The Antifungal Properties of Super-Hydrophobic Nanoparticles and Essential Oils on Different Material Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Superhydrophobic Nanoparticles
2.2. Essential Oils
2.3. Antifungal Ability of SHNPs and SHNP/EO Mixtures: Disc Diffusion Assay
2.4. Antifungal Ability of SHNPs and SHNP/EO Mixtures on Selected Materials
2.5. Application of Fungal Strains on the Surface of Selected Materials
2.6. Measurement of Antifungal Activities
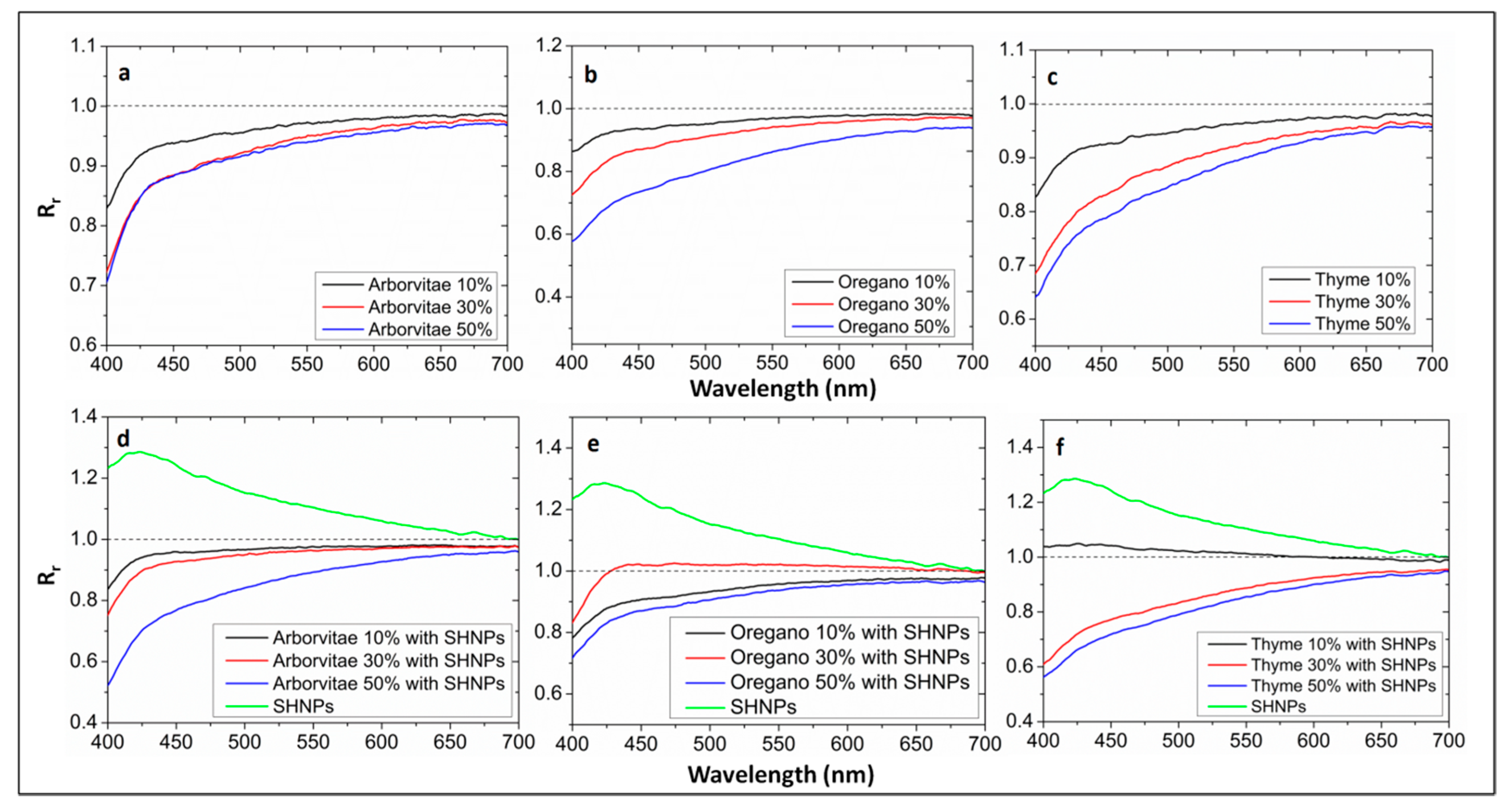
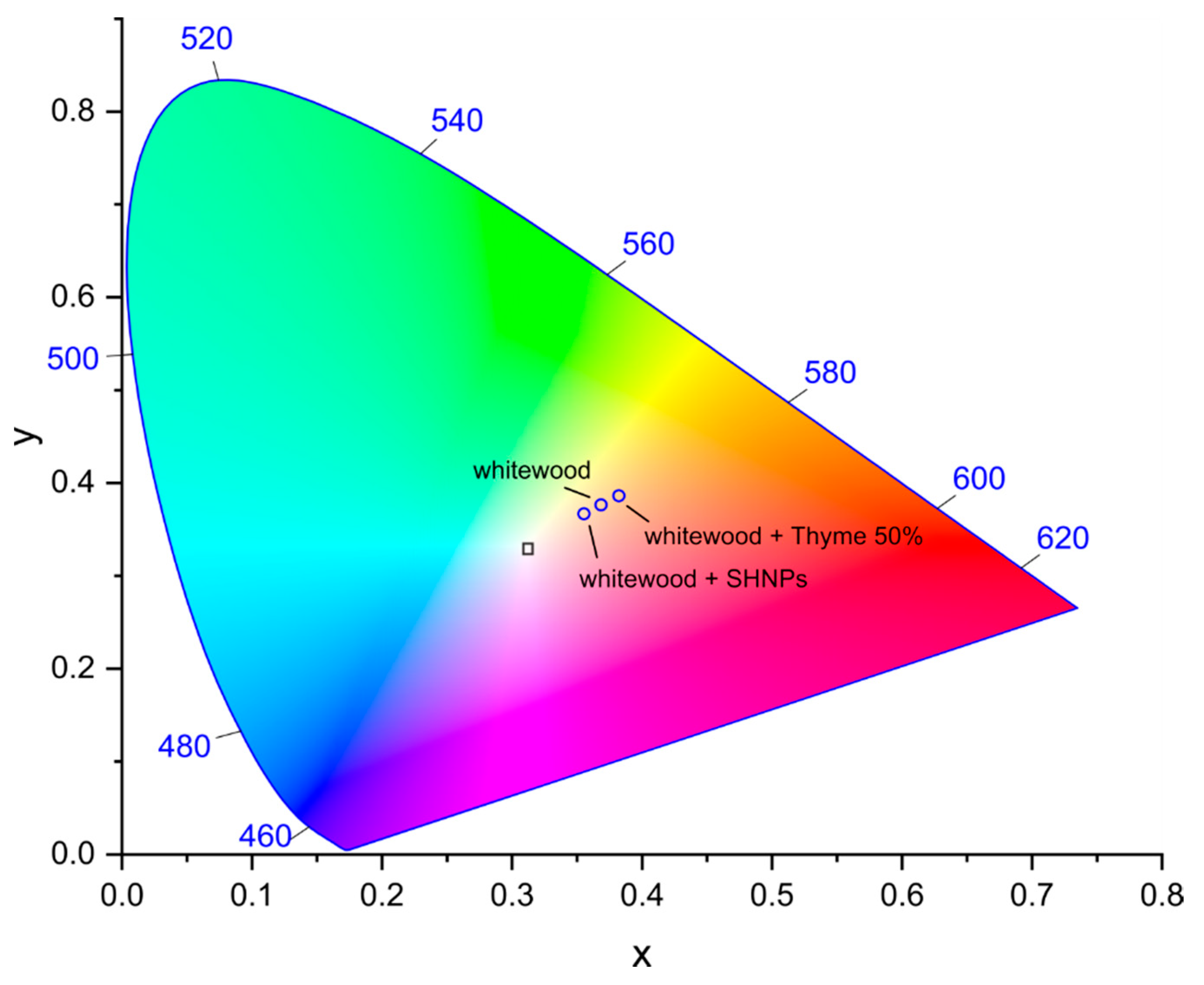
2.7. Measurement of Optical Properties
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carlborg, C.F.; van der Wijngaart, W. Sustained Superhydrophobic Friction Reduction at High Liquid Pressures and Large Flows. Langmuir 2011, 27, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Nosonovsky, M.; Bhushan, B. Roughness-Induced Superhydrophobicity: A Way to Design Non-Adhesive Surfaces. J. Phys. Condens. Matter 2008, 20, 225009. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Hierarchical Roughness Makes Superhydrophobic States Stable. Microelectron. Eng. 2007, 84, 382–386. [Google Scholar]
- Verplanck, N.; Coffinier, Y.; Thomy, V.; Boukherroub, R. Wettability Switching Techniques on Superhydrophobic Surfaces. Nanoscale Res. Lett. 2007, 2, 577–596. [Google Scholar] [CrossRef]
- Šiffalovič, P.; Jergel, M.; Benkovičová, M.; Vojtko, A.; Nádaždy, V.; Ivančo, J.; Bodík, M.; Demydenko, M.; Majková, E. Towards New Multifunctional Coatings for Organic Photovoltaics. Sol. Energy Mater. Sol. Cells 2014, 125, 127–132. [Google Scholar] [CrossRef]
- Teisala, H.; Tuominen, M.; Kuusipalo, J. Adhesion Mechanism of Water Droplets on Hierarchically Rough Superhydrophobic Rose Petal Surface. J. Nanomater. 2011, 2011, 33. [Google Scholar] [CrossRef]
- Daouk, R.K.; Dagher, S.M.; Sattout, E.J. Antifungal Activity of the Essential Oil of Origanum syriacum L. J. Food Prot. 1995, 58, 1147–1149. [Google Scholar] [CrossRef]
- Gakuubi, M.M.; Maina, A.W.; Wagacha, J.M. Antifungal Activity of Essential Oil of Eucalyptus camaldulensis Dehnh. against Selected Fusarium Spp. Int. J. Microbiol. 2017, 2017, 8761610. [Google Scholar] [CrossRef] [PubMed]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The Antibacterial and Antifungal Activity of Six Essential Oils and Their Cyto/Genotoxicity to Human HEL 12469 Cells. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Boskovic, M.; Zdravkovic, N.; Ivanovic, J.; Janjic, J.; Djordjevic, J.; Starcevic, M.; Baltic, M.Z. Antimicrobial Activity of Thyme (Tymus vulgaris) and Oregano (Origanum vulgare) Essential Oils against Some Food-Borne Microorganisms. Procedia Food Sci. 2015, 5, 18–21. [Google Scholar] [CrossRef]
- Mohareb, A.; Badawy, M.E.I.; Abdelgaleil, S.A.M. Antifungal Activity of Essential Oils Isolated from Egyptian Plants against Wood Decay Fungi. J. Wood Sci. 2013, 59, 499–505. [Google Scholar] [CrossRef]
- Han, X.; Parker, T.L. Arborvitae (Thuja plicata) Essential Oil Significantly Inhibited Critical Inflammation- and Tissue Remodeling-Related Proteins and Genes in Human Dermal Fibroblasts. Biochim. Open 2017, 4, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Santoro, G.F.; Das Graças Cardoso, M.; Guimarães, L.G.L.; Salgado, A.P.S.P.; Menna-Barreto, R.F.S.; Soares, M.J. Effect of Oregano (Origanum vulgare L.) and Thyme (Thymus vulgaris L.) Essential Oils on Trypanosoma cruzi (Protozoa: Kinetoplastida) Growth and Ultrastructure. Parasitol. Res. 2007, 100, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.L.; Stamford, T.L.M.; Lima, E.O.; Trajano, V.N. Effectiveness of Origanum vulgare L. Essential Oil to Inhibit the Growth of Food Spoiling Yeasts. Food Control 2007, 18, 409–413. [Google Scholar] [CrossRef]
- Magyar, D.; Vass, M.; Li, D. Biology of Microfungi; Li, D.-W., Ed.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Carmo, E.S.; Lima, E.D.O.; De Souza, E.L. The Potential of Origanum vulgare L. (Lamiaceae) Essential Oil in Inhibiting the Growth of Some Food-Related Aspergillus Species. Braz. J. Microbiol. 2008, 39, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Mith, H.; Duré, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial Activities of Commercial Essential Oils and Their Components against Food-Borne Pathogens and Food Spoilage Bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Hatakka, A.; Hammel, K.E. Fungal Biodegradation of Lignocelluloses. Ind. Appl. 2011, 319–340. [Google Scholar]
- Kraková, L.; Chovanová, K.; Selim, S.A.; Šimonovičová, A.; Puškarová, A.; Maková, A.; Pangallo, D. A Multiphasic Approach for Investigation of the Microbial Diversity and Its Biodegradative Abilities in Historical Paper and Parchment Documents. Int. Biodeterior. Biodegrad. 2012, 70, 117–125. [Google Scholar] [CrossRef]
- Nieminen, S.M.; Kärki, R.; Auriola, S.; Toivola, M.; Laatsch, H.; Laatikainen, R.; Hyvärinen, A.; von Wright, A. Isolation and Identification of Aspergillus Fumigatus Mycotoxins on Growth Medium and Some Building Materials Isolation and Identification of Aspergillus Fumigatus Mycotoxins on Growth Medium and Some Building Materials. Appl. Environ. Microbiol. 2002, 68, 4871–4875. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Verma, R.K. Fungi Diversity, Their Effects on Building Materials, Occupants and Control—A Brief Review. J. Sci. Ind. Res. 2010, 69, 657–661. [Google Scholar]
- Urzi, C. Microbial Deterioration of Rocks and Marble Monuments of the Mediterranean Basin: A Review. Corros. Rev. 2004, 22, 441–457. [Google Scholar] [CrossRef]
- Wiktor, V.; De Leo, F.; Urzì, C.; Guyonnet, R.; Grosseau, P.; Garcia-Diaz, E. Accelerated Laboratory Test to Study Fungal Biodeterioration of Cementitious Matrix. Int. Biodeterior. Biodegrad. 2009, 63, 1061–1065. [Google Scholar] [CrossRef]
- De Leo, F.; Cardiano, P.; De Carlo, G.; Lo Schiavo, S.; Urzì, C. Testing the Antimicrobial Properties of an Upcoming “Environmental-Friendly” Family of Ionic Liquids. J. Mol. Liq. 2017, 248, 81–85. [Google Scholar] [CrossRef]
- La Russa, M.F.; Macchia, A.; Ruffolo, S.A.; De Leo, F.; Barberio, M.; Barone, P.; Crisci, G.M.; Urzì, C. Testing the Antibacterial Activity of Doped TiO2 for Preventing Biodeterioration of Cultural Heritage Building Materials. Int. Biodeterior. Biodegrad. 2014, 96, 87–96. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; De Leo, F.; Ricca, M.; Arcudi, A.; Silvestri, C.; Bruno, L.; Urzì, C.; La Russa, M.F. Medium-Term in Situ Experiment by Using Organic Biocides and Titanium Dioxide for the Mitigation of Microbial Colonization on Stone Surfaces. Int. Biodeterior. Biodegrad. 2017, 123, 17–26. [Google Scholar] [CrossRef]
- Scheerer, S.; Ortega-Morales, O.; Gaylarde, C. Chapter 5 Microbial Deterioration of Stone Monuments—An Updated Overview, 1st ed.; Elsevier B.V. Inc.: Amsterdam, The Netherlands, 2009; Volume 66. [Google Scholar]
- Sadiki, M.; Barkai, H.; Saad, I.K. The Effect of the Thymus Vulgaris Extracts on the Physicochemical Characteristics of Cedar Wood Using Angle Contact Measurement. J. Adhes. Sci. Technol. 2014, 28, 1925–1934. [Google Scholar]
- Van Oss, C.J.; Ju, L.; Chaudhury, M.K.; Good, R.J. Estimation of the Polar Parameters of the Surface Tension of Liquids by Contact Angle Measurements on Gels. J. Colloid Interface Sci. 1988, 128, 313–319. [Google Scholar] [CrossRef]
- Vogler, E.A. Structure and Reactivity of Water at Biomaterial Surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Law, K.-Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Ohta, N.; Robertson, A.R. Colorimetry: Fundamentals and Applications; John Wiley & Sons Ltd.: West Sussex, England, 2005. [Google Scholar]
- Panek, M.; Reinprecht, L.; Hulla, M. Ten Essential Oils for Beech Wood Protection—Efficacy Against Wood-Destroying Fungi and Moulds, and Effect on Wood Discoloration. BioResources 2014, 9, 5588–5603. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, M.; Xu, Y.; Wang, S.; Liu, F.; Ma, M.; Zang, D.; Gao, Z. One-Step Synthesis of Unique Silica Particles for the Fabrication of Bionic and Stably Superhydrophobic Coatings on Wood Surface. Adv. Powder Technol. 2014, 25, 530–535. [Google Scholar] [CrossRef]
- de Ferri, L.; Paolo, P.; Lorenzi, A.; Montenero, A.; Salvioli-mariani, E. Study of Silica Nanoparticles—Polysiloxane Hydrophobic Treatments for Stone-Based Monument Protection. J. Cult. Herit. 2011, 12, 356–363. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Tuning the Wetting Properties of Siloxane-Nanoparticle Coatings to in- Duce Superhydrophobicity and Superoleophobicity for Stone Protection. Mater. Des. 2016, 108, 736–744. [Google Scholar] [CrossRef]
- Facio, D.S.; Ordoñez, J.A.; Gil, M.L.A.; Carrascosa, L.A.M.; Mosquera, M.J. Combining SiO2 Composite and Fluorinated Alkoxysilane: Application on Decayed Biocalcareous Stone from an 18th Century Cathedral. Coatings 2018, 8, 170. [Google Scholar] [CrossRef]
- Frigione, M.; Lettieri, M. Novel Attribute of Organic—Inorganic Hybrid. Coatings 2018, 8, 319. [Google Scholar] [CrossRef]
- Li, K.; Zeng, X.; Li, H.; Lai, X. Fabrication and Characterization of Stable Superhydrophobic Fluorinated-Polyacrylate/Silica Hybrid Coating. Appl. Surf. Sci. 2014, 298, 214–220. [Google Scholar] [CrossRef]
- Teisala, H.; Tuominen, M.; Kuusipalo, J. Superhydrophobic Coatings on Cellulose-Based Materials: Fabrication, Properties, and Applications. Adv. Mater. Interfaces 2014, 1, 1300026. [Google Scholar] [CrossRef]

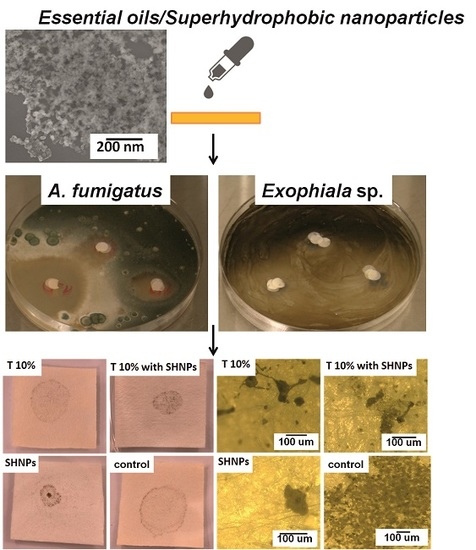
| A. Fumigatus * | E. Xenobiotica * | |
|---|---|---|
| SHNPs | - | - |
| SHNPs/Oregano 10% | 20 | 18 |
| SHNPs/Oregano 30% | 22 | 20 |
| SHNPs/Oregano 50% | 30 | 30 |
| SHNPs/Thyme 10% | 15 | 10 |
| SHNPs/Thyme 30% | 22 | 12 |
| SHNPs/Thyme 50% | 25 | 20 |
| SHNPs/Arborvitae 10% | 10 | 10 |
| SHNPs/Arborvitae 30% | 20 | 15 |
| SHNPs/Arborvitae 50% | 22 | 20 |
| Untreated Samples | |||||
| Material | Contact angle (°) | ||||
| Whitewood | - | ||||
| Sandstone | - | ||||
| Paper | - | ||||
| Treated samples | |||||
| Silicon wafer with SHNPs | 171.10 ± 1.50 | ||||
| Whitewood | Sandstone | Paper | |||
| EOs and mixtures | Contact angle (°) | EOs and mixtures | Contact angle (°) | EOs and mixtures | Contact angle (°) |
| A 10% | - | A 10% | - | A 10% | - |
| A 30%/ | 35.59 ± 2.04 | A 30%/ | 42.13 ± 3.55 | A 30%/ | - |
| A 50% | 49.51 ± 2.68 | A 50% | 49.95 ± 3.55 | A 50% | - |
| O10% | - | O10% | - | O 10% | - |
| O 30% | 34.84 ± 1.28 | O 30% | 42.15 ± 4.06 | O 30% | - |
| O 50% | 38.53 ± 4.88 | O 50% | 43.46 ± 2.59 | O 50% | - |
| T 10% | - | T 10% | - | T 10% | - |
| T 30% | 36.15 ± 3.17 | T 30% | 48.84 ± 3.89 | T 30% | - |
| T 50% | 50.43 ± 2.94 | T 50% | 44.47 ± 2.85 | T 50% | - |
| SHNPs | 124.14 ± 11.89 | SHNPs | 106.32 ± 14.37 | SHNPs | 115.58 ± 2.87 |
| A 10%/SHNPs | 91.08 ± 8.16 | A 10%/SHNPs | 100.95 ± 16.23 | A 10%/SHNPs | - |
| A 30%/SHNPs | 66.63 ± 4.44 | A 30%/SHNPs | 98.92 ± 10.57 | A 30%/SHNPs | 69.92 ± 3.73 |
| A 50%/SHNPs | 59.17 ± 2.93 | A 50%/SHNPs | 72.38 ± 8.96 | A 50%/SHNPs | 85.92 ± 1.55 |
| O 10%/SHNPs | 129.05 ± 40.77 | O10%/SHNPs | 108.03 ± 13.27 | O 10%/SHNPs | - |
| O 30%/SHNPs | 73.22 ± 4.52 | O 30%/SHNPs | 70.77 ± 2.33 | O 30%/SHNPs | - |
| O 50%/SHNPs | 55.34 ± 5.76 | O 50%/SHNPs | 71.57 ± 4.85 | O 50%/SHNPs | 72.50 ± 2.87 |
| T 10%/SHNPs | 66.58 ± 8.25 | T 10%/SHNPs | 133.68 ± 26.33 | T 10%/SHNPs | - |
| T 30%/SNHPs | 65.19 ± 4.86 | T 30%/SNHPs | 84.08 ± 2.99 | T 30%/SNHPs | 71.98 ± 2.07 |
| T 50%/SHNPs | 62.05 ± 7.03 | T 50%/SHNPs | 95.90 ± 11.88 | T 50%/SHNPs | 98.49 ± 1.11 |
| Localized Fungal Area on Surface (mm2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Whitewood | Sandstone | Paper | |||||||
| 10% | 30% | 50% | 10% | 30% | 50% | 10% | 30% | 50% | |
| Arborvitae oil | 3.1 | 3.8 | 11.8 | - | 11.9 | 10.4 | 22.3 | 21.5 | 25.7 |
| Oregano oil | 1.5 | 5 | 8.1 | 0.9 | 4.6 | 3.8 | 58.2 | 40.3 | 33.2 |
| Thyme oil | 0.8 | - | 3.9 | - | 2.2 | 2.7 | 51.7 | 32.5 | 26 |
| SHNPs | 0.9 | 3.5 | 38 | ||||||
| SHNPs/arborvitae | 1.9 | 1.3 | 1.3 | 4.7 | 20.3 | 25.4 | 58.4 | 36.3 | 31.9 |
| SHNPs/oregano | 4.5 | 2.9 | 2.5 | - | 18.5 | 23.8 | 43.1 | 40.5 | 19.1 |
| SHNPs/thyme | 2.5 | 2.5 | 1.4 | - | 28 | 22.4 | 31.3 | 23.6 | 16.2 |
| control | 28.9 | 20 | 89.4 | ||||||
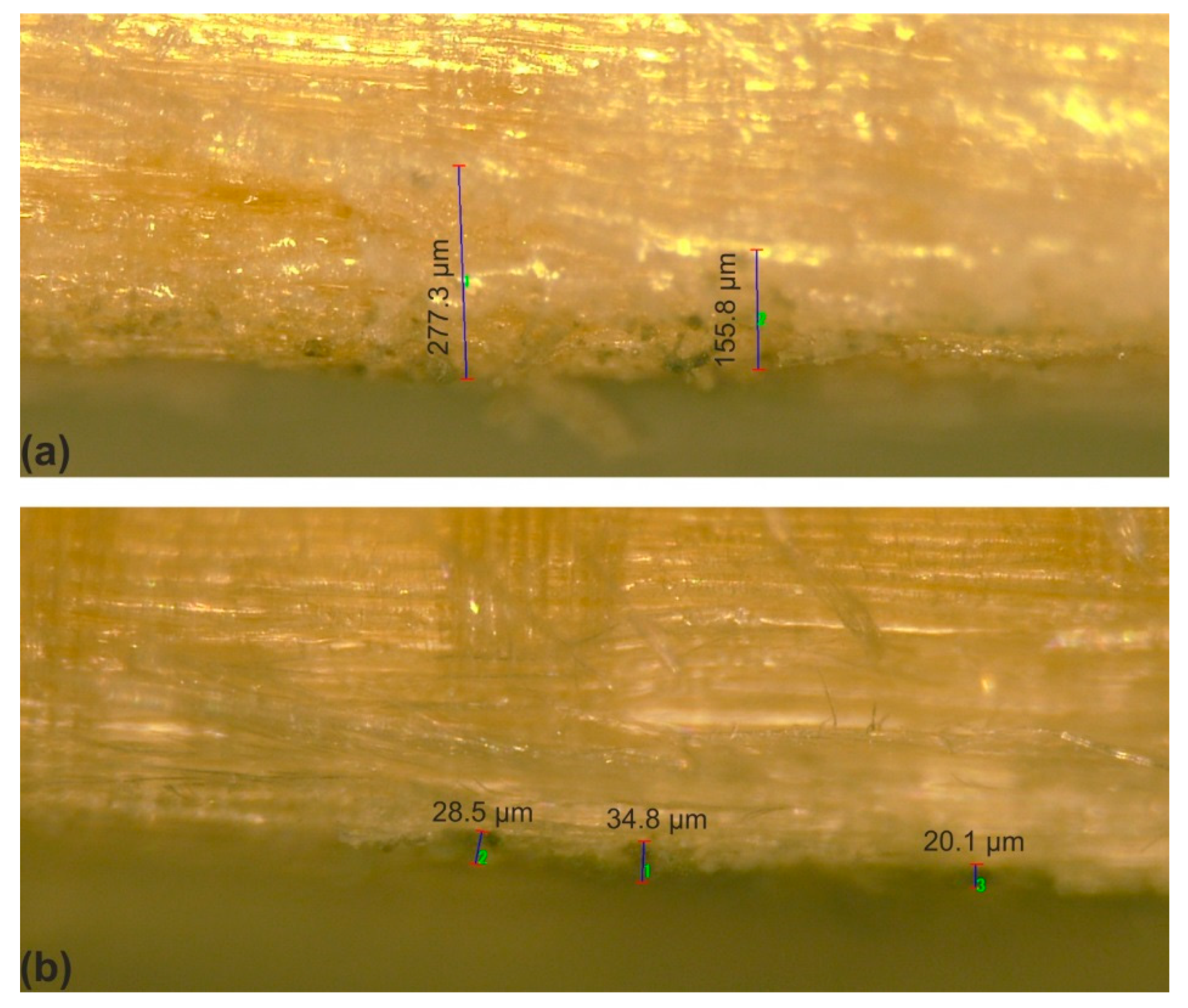
| Penetration Depth (µm) into the Structure of Whitewood | |||
|---|---|---|---|
| 10% | 30% | 50% | |
| Arborvitae oil | 127.9 | 105.4 | 83.3 |
| Oregano oil | 312.0 | 62.0 | 62.0 |
| Thyme oil | 171.5 | 69.0 | 53.0 |
| SHNPs | 34.8 | ||
| SHNPs/arborvitae | 315.0 | 107.4 | 53.4 |
| SHNPs/oregano | 209.9 | 117.5 | 108.8 |
| SHNPs/thyme | 277.3 | 156.1 | 125.9 |
| control | 138 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benkovičová, M.; Kisová, Z.; Bučková, M.; Majková, E.; Šiffalovič, P.; Pangallo, D. The Antifungal Properties of Super-Hydrophobic Nanoparticles and Essential Oils on Different Material Surfaces. Coatings 2019, 9, 176. https://doi.org/10.3390/coatings9030176
Benkovičová M, Kisová Z, Bučková M, Majková E, Šiffalovič P, Pangallo D. The Antifungal Properties of Super-Hydrophobic Nanoparticles and Essential Oils on Different Material Surfaces. Coatings. 2019; 9(3):176. https://doi.org/10.3390/coatings9030176
Chicago/Turabian StyleBenkovičová, Monika, Zuzana Kisová, Mária Bučková, Eva Majková, Peter Šiffalovič, and Domenico Pangallo. 2019. "The Antifungal Properties of Super-Hydrophobic Nanoparticles and Essential Oils on Different Material Surfaces" Coatings 9, no. 3: 176. https://doi.org/10.3390/coatings9030176
APA StyleBenkovičová, M., Kisová, Z., Bučková, M., Majková, E., Šiffalovič, P., & Pangallo, D. (2019). The Antifungal Properties of Super-Hydrophobic Nanoparticles and Essential Oils on Different Material Surfaces. Coatings, 9(3), 176. https://doi.org/10.3390/coatings9030176