Physical and Electrochemical Performances of Cold Sprayed Pb Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Powders Used for Coating
2.2. Cold Spraying and Parameters
2.3. Characters of Cold Sprayed Pb Coating
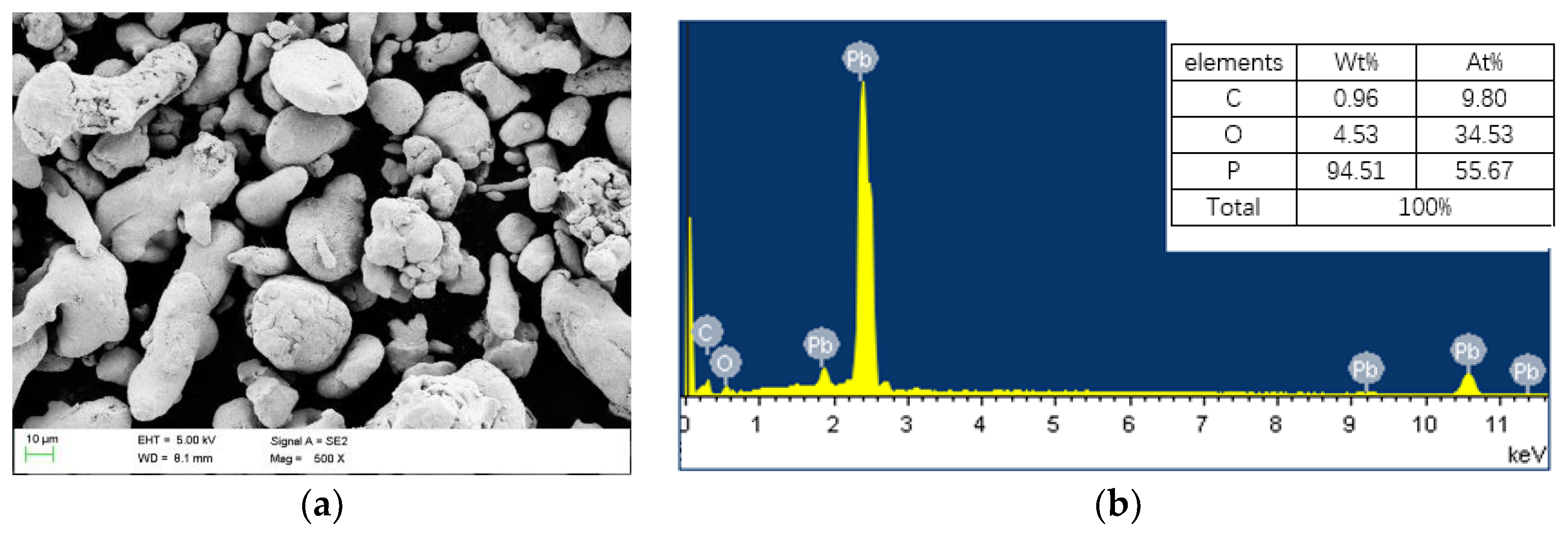
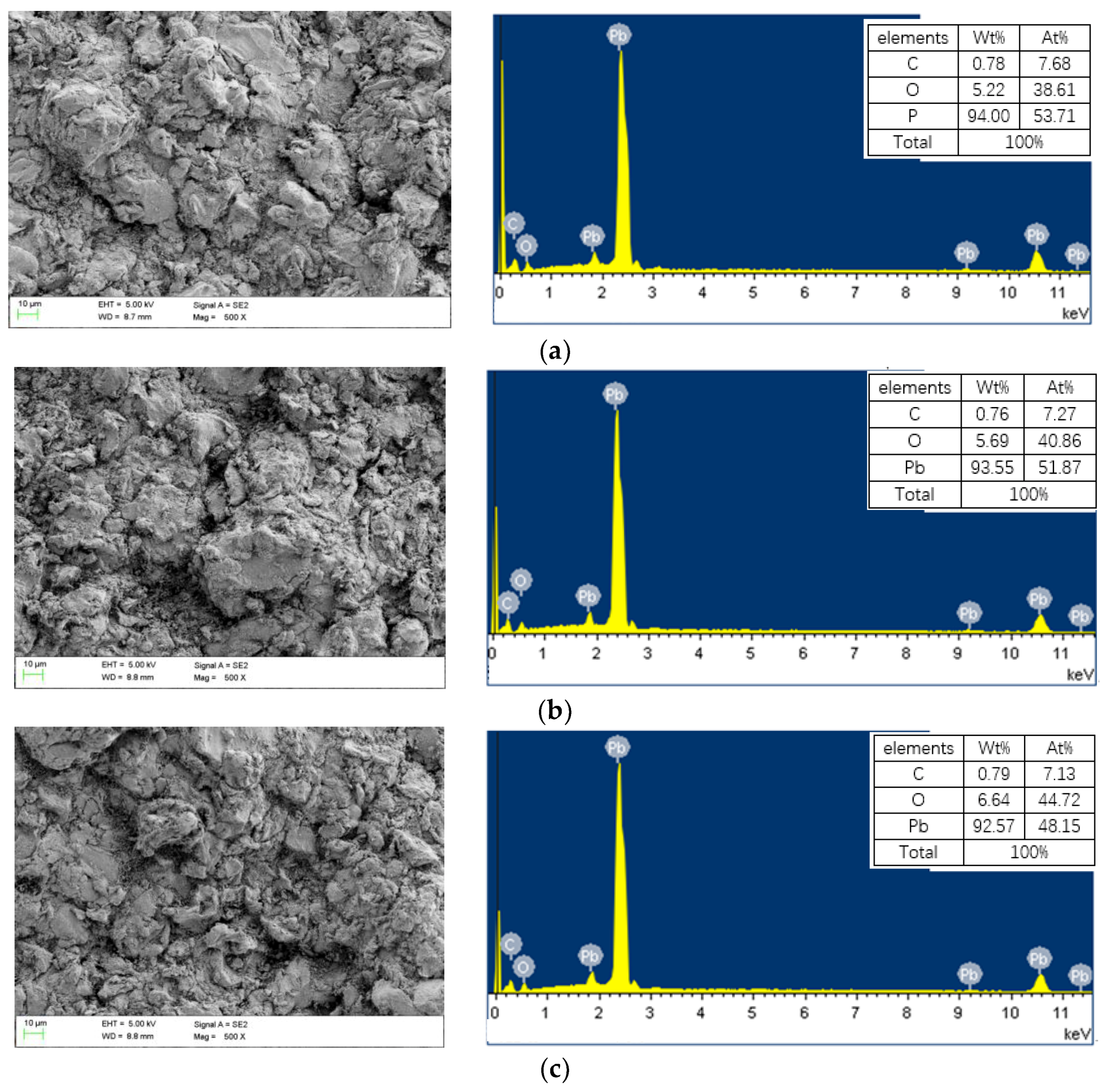
2.3.1. Imaging
2.3.2. Bonding Strength
2.4. Electrochemical Behaviour
2.4.1. Potentiodynamic Polarization Test
2.4.2. Cyclic Voltammetry
2.4.3. Accelerating Electrolysis Test
3. Results
3.1. Characteristics of Coating Thickness around the Holes
3.2. Pull-off Bonding Strength of Pb Coating on Steel Substrate
3.3. Electrochemical Performance of Cold Sprayed Pb Coating
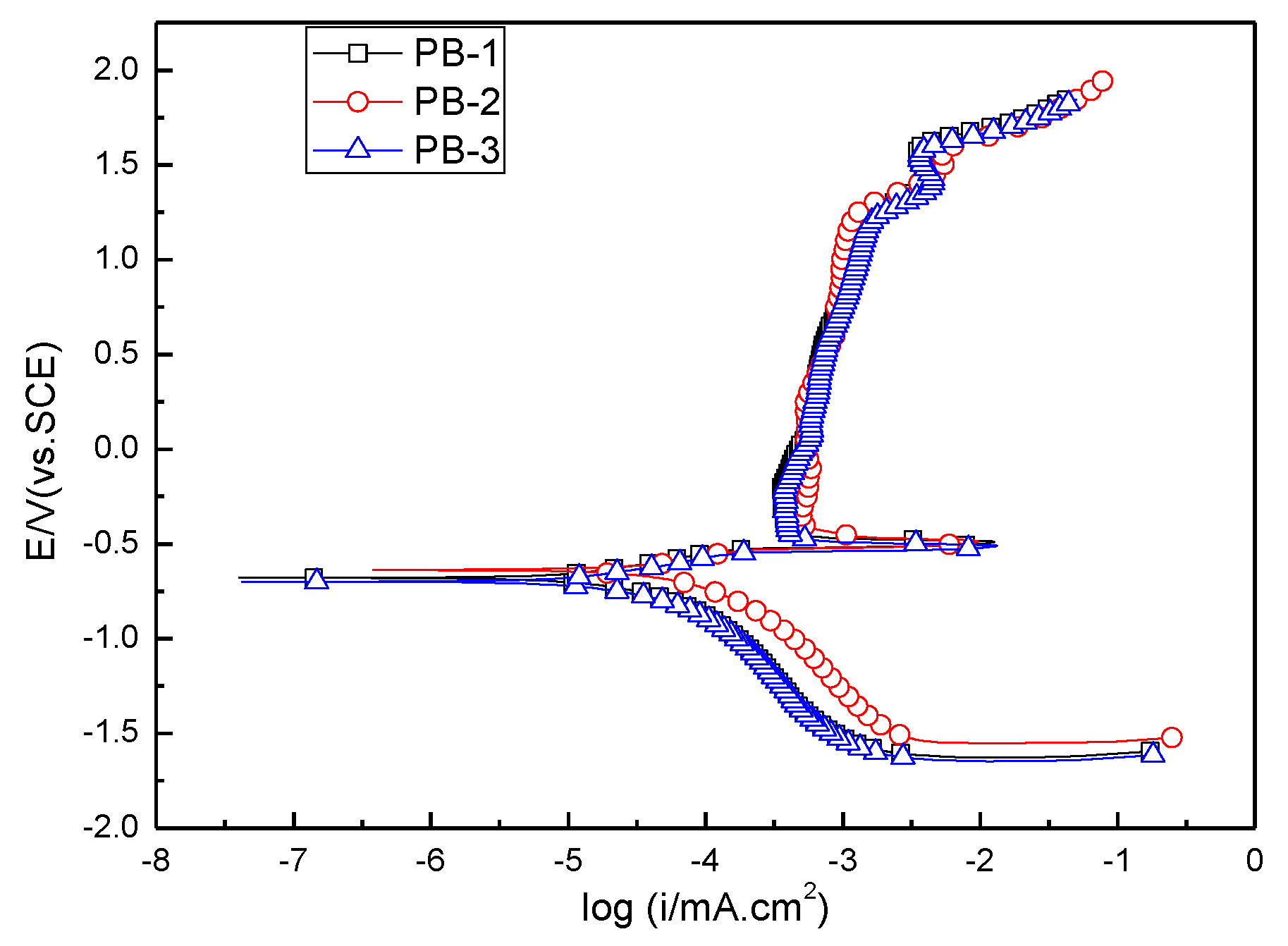
3.3.1. Potentiodynamic Polarization Test
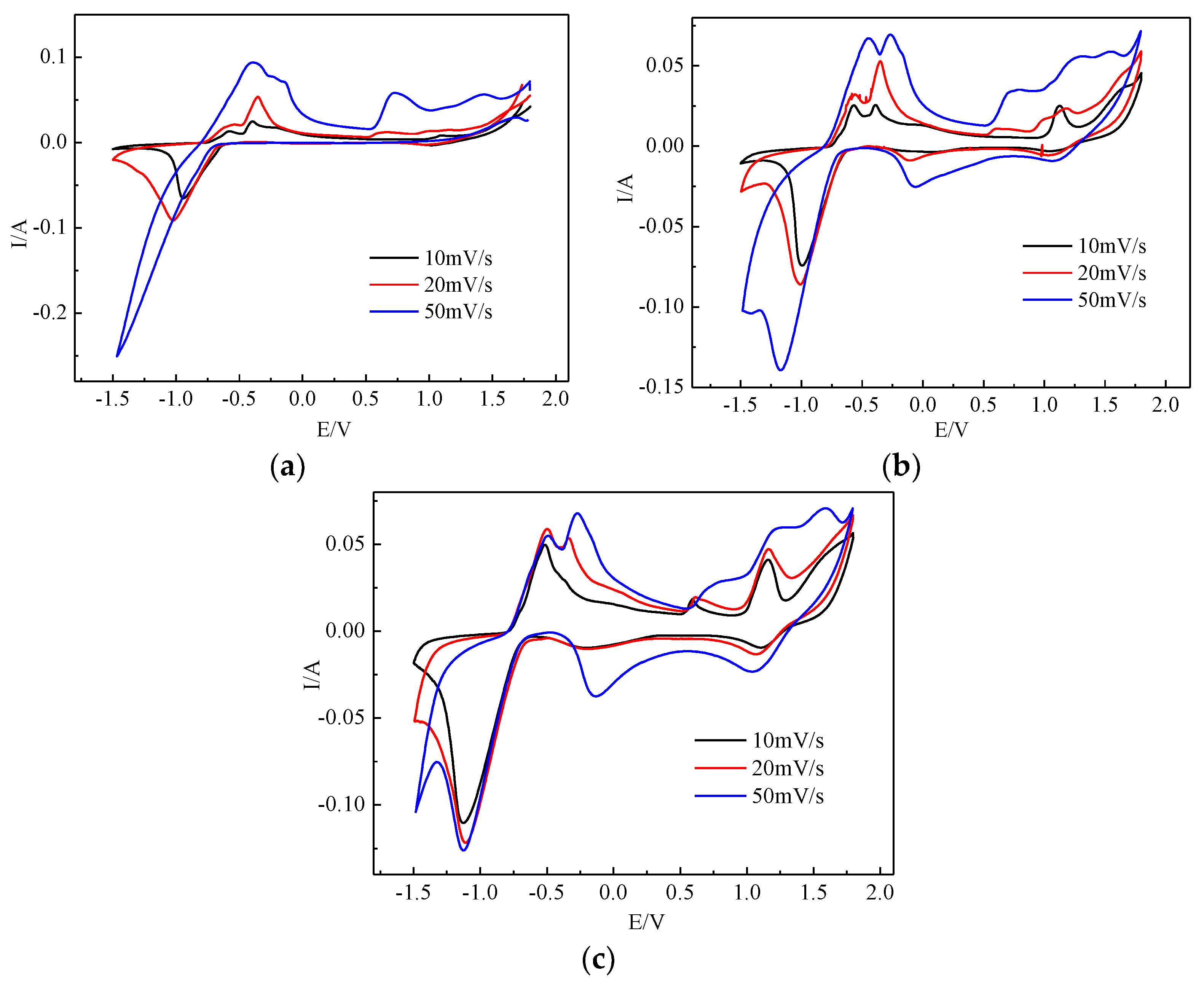
3.3.2. Cyclic Voltammetry Behavior of PbO2 Electrodes
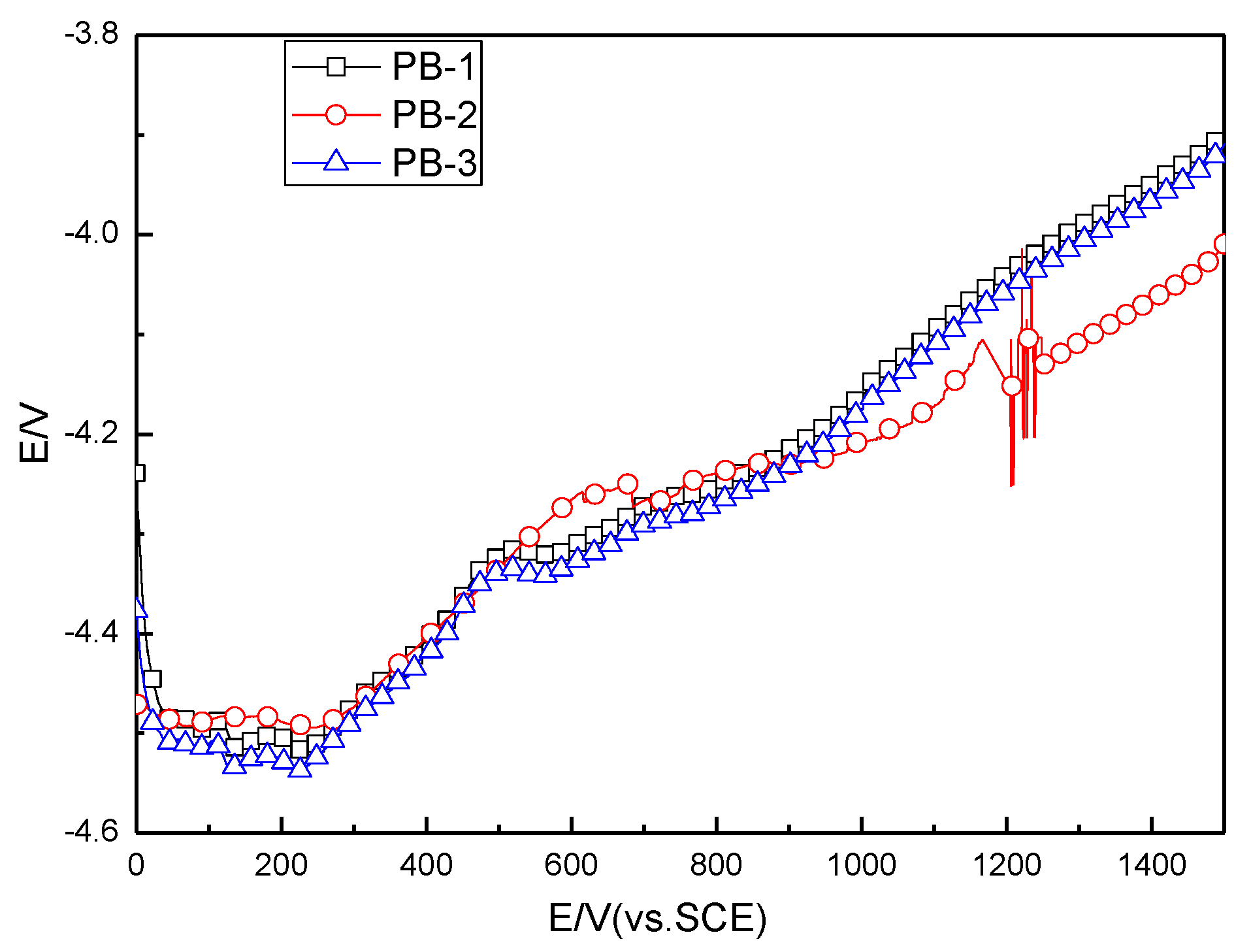
3.3.3. Accelerated Electrolytic Life of PbO2 Anode
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.N.; Zhuang, X.Y.; Li, H.Y.; Ai, L.M.; Wang, Y.H.; Wang, Q.; Chen, H.Y.; Xia, J.X.; Xing, X. Fabrication and water treatment application of high efficient PbO2 electrode. Key Eng. Mater. 2017, 727, 821–829. [Google Scholar] [CrossRef]
- Hämeenoja, E.; Laitinen, T.; Sundholm, G. The formation of soluble Pb(iv) and Pb(ii) species in the reactions of the PbSO4/PbO2, electrode. Electrochim. Acta 1987, 32, 187–189. [Google Scholar] [CrossRef]
- Hao, X.; Dan, S.; Qian, Z.; Honghui, Y.; Yan, W. Preparation and characterization of PbO2 electrodes from electro-deposition solutions with different copper concentration. RSC Adv. 2014, 4, 25011. [Google Scholar] [CrossRef]
- Ueda, M.; Watanabe, A.; Kamey Ama, T. Performance characteristics of a new type of lead dioxide coated titanium anode. J. Appl. Electrochem. 1995, 25, 817–822. [Google Scholar] [CrossRef]
- Pavlov, D.; Monahov, B. Temperature dependence of the oxygen evolution reaction on the Pb/PbO2 electrode. J. Electrochem. Soc. 1998, 145, 70–77. [Google Scholar] [CrossRef]
- Hampson, N.A.; Jones, P.C.; Phillips, R.F. Electrochemical reactions at PbO2 electrodes. Part IV. electrochemical. Can. J. Chem. 2011, 47, 2171–2179. [Google Scholar] [CrossRef]
- Fengbang, S.; Buming, C.; Zhongcheng, G.; Siqi, D.; Yali, H. Preparation and characterization of β-PbO_2-Tio_2-Co_3O_4 composite coating on stainless steel. Chin. J. App. Chem. 2012, 29, 691–696. [Google Scholar]
- Velichenko, A.B.; Knysh, V.A.; Luk’yanenko, T.V.; Danilov, F.I.; Devilliers, D. PbO2-TiO2 composite electrodes. Prot. Met. Phys. Chem. Surf. 2009, 45, 327–332. [Google Scholar] [CrossRef]
- Zhou, X.Z.; Liu, S.Q.; Yu, H.X.; Xu, A.L.; Li, J.S.; Sun, X.Y.; Shen, J.Y.; Han, W.Q.; Wang, L.J. Electrochemical oxidation of pyrrole, pyrazole and tetrazole using a TiO2 nanotubes based SnO2-Sb/3D highly ordered macro-porous PbO2 electrode. J. Electroanal. Chem. 2018, 826, 181–190. [Google Scholar] [CrossRef]
- Kumar, S.; Vidyasagar, V.; Jyothirmayi, A.; Joshi, S.V. Effect of heat treatment on mechanical properties and corrosion performance of cold-sprayed tantalum coatings. J. Therm. Spray Technol. 2016, 25, 745–756. [Google Scholar] [CrossRef]
- Koivuluoto, H.; NiKki, J.; Vuoristo, P. Corrosion properties of cold-sprayed tantalum coatings. J. Therm. Spray Technol. 2009, 18, 75–82. [Google Scholar] [CrossRef]
- Papyrin, A. Cold spray technology. Adv. Mater. Process. 2001, 159, 49–51. [Google Scholar]
- Goldbaum, D.; Poirier, D.; Irissou, E.; Legoux, J.G.; Moreau, C. Review on cold spray process and technology US patents. In Modern Cold Spray; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Hussain, T.; Mccartney, D.G.; Shipway, P.H.; Zhang, D. Bonding mechanisms in cold spraying: The contributions of metallurgical and mechanical components. J. Therm. Spray Technol. 2009, 18, 364–379. [Google Scholar] [CrossRef]
- Schmidt, T. Development of a generalized parameter window for cold spray deposition. Acta Mater. 2006, 54, 729–742. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Bolelli, G.; Lusvarghi, L.; Casadei, F.; Vuoristo, P. Corrosion resistance of cold-sprayed Ta coatings in very aggressive conditions. Surf. Coat. Technol. 2010, 205, 1103–1107. [Google Scholar] [CrossRef]
- ASTM D4541-02 Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers; ASTM International: West Conshohocken, PA, USA, 2010.
- Grujicic, M.; Zhao, C.L.; Tong, C.; DeRosset, W.S.; Helfritch, D. Analysis of the impact velocity of powder particles in the cold-gas dynamic-spray process. Mater. Sci. Eng. A 2004, 368, 222–230. [Google Scholar] [CrossRef]
- Huang, R.; Fukanuma, H. Study of the influence of particle velocity on adhesive strength of cold spray deposits. J. Thermal Spray Technol. 2012, 21, 541–549. [Google Scholar] [CrossRef]
- Dong, Y.J.; Lin, H.B.; Liu, X.B.; Ren, X.B.; Jiang, M. A cyclic voltammetric study of phenol on Ti/PbO2 electrode in H2SO4 solution. Acta Chim. Sin. 2007, 65, 2257–2260. [Google Scholar]
- Tang, W.; Chen, Q.; Chen, Z.; Zhang, X.; Chai, W.; He, Z. Numerical simulation for effect of powder carrier gas on flow field and particle impact velocity in cold spraying. J. Xi’an Jiaotong Univ. 2012, 46, 82–86. (In Chinese) [Google Scholar]
- Silva, F.S.D.; Cinca, N.; Dosta, S.; Cano, I.G.; Couto, M.; Guilemany, J.M.; Benedetti, A.V. Corrosion behavior of WC-Co coatings deposited by cold gas spray onto AA 7075-T6. Corros. Sci. 2018, 136, 231–243. [Google Scholar] [CrossRef]
- Almangour, B.; Mongrain, R.; Irissou, E.; Yue, S. Improving the strength and corrosion resistance of 316L stainless steel for biomedical applications using cold spray. Surf. Coat. Technol. 2013, 216, 297–307. [Google Scholar] [CrossRef]
- Couto, M.; Dosta, S.; Guilemany, J.M. Comparison of the mechanical and electrochemical properties of WC-17 and 12Co coatings onto Al7075-T6 obtained by high velocity oxy-fuel and cold gas spraying. Surf. Coat. Technol. 2015, 268, 180–189. [Google Scholar] [CrossRef]
- Kumar, S.; Jyothirmayi, A.; Wasekar, N.; Joshi, S.V. Influence of annealing on mechanical and electrochemical properties of cold sprayed niobium coatings. Surf. Coat. Technol. 2016, 296, 124–135. [Google Scholar] [CrossRef]



| Parameters | Value |
|---|---|
| Expansion ratio | 2.50 |
| Dimension of nozzle throat | 2 mm × 4 mm |
| Length of converging part | 10 mm |
| Length of diverging part | 100 mm |
| Dimension of exit | 2 mm × 10 mm |
| Standoff distant from nozzle exit to substrate | 25.0 mm |
| Carrier gas | Compressed air |
| Pressure in prechamber | 1.6 MPa |
| Temperature in prechamber | 298, 348, 398 K |
| Powder feeding rate | 1.50 g/s |
| Spraying angle | 90° (Perpendicular) |
| Transverse speed of nozzle | 20 mm/s |
| Sample Numbers | Pb-1 | Pb-2 | Pb-3 |
|---|---|---|---|
| Bonding strength | 7.42 | 6.71 | 6.82 |
| 6.66 | 7.75 | 6.52 | |
| 6.25 | 7.55 | 7.35 | |
| Average bonding strength | 6.78 ± 0.59 | 7.34 ± 0.55 | 6.90 ± 0.42 |
| Sample Numbers | Pb-1 | Pb-2 | Pb-3 |
|---|---|---|---|
| Weight losses | 5.115 | 5.013 | 4.995 |
| 5.117 | 5.022 | 4.966 | |
| 5.125 | 5.021 | 4.976 | |
| Average weight loss | 5.119 ± 0.005 | 5.019 ± 0.005 | 4.979 ± 0.015 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Zhuang, Y.; Fu, W. Physical and Electrochemical Performances of Cold Sprayed Pb Electrodes. Coatings 2019, 9, 174. https://doi.org/10.3390/coatings9030174
Huang G, Zhuang Y, Fu W. Physical and Electrochemical Performances of Cold Sprayed Pb Electrodes. Coatings. 2019; 9(3):174. https://doi.org/10.3390/coatings9030174
Chicago/Turabian StyleHuang, Guosheng, Yaowei Zhuang, and Wei Fu. 2019. "Physical and Electrochemical Performances of Cold Sprayed Pb Electrodes" Coatings 9, no. 3: 174. https://doi.org/10.3390/coatings9030174
APA StyleHuang, G., Zhuang, Y., & Fu, W. (2019). Physical and Electrochemical Performances of Cold Sprayed Pb Electrodes. Coatings, 9(3), 174. https://doi.org/10.3390/coatings9030174




