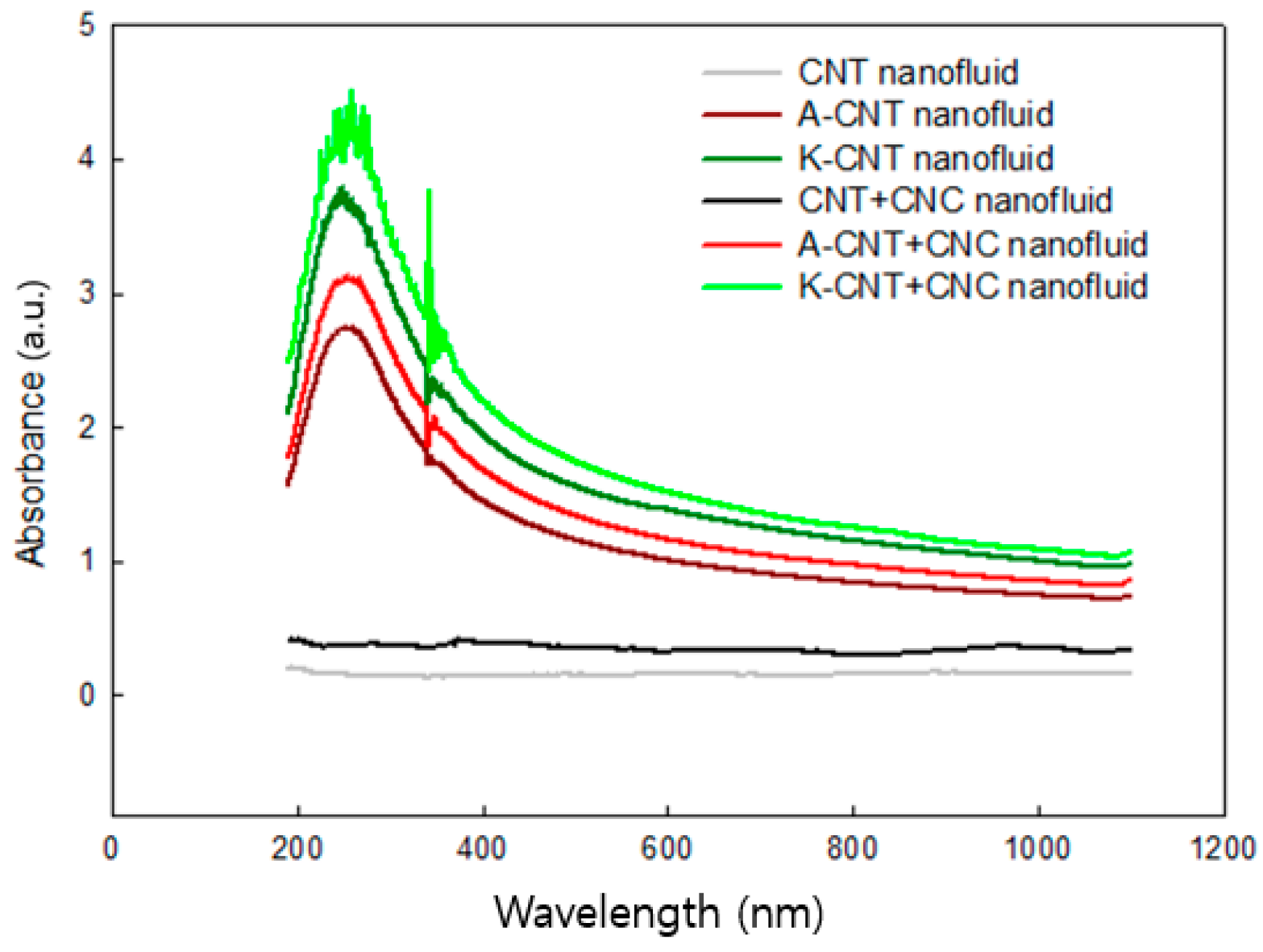
3.2. Material Dispersion and Light Absorption
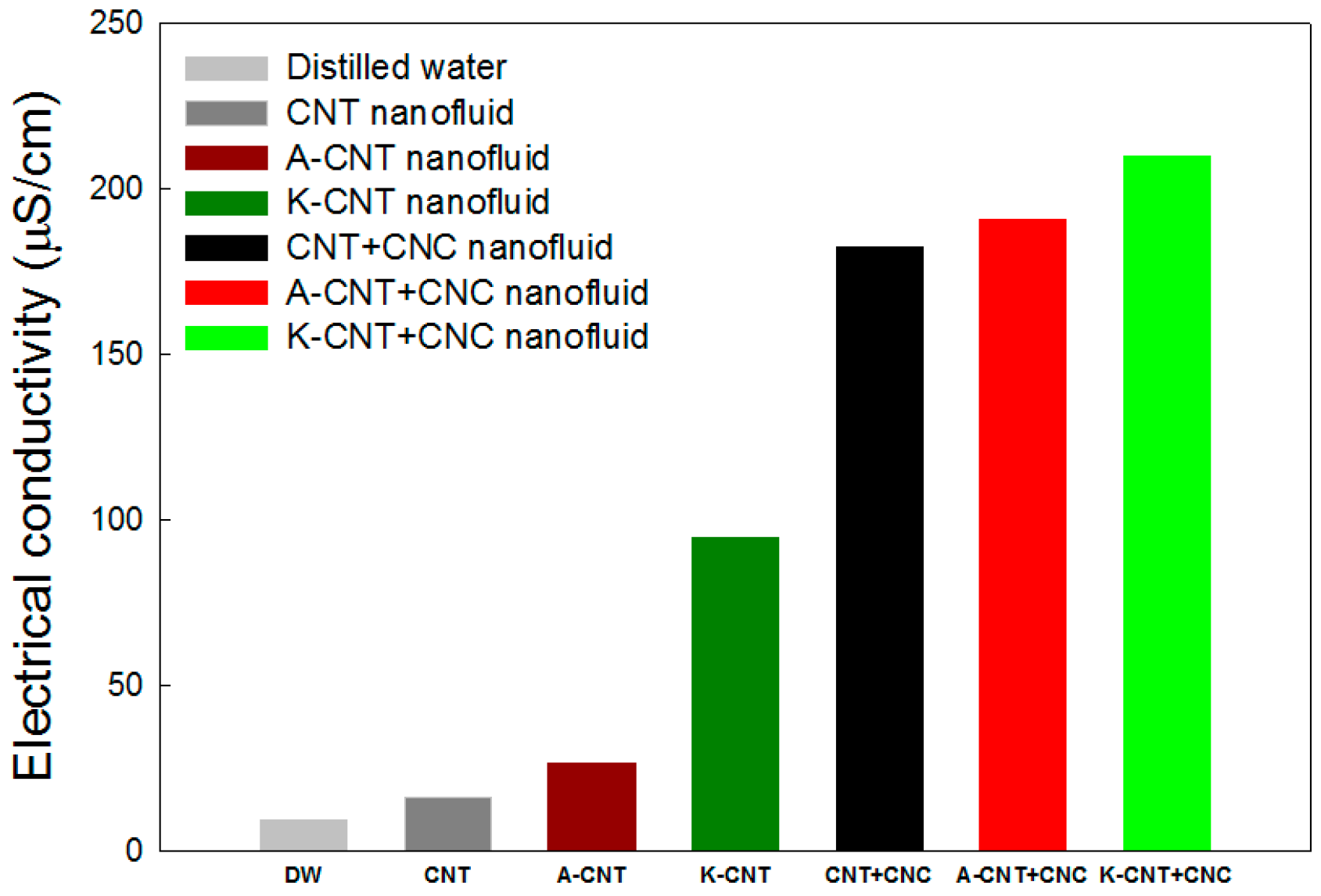
3.3. Electrical Conductivity
3.4. DSSC Photovoltaic Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Müller, E.; Liska, P.; Vlachopoulos, N.; Grätzel, M. Conversion of light to electricity by cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X = Cl−, Br−, I−, CN−, and SCN−) on nanocrystalline titanium dioxide electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Lee, Y.; Chae, J.; Kang, M. Comparison of the photovoltaic efficiency on DSSC for nanometer sized TiO2 using a conventional sol-gel and solvothermal methods. J. Ind. Eng. Chem. 2010, 16, 609–614. [Google Scholar] [CrossRef]
- Chappel, S.; Chen, S.G.; Zaban, A. TiO2-coated nanoporous SnO2 electrodes for dye-sensitized solar cells. Langmuir 2002, 18, 3336–3342. [Google Scholar] [CrossRef]
- Bedja, I.; Kamat, P.V.; Hua, X.; Lappin, A.G.; Hotchandani, S. Photosensitization of nanocrystalline ZnO films by bis(2,2′-bipyridine)(2,2′-bipyridine-4,4′-dicarboxylic acid)ruthenium(II). Langmuir 1997, 13, 2398–2408. [Google Scholar] [CrossRef]
- Keis, K.; Bauer, C.; Boschloo, G.; Hagfeldt, A.; Westermark, K.; Rensmo, H.; Siegbahn, H. Nanostructured ZnO electrodes for dye-sensitized solar cell applications. J. Photochem. Photobiol. A Chem. 2002, 148, 57–64. [Google Scholar] [CrossRef]
- Chappel, S.; Zaban, A. Nanoporous SnO2 electrodes for dye-sensitized solar cells: Improved cell performance by the synthesis of 18 nm SnO2 colloids. Sol. Energy Mater. Sol. Cells 2002, 71, 141–152. [Google Scholar] [CrossRef]
- Guo, P.; Aegerter, M.A. RU (II) sensitized Nb2O5 solar cell made by the sol-gel process. Thin Solid Films 1999, 351, 290–294. [Google Scholar] [CrossRef]
- Murakami, T.N.; Grätzel, M. Counter electrodes for DSC: Application of functional materials as catalysts. Inorganica Chimica Acta 2008, 361, 572–580. [Google Scholar] [CrossRef]
- Congiu, M.; Bonomo, M.; Marco, M.L.D.; Dowling, D.P.; Di Carlo, A.; Dini, D.; Graeff, C.F. Cobalt sulfide as counter electrode in p-type dye-sensitized solar cells. ChemistrySelect 2016, 1, 2808–2815. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, Y.; Yang, L.; Mohammadi, H.; Vlachopoulos, N.; Sun, L.; Hagfeldt, A. Electrochemically polymerized poly(3,4-phenylenedioxythiophene) as efficient and transparent counter electrode for dye sensitized solar cells. Electrochim. Acta 2019, 300, 482–488. [Google Scholar] [CrossRef]
- Hu, L.; Hecht, D.S.; Gruner, G. Carbon nanotube thin films: Fabrication, properties, and applications. Chem. Rev. 2010, 110, 5790–5844. [Google Scholar] [CrossRef] [PubMed]
- Dan, B.; Irvin, G.C.; Pasquali, M. Continuous and scalable fabrication of transparent conducting carbon nanotube films. ACS Nano 2009, 3, 835–843. [Google Scholar] [CrossRef]
- Hu, L.; Hecht, D.S.; Grüner, G. Percolation in transparent and conducting carbon nanotube networks. Nano Lett. 2004, 4, 2513–2517. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef]
- Hong, W.; Xu, Y.; Lu, G.; Li, C.; Shi, G. Transparent graphene/PEDOT–PSS composite films as counter-electrodes of dye-sensitized solar cells. Electrochem. Commun. 2008, 10, 1555–1558. [Google Scholar] [CrossRef]
- Hu, L.; Hecht, D.S.; Grüner, G. Infrared transparent carbon nanotube thin films. Appl. Phys. Lett. 2009, 94, 081103. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Bai, X.; Sun, X.; Wang, X.; Wang, E.; Dai, H. Highly conducting graphene sheets and Langmuir-Blodgett films. Nat. Nanotechnol. 2008, 3, 538–542. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef]
- Son, Y.W.; Cohen, M.L.; Louie, S.G. Energy gaps in graphene nanoribbons. Phys. Rev. Lett. 2006, 97, 216803. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.J.; Mullen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef]
- Wu, J.B.; Becerril, H.A.; Bao, Z.N.; Liu, Z.F.; Chen, Y.S.; Peumans, P. Organic solar cells with solution-processed graphene transparent electrodes. Appl. Phys. Lett. 2008, 92, 263302. [Google Scholar] [CrossRef]
- Ju, S.; Li, J.F.; Liu, J.; Chen, P.C.; Ha, Y.G.; Ishikawa, F.; Chang, H.; Zhou, C.W.; Facchetti, A.; Janes, D.B.; et al. Transparent active matrix organic light-emitting diode displays driven by nanowire transistor circuitry. Nano Lett. 2008, 8, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Higgins, T.M.; Lyons, P.E.; Doherty, E.M.; Nirmalraj, P.N.; Blau, W.J.; Boland, J.J.; Coleman, J.N. Silver nanowire networks as flexible, transparent, conducting films: extremely high DC to optical conductivity ratios. ACS Nano 2009, 3, 1767–1774. [Google Scholar] [CrossRef]
- Tung, V.C.; Allen, M.J.; Yang, Y.; Kaner, R.B. High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 2009, 4, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Tung, V.C.; Chen, L.M.; Allen, M.J.; Wassei, J.K.; Nelson, K.; Kaner, R.B.; Yang, Y. Low-temperature solution processing of graphene-carbon nanotube hybrid materials for high-performance transparent conductors. Nano Lett. 2009, 9, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Kang, J.; Liu, Y.; He, H. Economical and highly efficient Pt-free counter electrode for dye-sensitized solar cells. Colloids Surf. A Physicochem. Eng. Asp. 2019, 566, 1–10. [Google Scholar] [CrossRef]
- Lee, S.U.; Choi, W.S.; Hong, B. A comparative study of dye-sensitized solar cells added carbon nanotubes to electrolyte and counter electrodes. Sol. Energy Mater. Sol. Cells 2010, 94, 680–685. [Google Scholar] [CrossRef]
- Luo, J.; Niu, H.J.; Wu, W.J.; Wang, C.; Bai, X.D.; Wang, W. Enhancement of the efficiency of dye-sensitized solar cell with multi-wall carbon nanotubes/polythiophene composite counter electrodes prepared by electrodeposition. Solid State Sci. 2012, 14, 145–149. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G. Electrolytes in dye-sensitized solar cells. Chem. Rev. 2015, 115, 2136–2173. [Google Scholar] [CrossRef]
- Mohanty, S.P.; Bhargava, P. Impact of electrolytes based on different solvents on thelong term stability of dye sensitized solar cells. Electrochim. Acta 2015, 168, 111–115. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Nguyen, T.D.T.; Nguyen, V.S.; Dang, D.T.X.; Le, H.M.; Wei, T.C.; Tran, P.H. Application of deep eutectic solvent from phenol and choline chloride in electrolyte to improve stability performance in dye-sensitized solar cells. J. Mol. Liq. 2019, 277, 157–162. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, A.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Datye, A.; Wu, K.H.; Gomes, G.; Monroy, V.; Lin, H.T.; Jozef, V.; Vanmeensel, K. Synthesis, microstructure and mechanical properties of Yttria Stabilized Zirconia (3YTZP)—Multi-Walled Nanotube (MWNTs) nanocomposite by direct in-situ growth of MWNTs on Zirconia particles. Compos. Sci. Technol. 2010, 70, 2086–2092. [Google Scholar] [CrossRef]
- Lee, K.M.; Hu, C.W.; Chen, H.W.; Ho, K.C. Incorporating carbon nanotube in a low-temperature fabrication process for dye-sensitized TiO2 solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 1628–1633. [Google Scholar] [CrossRef]
- Park, O.K.; Jeevananda, T.; Kim, N.H.; Kim, S.I.; Lee, J.H. Effects of surface modification on the dispersion and electrical conductivity of carbon nanotube/polyaniline composites. Scr. Mater. 2009, 60, 551–554. [Google Scholar] [CrossRef]
- Choi, H.J.; Shin, J.E.; Lee, G.W.; Park, N.G.; Kim, K.; Hong, S.C. Effect of surface modification of multi-walled carbon nanotubes on the fabrication and performance of carbon nanotube based counter electrodes for dye-sensitized solar cells. Curr. Appl. Phys. 2010, 10, S165–S167. [Google Scholar] [CrossRef]
- Ramasamy, E.; Lee, W.J.; Lee, D.Y.; Song, J.S. Spray coated multi-wall carbon nanotube counter electrode for tri-iodide (I3−) reduction in dye-sensitized solar cells. Electrochem. Commun. 2008, 10, 1087–1089. [Google Scholar] [CrossRef]
- Lee, W.J.; Ramasamy, E.; Lee, D.Y.; Song, J.S. Efficient dye-sensitized solar cells with catalytic multiwall carbon nanotube counter electrodes. Appl. Mater. Interfaces 2009, 1, 1145–1149. [Google Scholar] [CrossRef]
- Nam, J.G.; Park, Y.J.; Kim, B.S.; Lee, J.S. Enhancement of the efficiency of dye-sensitized solar cell by utilizing carbon nanotube counter electrode. Scr. Mater. 2010, 62, 148–150. [Google Scholar] [CrossRef]
- Ramachandran, K.; Kadirgama, K.; Ramasamy, D.; Azmi, W.H.; Tarlochan, F. Investigation on effect thermal conductivity and relative viscosity of cellulose nanocrystal as a nanofluidic thermal transport through a combined experimental—Statistical approach by using response surface methodology. Appl. Therm. Eng. 2017, 122, 473–483. [Google Scholar] [CrossRef]
- Ramachandran, K.; Hussein, A.M.; Kadirgama, K.; Ramasamy, D.; Azmi, W.H.; Tarlochan, F.; Kadirgama, G. Thermophysical properties measurement of nano cellulose in ethylene glycol/water. Appl. Therm. Eng. 2017, 123, 1158–1165. [Google Scholar] [CrossRef]
- Myekhlai, M.; Munkhbayar, B.; Lee, T.; Tanshen, M.R.; Chung, H.; Jeong, H. Experimental investigation of the mechanical grinding effect on graphene structure. RSC Adv. 2014, 4, 2495–2500. [Google Scholar] [CrossRef]
- Munkhbayar, B.; Nine, M.J.; Jeoun, J.; Bat-Erdene, M.; Chung, H.; Jeong, H. Influence of dry and wet ball milling on dispersion characteristics of the multi-walled carbon nanotubes in aqueous solution with and without surfactant. Powder Technol. 2013, 234, 132–140. [Google Scholar] [CrossRef]
- Hong, C.E.; Lee, J.H.; Kalappa, P.; Advani, S.G. Effects of oxidative conditions on properties of multi-walled carbon nanotubes in polymer nanocomposites. Compos. Sci. Technol. 2007, 67, 1027–1034. [Google Scholar] [CrossRef]
- Zhang, L.; Ni, Q.Q.; Fu, Y.; Natsuki, T. One-step preparation of water-soluble single-walled carbon nanotubes. Appl. Surf. Sci. 2009, 255, 7095–7099. [Google Scholar] [CrossRef]
- Munkhbayar, B.; Hwang, S.; Kim, J.; Bae, K.; Ji, M.; Chung, H.; Jeong, H. Photovoltaic performance of dye-sensitized solar cells with various MWCNT counter electrode structures produced by different coating methods. Electrochim. Acta 2012, 80, 100–107. [Google Scholar] [CrossRef]
- Dhungel, S.K.; Park, J.G. Optimization of paste formulation for TiO2 nanoparticles with wide range of size distribution for its application in dye sensitized solar cells. Renew. Energy 2010, 35, 2776–2780. [Google Scholar] [CrossRef]
- Tsoukleris, D.S.; Arabatzis, I.M.; Chatzivasiloglou, E.; Kontos, A.I.; Belessi, V.; Bernard, M.C.; Falaras, P. 2-Ethyl-1-hexanol based screen-printed titania thin films for dye-sensitized solar cells. Sol. Energy. 2005, 79, 422–430. [Google Scholar] [CrossRef]
- Jhong, H.R.; Wong, D.S.H.; Wan, C.C.; Wang, Y.Y.; Wei, T.C. A novel deep eutectic solvent-based ionic liquid used as electrolyte for dye-sensitized solar cells. Electrochem. Commun. 2009, 11, 209–211. [Google Scholar] [CrossRef]
- Yudianti, R.; Onggo, H.; Saito, Y.; Iwata, T.; Azuma, J.I. Analysis of functional group sited on multi-wall carbon nanotube surface. Open Mater. Sci. J. 2011, 5, 242–247. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, Y.; Yu, T.; Shen, Z. Raman spectroscopy and imaging of graphene. Nano Res. 2008, 1, 273–291. [Google Scholar] [CrossRef]
- Gupta, A.; Chen, G.; Joshi, P.; Tadigadapa, S.; Eklund, P.C. Raman scattering from high-frequency phonons in supported n-graphene layer films. Nano Lett. 2006, 6, 2667–2673. [Google Scholar] [CrossRef]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially resolved Raman spectroscopy of single- and few-layer graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef]
- Wang, X.; Huang, P.; Feng, L.; He, M.; Guo, S.; Shen, G.; Cui, D. Green controllable synthesis of silver nanomaterials on graphene oxide sheets via spontaneous reduction. RSC Adv. 2012, 2, 3816–3822. [Google Scholar] [CrossRef]
- Yoo, H.J.; Kim, K.H.; Yadav, S.K.; Cho, J.W. Effects of carbon nanotube functionalization and annealing on crystallization and mechanical properties of melt-spun carbon nanotubes/poly(ethylene terephthalate) fibers. Compos. Sci. Technol. 2012, 72, 1834–1840. [Google Scholar] [CrossRef]
- Munkhbayar, B.; Bat-Erdene, M.; Ochirkhuyag, B.; Sarangerel, D.; Battsengel, B.; Chung, H.; Jeong, H. An experimental study of the planetary ball milling effect on dispersibility and thermal conductivity of MWCNTs-based aqueous nanofluids. Mater. Res. Bull. 2012, 47, 4187–4196. [Google Scholar] [CrossRef]
- Shim, J.W.; Park, S.J.; Ryu, S.K. Effect of modification with HNO3 and NaOH on metal adsorption by pitch-based activated carbon fibers. Carbon. 2001, 39, 1635–1642. [Google Scholar] [CrossRef]
- Nasiri, A.; Shariaty-Niasar, M.; Rashidi, A.M.; Khodafarin, R. Effect of CNT structures on thermal conductivity and stability of nanofluid. Inter. J. Heat Mass Transf. 2012, 55, 1529–1535. [Google Scholar] [CrossRef]
- Yadav, S.K.; Mahapatra, S.S.; Yoo, H.J.; Cho, J.W. Synthesis of multi-walled carbon nanotube/polyhedral oligomeric silsesquioxane nanohybrid by utilizing click chemistry. Nanoscale Res. Lett. 2011, 6, 122. [Google Scholar] [CrossRef]
- Wei, J.; Lv, R.; Guo, N.; Wang, H.; Bai, X.; Mathkar, A.; Kang, F.; Zhu, H.; Wang, K.; Wu, D.; et al. Preparation of highly oxidized nitrogen-doped carbon nanotubes. Nanotechnology 2012, 23, 155601. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Agarwal, S.; Saleh, T.A. Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J. Hazard. Mater. 2011, 185, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Grossiord, N.; Koning, C.E.; Loos, J. Controlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solution. Carbon 2007, 45, 618–623. [Google Scholar] [CrossRef]
- Hamada, N.; Sawada, S.I.; Oshiyama, A. New one-dimensional conductors: Graphitic microtubules. Phys. Rev. Lett. 1992, 68, 1579. [Google Scholar] [CrossRef]
- Saito, R.; Fujita, M.; Dresselhaus, G.; Dresselhaus, U.M. Electronic structure of chiral graphene tubules. Appl. Phys. Lett. 1992, 60, 2204–2206. [Google Scholar] [CrossRef]
- Bordi, F.; Cametti, C.; Codastefano, P.; Tartaglia, P. Electrical conductivity of colloidal systems during irreversible aggregation. Phys. A Stat. Mech. Appl. 1990, 164, 663–672. [Google Scholar] [CrossRef]
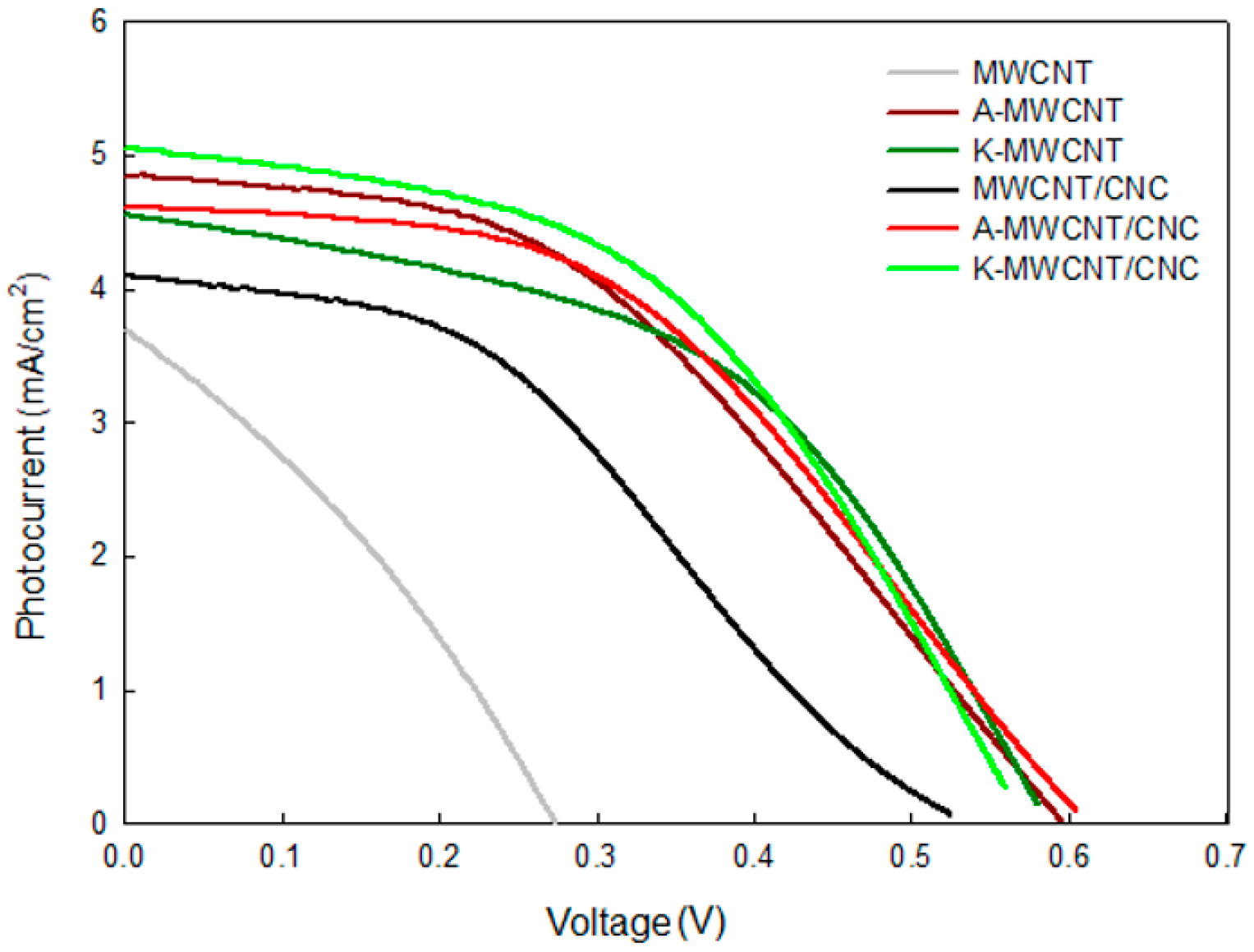
| Counter Electrode | Jsc (mA/cm−2) | Voc (V) | FF | η (%) |
|---|---|---|---|---|
| CNT | 3.684 | 0.2738 | 0.3172 | 0.2051 |
| A-CNT | 5.066 | 0.5794 | 0.413 | 1.214 |
| K-CNT | 4.48 | 0.5796 | 0.4877 | 1.266 |
| CNT/CNC | 4.363 | 0.5025 | 0.367 | 0.517 |
| A-CNT/CNC | 4.812 | 0.5909 | 0.44 | 1.252 |
| K-CNT/CNC | 5.092 | 0.5592 | 0.04747 | 1.352 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Dovjuu, O.; Choi, S.-H.; Jeong, H.; Park, J.-T. Photovoltaic Characteristics of Multiwalled Carbon Nanotube Counter-Electrode Materials for Dye-Sensitized Solar Cells Produced by Chemical Treatment and Addition of Dispersant. Coatings 2019, 9, 250. https://doi.org/10.3390/coatings9040250
Kim S, Dovjuu O, Choi S-H, Jeong H, Park J-T. Photovoltaic Characteristics of Multiwalled Carbon Nanotube Counter-Electrode Materials for Dye-Sensitized Solar Cells Produced by Chemical Treatment and Addition of Dispersant. Coatings. 2019; 9(4):250. https://doi.org/10.3390/coatings9040250
Chicago/Turabian StyleKim, Sedong, Otgonbayar Dovjuu, Soon-Ho Choi, Hyomin Jeong, and Ji-Tae Park. 2019. "Photovoltaic Characteristics of Multiwalled Carbon Nanotube Counter-Electrode Materials for Dye-Sensitized Solar Cells Produced by Chemical Treatment and Addition of Dispersant" Coatings 9, no. 4: 250. https://doi.org/10.3390/coatings9040250
APA StyleKim, S., Dovjuu, O., Choi, S.-H., Jeong, H., & Park, J.-T. (2019). Photovoltaic Characteristics of Multiwalled Carbon Nanotube Counter-Electrode Materials for Dye-Sensitized Solar Cells Produced by Chemical Treatment and Addition of Dispersant. Coatings, 9(4), 250. https://doi.org/10.3390/coatings9040250





