Design and Development of Oleoresins Rich in Carotenoids Coated Microbeads
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Extraction of Oleoresins from Sea Buckthorn Fruits (Hippophae rhamnoides)
2.3. Fractions Analysis
2.4. Analysis of Biopolymers
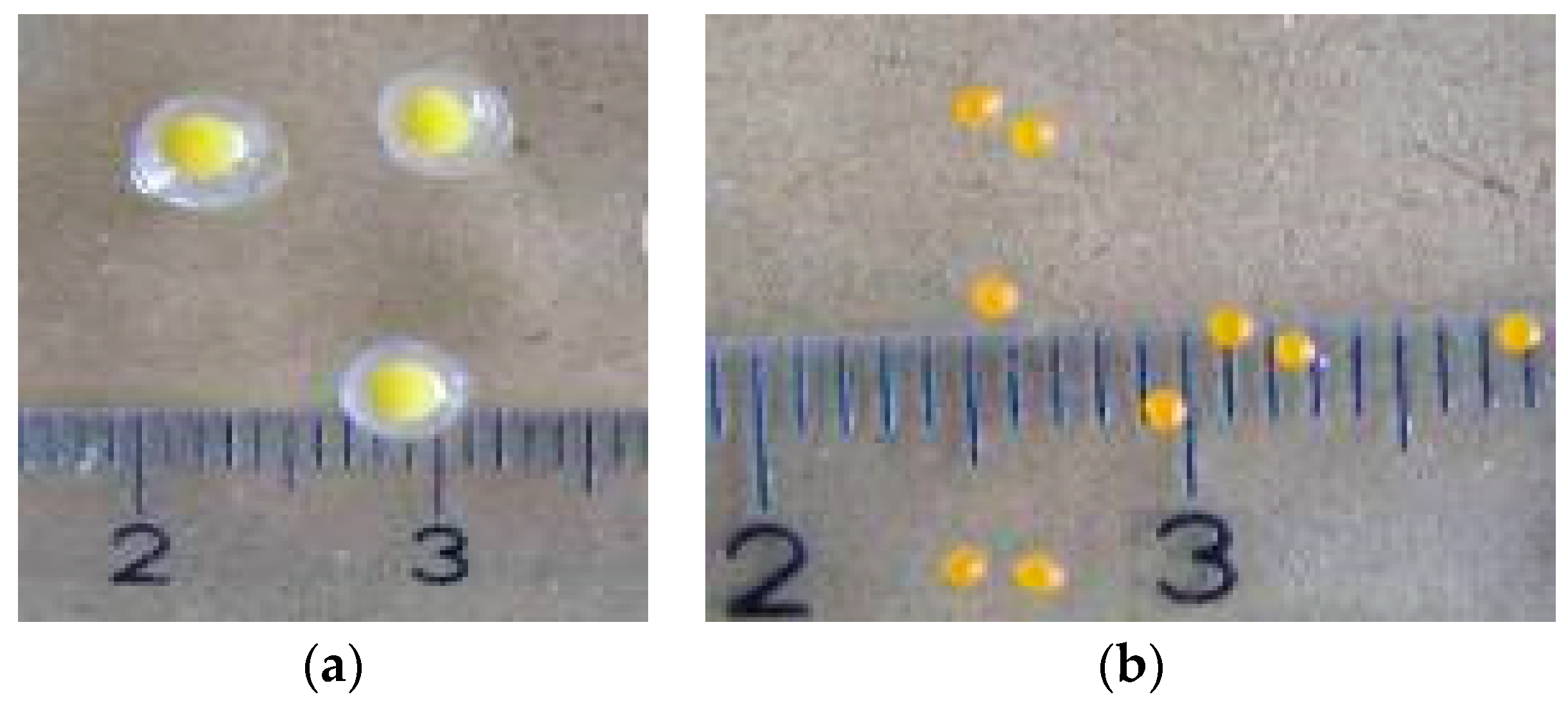
2.5. Coated Microbeads Preparation
2.6. Determination of Encapsulation Efficiency of the Oleoresins
2.7. UV-Vis Analysis
2.8. Analysis of Coated Microbeads for Encapsulation of Oleoresins
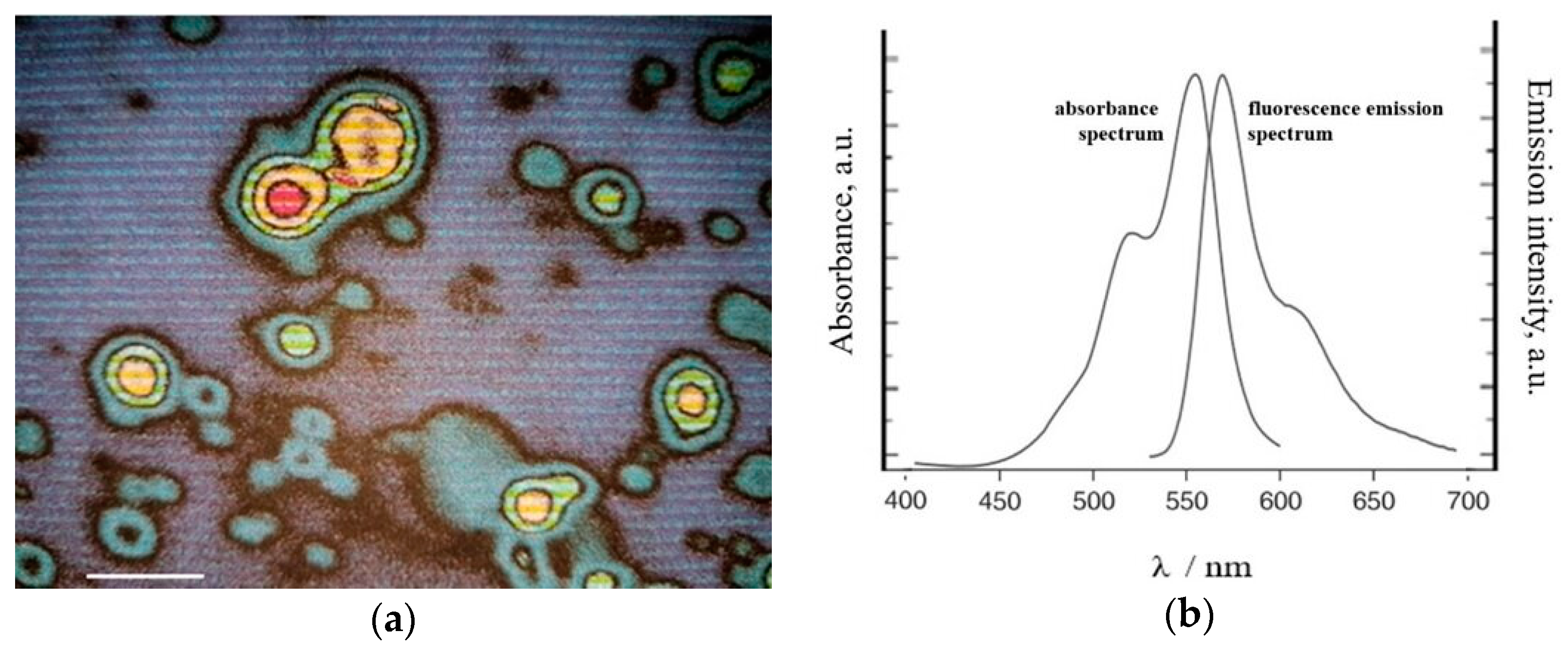
2.8.1. Fluorescence Method
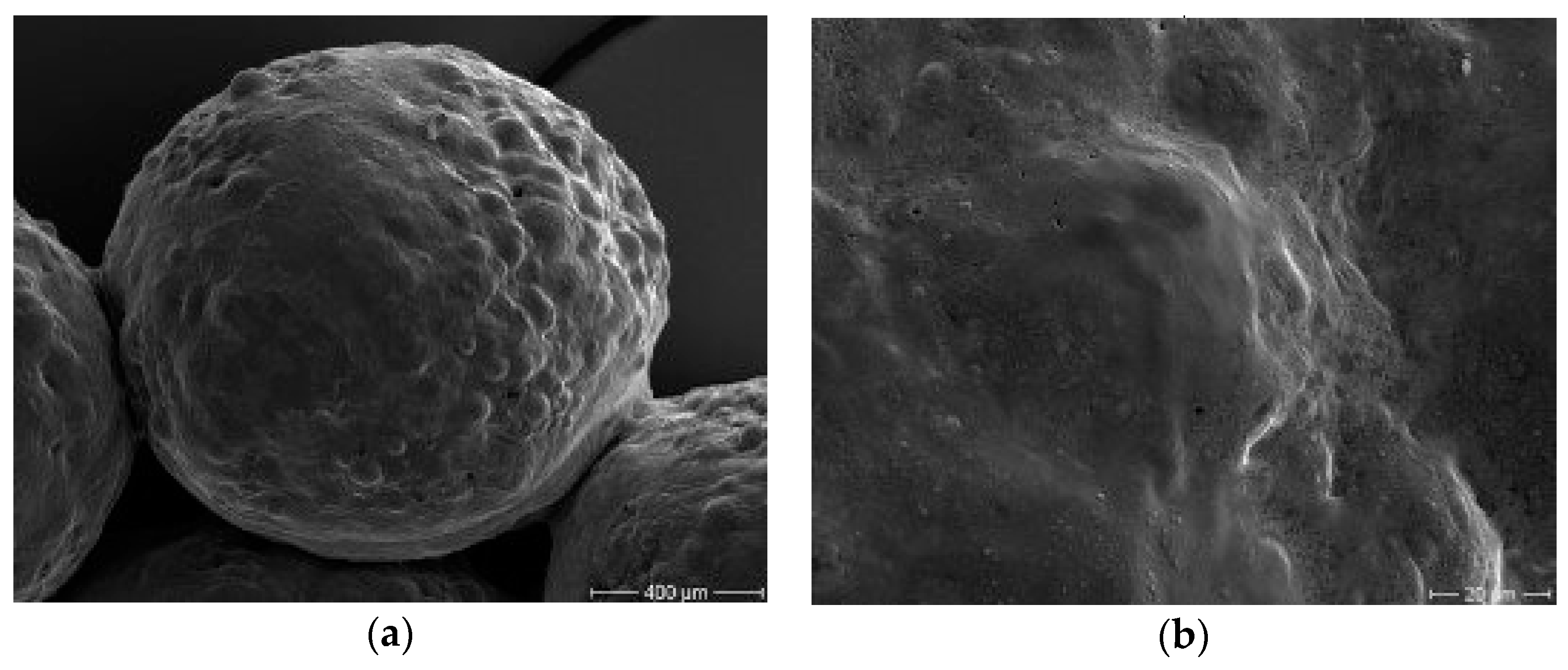
2.8.2. Scanning Electron Microscopy (SEM)
2.8.3. Microbeads Stability in Different Solutions with Different pH
3. Results and Discussion
3.1. Characterization of Fractions
3.2. Characterization of Biopolymers
3.3. Oleoresins Encapsulation Efficiency Measured by β-Carotene Content
3.4. Characterization of Coated Microbeads Containing Oleoresins Encapsulated
3.4.1. Fluorescence Characterization
3.4.2. Microbeads Surface Morphology
3.5. Microbeads Stability in Different Solutions with Different pH
- pH = 2 to mimic stomach pH: consisted of 0.1 N HCl;
- pH = 4.5 to mimic intestinal fluid: mixing solution pH 1.2 and solution pH 7.4 in a ratio 39:61; pH adjusted to 4.5 ± 0.1.
- pH = 5–6.5 to mimic duodenum and proximal jejunum pH;
- pH = 7.1–7.4 to mimic saliva and colon pH: consisted of 1.074 g KH2PO4 in 30 mL of 0.2 N NaOH;
- pH = 6.5–8 to mimic large bowel pH.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zielińska, A.; Nowak, I. Abundance of active ingredients in sea-buckthorn oil. Lipids Health Dis. 2017, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) oil. J. Ethnopharmacol. 2018, 213, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Dulf, F.V. Fatty acids in berry lipids of six sea buckthorn (Hippophae rhamnoides L., subspecies carpatica) cultivars grown in Romania. Chem. Cent. J. 2012, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Kallio, H.P.; Yang, B. Health effects of sea buckthorn berries; research and. strategies at the University of Turku, Finland. Acta Hortic. 2014, 1017, 343–349. [Google Scholar] [CrossRef]
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem. Toxicol. 2016, 97, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Exporting Oleoresins for Food to Europe. Available online: https://www.cbi.eu/market-information/natural-food-additives/oleoresins (accessed on 20 January 2019).
- Nishinari, K. Some thoughts on the definition of a gel. In Gels: Structures, Properties, and Functions; Tokita, M., Nishinari, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 87–94. [Google Scholar]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Paşcalău, V.; Popescu, V.; Popescu, G.L.; Dudescu, M.C.; Borodi, V.; Dinescu, A.; Perhaiţa, I.; Paul, M. The alginate/k-carrageenan ratio’s influence on the properties of the cross-linked composite films. J. Alloy. Compd. 2012, 536, S418–S423. [Google Scholar] [CrossRef]
- Poncelet, D. Microencapsulation: Fundamentals, methods and applications. In Surface Chemistry in Biomedical and Environmental Science; Blitz, J.P., Gun’ko, V.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 23–34. [Google Scholar]
- Martins, E.; Poncelet, P.; Rodrigues, R.C.; Renard, D. Oil encapsulation techniques using alginate as encapsulating agent: Applications and drawbacks. J. Microencapsul. 2016, 34, 754–771. [Google Scholar] [CrossRef]
- Strasdat, B.; Bunjes, H. Incorporation of lipid nanoparticles into calcium alginate beads and characterization of the encapsulated particles by differential scanning calorimetry. Food Hydrocoll. 2013, 30, 567–575. [Google Scholar] [CrossRef]
- Cojocneanu Petric, R.; Braicu, C.; Raduly, L.; Zanoaga, O.; Dragos, N.; Monroig, P.; Dumitrascu, D.; Berindan-Neagoe, I. Phytochemicals modulate carcinogenic signaling pathways in breast and hormone-related cancers. Onco Targ. Ther. 2015, 8, 2053–2066. [Google Scholar] [CrossRef]
- Cioroi, M.; Chiriac, E.R.; Stefan, C.S. determination of acidity, total polyphenols content, calcium, magnesium and phosphorous in sea buckthorn berries. Rev. Chim. (Bucharest) 2017, 68, 300–303. [Google Scholar]
- Trif, M.; Ansorge-Schumacher, M.B.; Socaciu, C.; Diehl, H.A. Bioencapsulated seabuckthorn oil: Controlled release rates in different solvents. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2008, 65, 415–420. [Google Scholar] [CrossRef]
- Trif, M.; Socaciu, C. Evaluation of effiency, release and oxidation stability of seabuckthorn microencapsulated oil using Fourier transformed infrared spectroscopy. Chem. Listy 2008, 102, s1198–s1199. [Google Scholar]
- Laos, K.; Lõugas, T.; Mändmets, A.; Vokk, V. Encapsulation of β-carotene from sea buckthorn (Hippophaë rhamnoides L.) juice in furcellaran beads. Innova. Food Sci. Emerg. Technol. 2007, 8, 395–398. [Google Scholar] [CrossRef]
- Bindea, M.; Rusu, B.; Rusu, A.; Trif, M.; Leopold, L.F.; Dulf, F.; Vodnar, D.C. Valorification of crude glycerol for pure fractions of docosahexaenoic acid and β-carotene production by using Schizochytrium limacinum and Blakeslea trispora. Microb. Cell Fact. 2018, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- González-Casado, S.; Martín-Belloso, O.; Elez-Martínez, P.; Soliva-Fortuny, R. Application of pulsed electric fields to tomato fruit for enhancing the bioaccessibility of carotenoids in derived products. Food Funct. 2018, 9, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Oms-Oliu, G.; Odriozola-Serrano, I.; Lamuela-Raventós, R.M.; Martín-Belloso, O.; Elez-Martínez, P. Metabolite profiling of phenolic and carotenoid contents in tomatoes after moderate-intensity pulsed electric field treatments. Food Chem. 2013, 136, 199–205. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Parisi, F.; Milioto, S.; Fakhrullin, R.; Lazzara, G. Core/shell gel beads with embedded halloysite nanotubes for controlled drug release. Coatings 2019, 9, 70. [Google Scholar] [CrossRef]
- Lyn, M.E.; Ying, D. Drying model for calcium alginate beads. Ind. Eng. Chem. Res. 2010, 49, 1986–1990. [Google Scholar] [CrossRef]
- Fernandez-Arrojo, L.; Rodriguez-Colinas, B.; Gutierrez-Alonso, P.; Fernandez-Lobato, M.; Alcalde, M.; Ballesteros, A.O.; Plou, F.J. Dried alginate-entrapped enzymes (DALGEEs) and their application to the production of fructooligosaccharides. Process Biochem. 2013, 48, 677–682. [Google Scholar] [CrossRef]
- Van de Waterbeemd, H.; Testa, B.; Mannhold, R.; Kubinyi, H.; Folkers, G. Drug Bioavailability: Estimation of Solubility, Permeability, Absorption and Bioavailability, 2nd ed.; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Krisanti, E.; Astuty, R.M.; Mulia, K. Microencapsulation of oleoresin from red ginger (Zingiber officinale var. Rubrum) in chitosan and alginate for fresh milk preservatives. AIP Conf. Proc. 2017, 1817, 030016. [Google Scholar] [CrossRef]
- Yulvianti, M.; Barleany, D.R.; Ernayati, W. Encapsulation red ginger oleoresin (Zingiber officinale) with chitosan-alginate as wall material using spray drying. Res. J. Appl. Sci. Eng. Technol. 2015, 10, 1370–1378. [Google Scholar] [CrossRef]
- Shaikh, J.; Bhosale, R.; Singhal, R. Microencapsulation of black pepper oleoresin. Food Chem. 2006, 94, 105–110. [Google Scholar] [CrossRef]
- Krithika, V.; Radhai Sri, S.; Ravindra, N.; Thirupathi, V. Microencapsulation of paprika (Capsicum annum L) oleoresin by spray drying. Int. J. Sci. Eng. Res. 2014, 5, 971–980. [Google Scholar]
- Vaidya, S.; Bhosale, R.; Singhal, R.S. Microencapsulation of cinnamon oleoresin by spray drying using different wall materials. Dry. Technol. 2016, 24, 983–992. [Google Scholar] [CrossRef]
- Rosa, J.M.; Bonato, L.B.; Mancuso, C.B.; Martinelli, L.; Okura, M.H.; Malpass, G.R.P.; Granato, A.C. Antimicrobial wound dressing films containing essential oils and oleoresins of pepper encapsulated in sodium alginate films. Ciência Rural 2018, 48, e20170740. [Google Scholar] [CrossRef]
- Ahirrao, S.P.; Gide, P.S.; Shrivastav, B.; Sharma, P. Ionotropic gelation: A promising cross linking technique for hydrogels. Res. Rev. J. Pharm. Nanotechnol. 2014, 2, 1–6. [Google Scholar]
- Zhang, Y.; Zhang, H.; Wang, F.; Wang, L.-X. Preparation and properties of ginger essential oil β-cyclodextrin/chitosan inclusion complexes. Coatings 2018, 8, 305. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trif, M.; Vodnar, D.C.; Mitrea, L.; Rusu, A.V.; Socol, C.T. Design and Development of Oleoresins Rich in Carotenoids Coated Microbeads. Coatings 2019, 9, 235. https://doi.org/10.3390/coatings9040235
Trif M, Vodnar DC, Mitrea L, Rusu AV, Socol CT. Design and Development of Oleoresins Rich in Carotenoids Coated Microbeads. Coatings. 2019; 9(4):235. https://doi.org/10.3390/coatings9040235
Chicago/Turabian StyleTrif, Monica, Dan Cristian Vodnar, Laura Mitrea, Alexandru Vasile Rusu, and Claudia Terezia Socol. 2019. "Design and Development of Oleoresins Rich in Carotenoids Coated Microbeads" Coatings 9, no. 4: 235. https://doi.org/10.3390/coatings9040235
APA StyleTrif, M., Vodnar, D. C., Mitrea, L., Rusu, A. V., & Socol, C. T. (2019). Design and Development of Oleoresins Rich in Carotenoids Coated Microbeads. Coatings, 9(4), 235. https://doi.org/10.3390/coatings9040235








