Hydrothermal Synthesis of Protective Coating on Mg Alloy for Degradable Implant Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Characterization
3. Results and Discussion
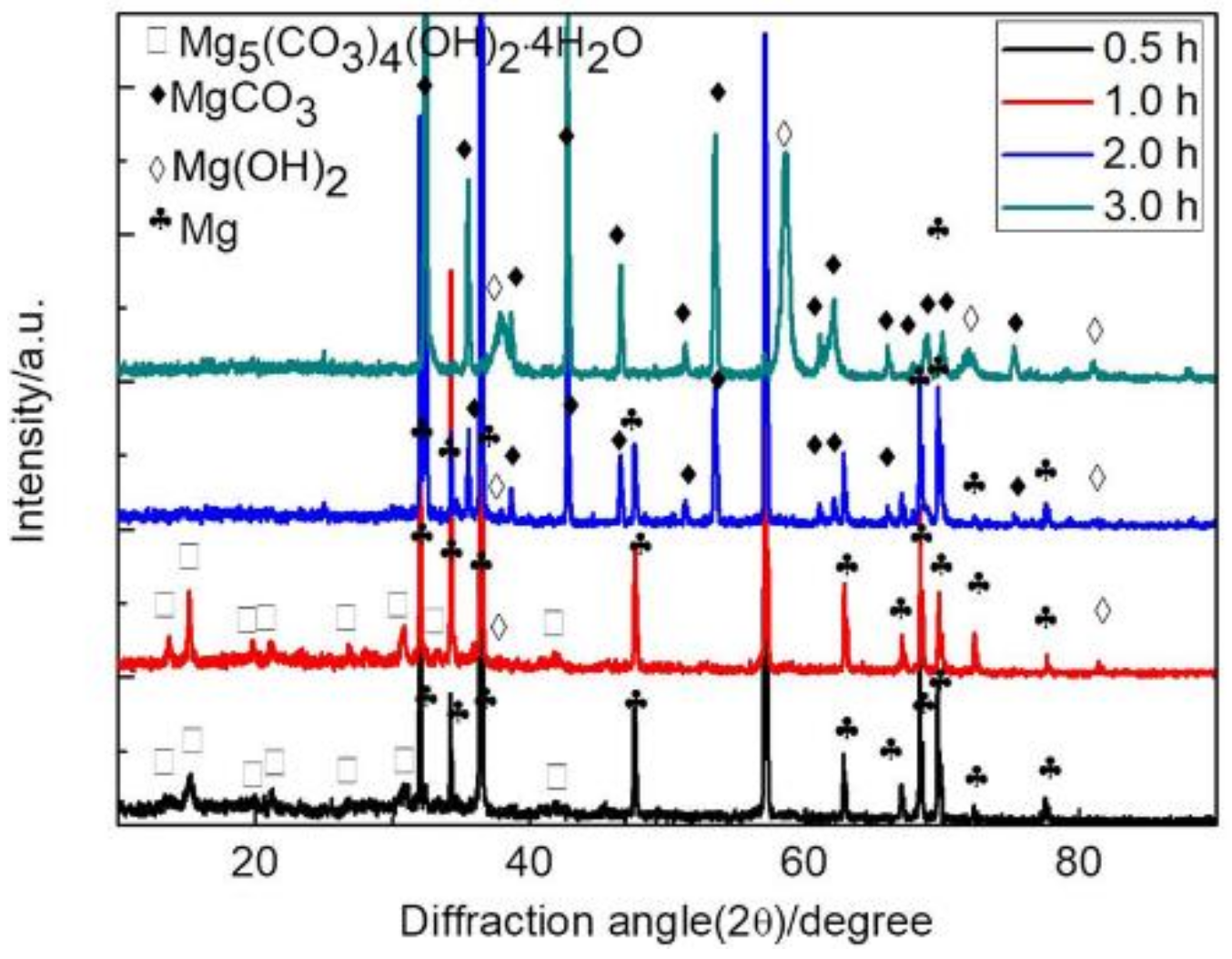
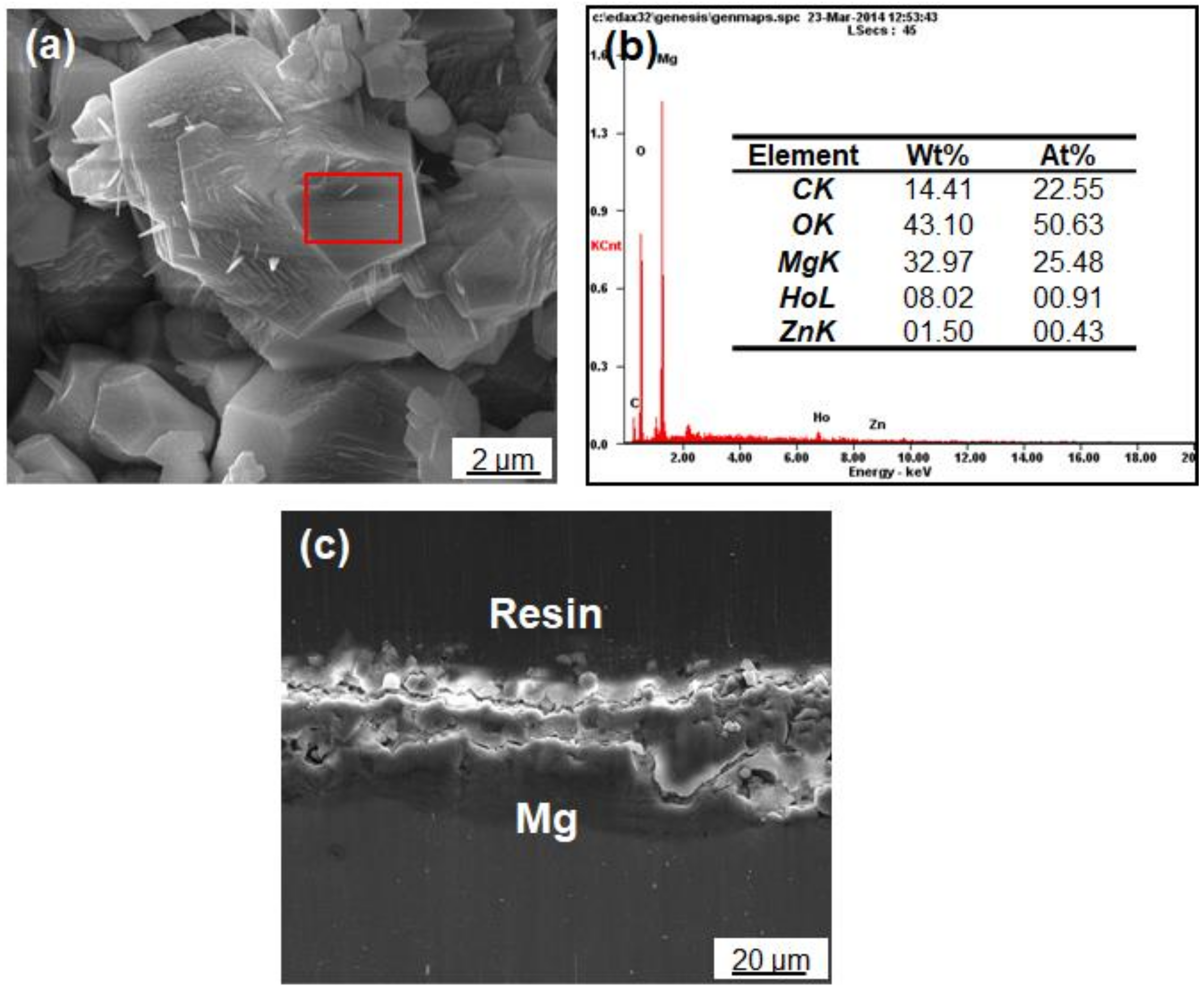
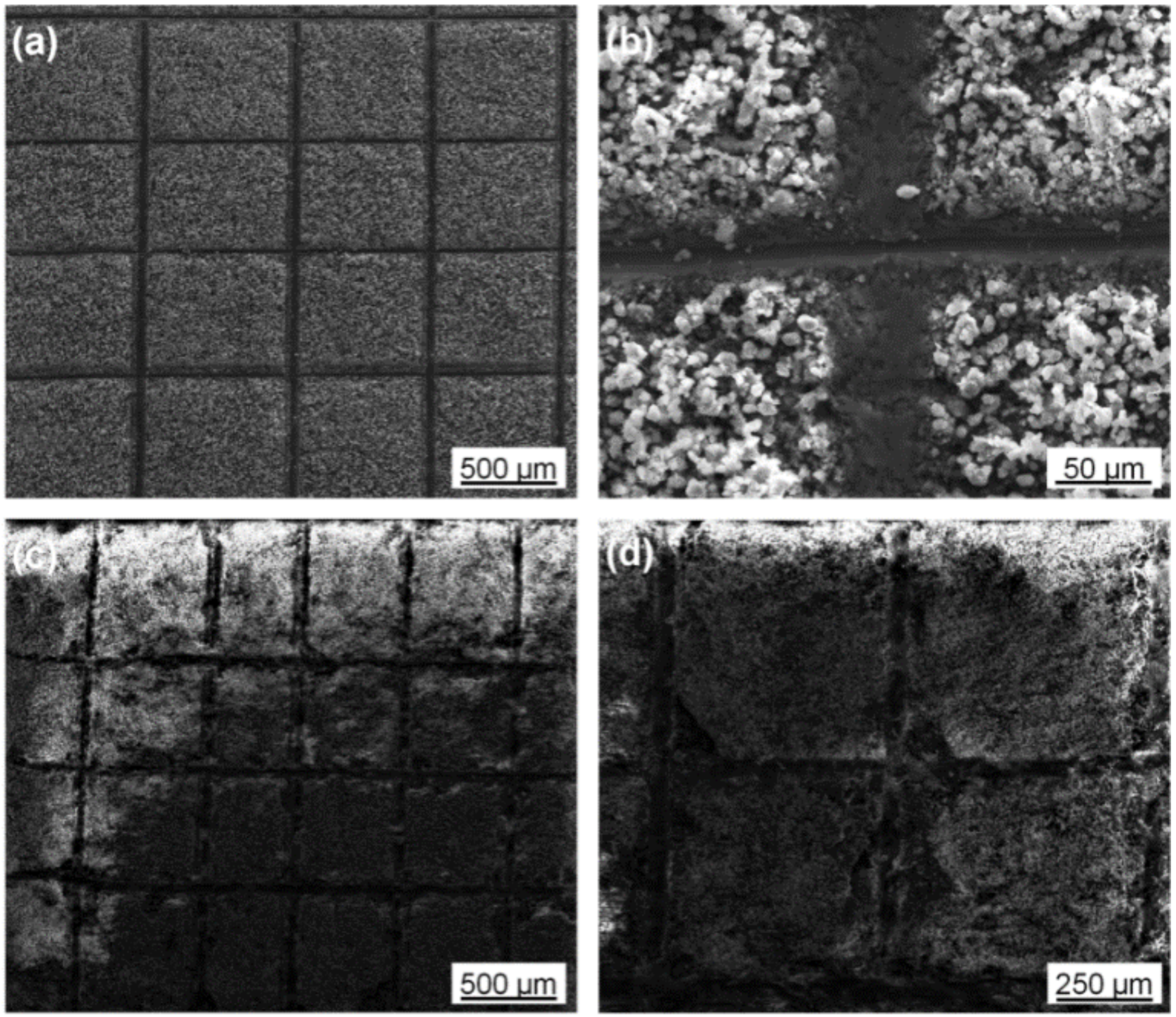
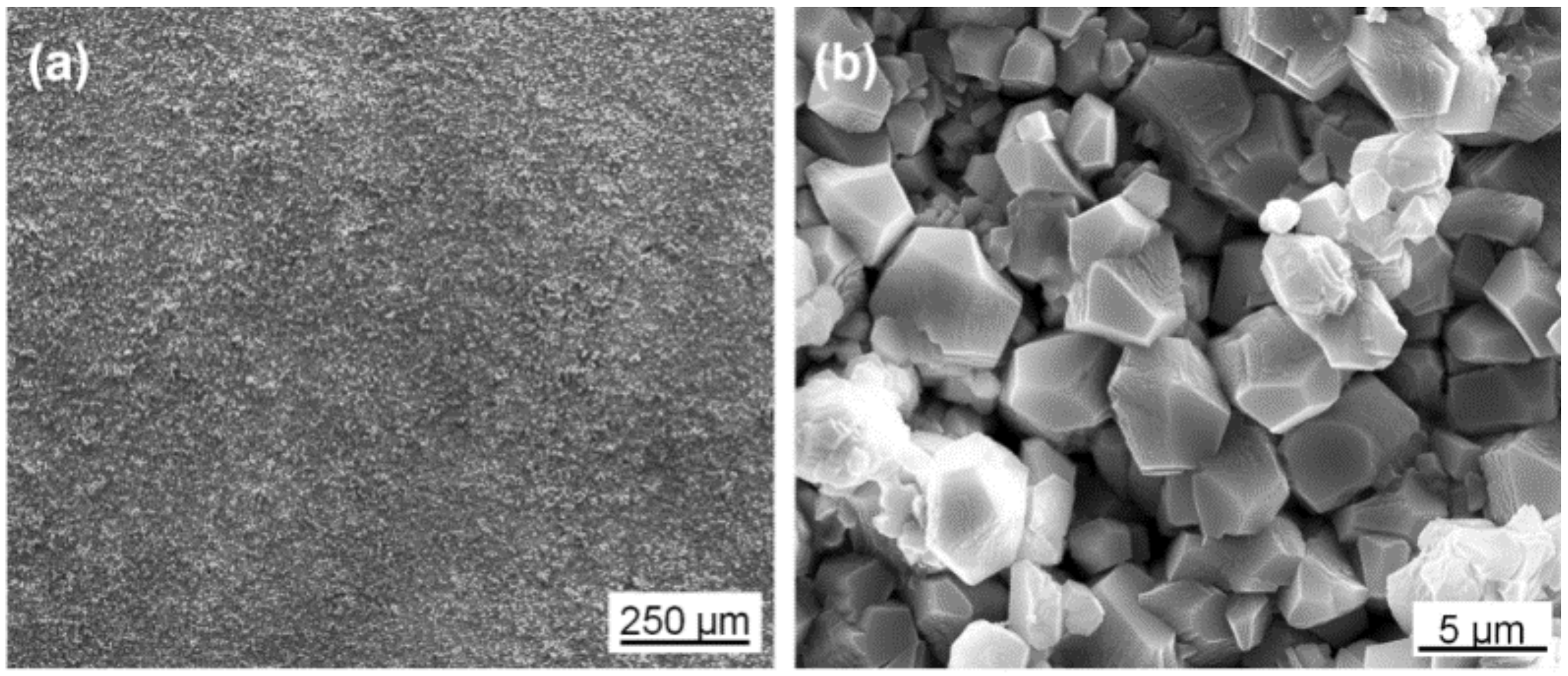
3.1. Morphology and Composition
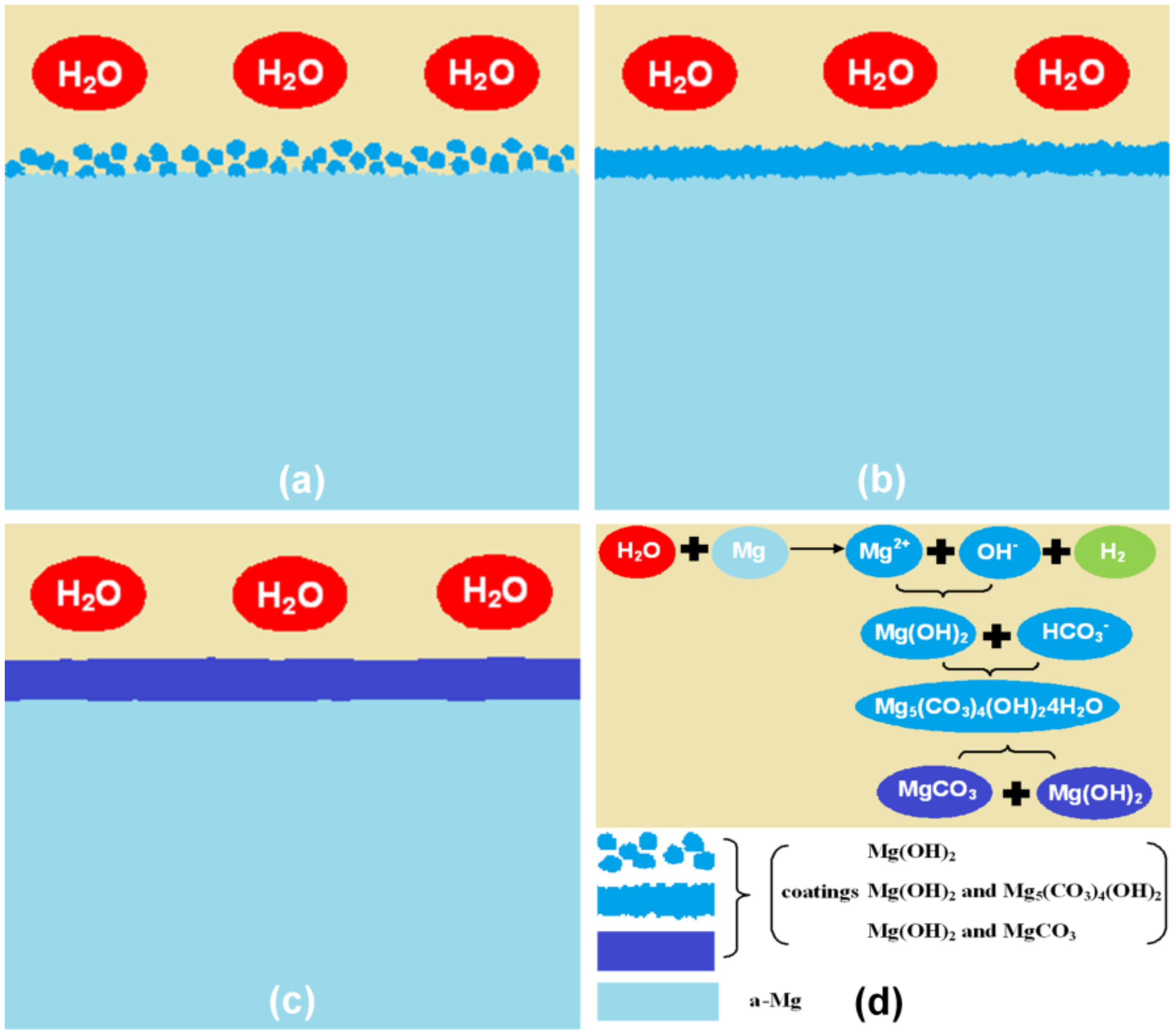
3.2. Formation Mechanism
3.3. Mechanical Strength
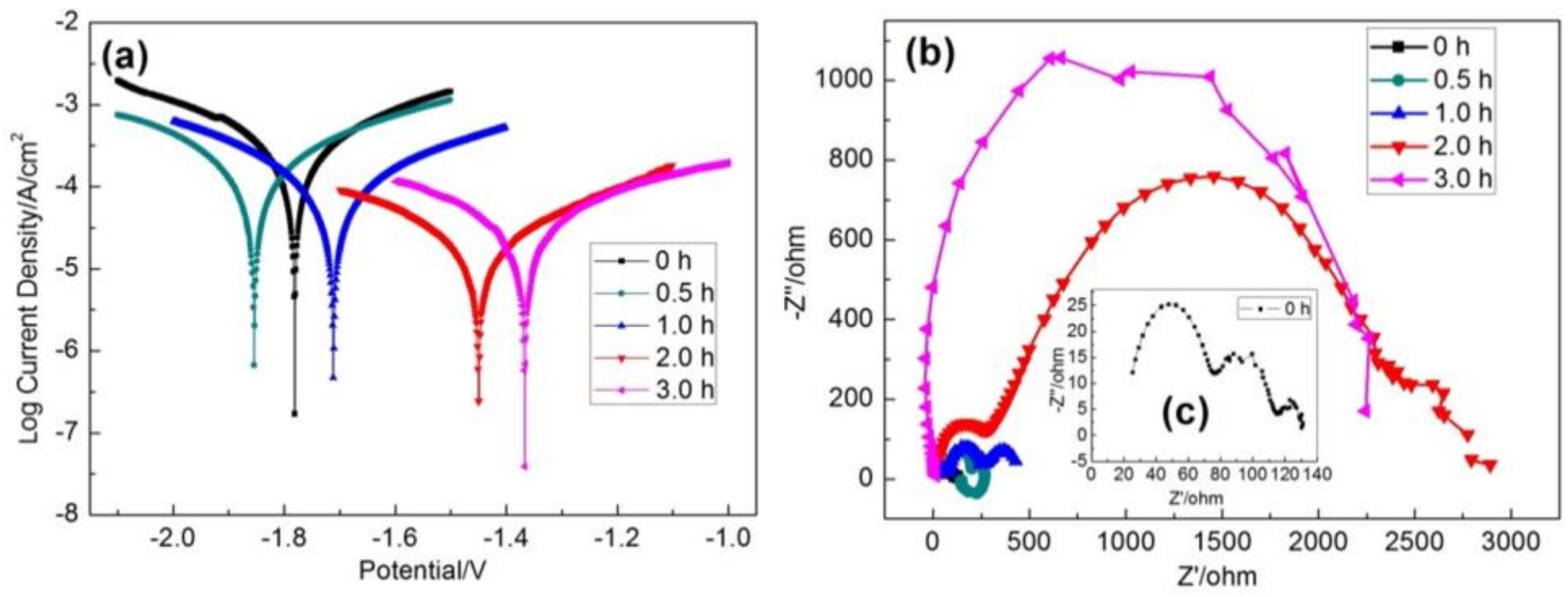
3.4. Electrochemistry
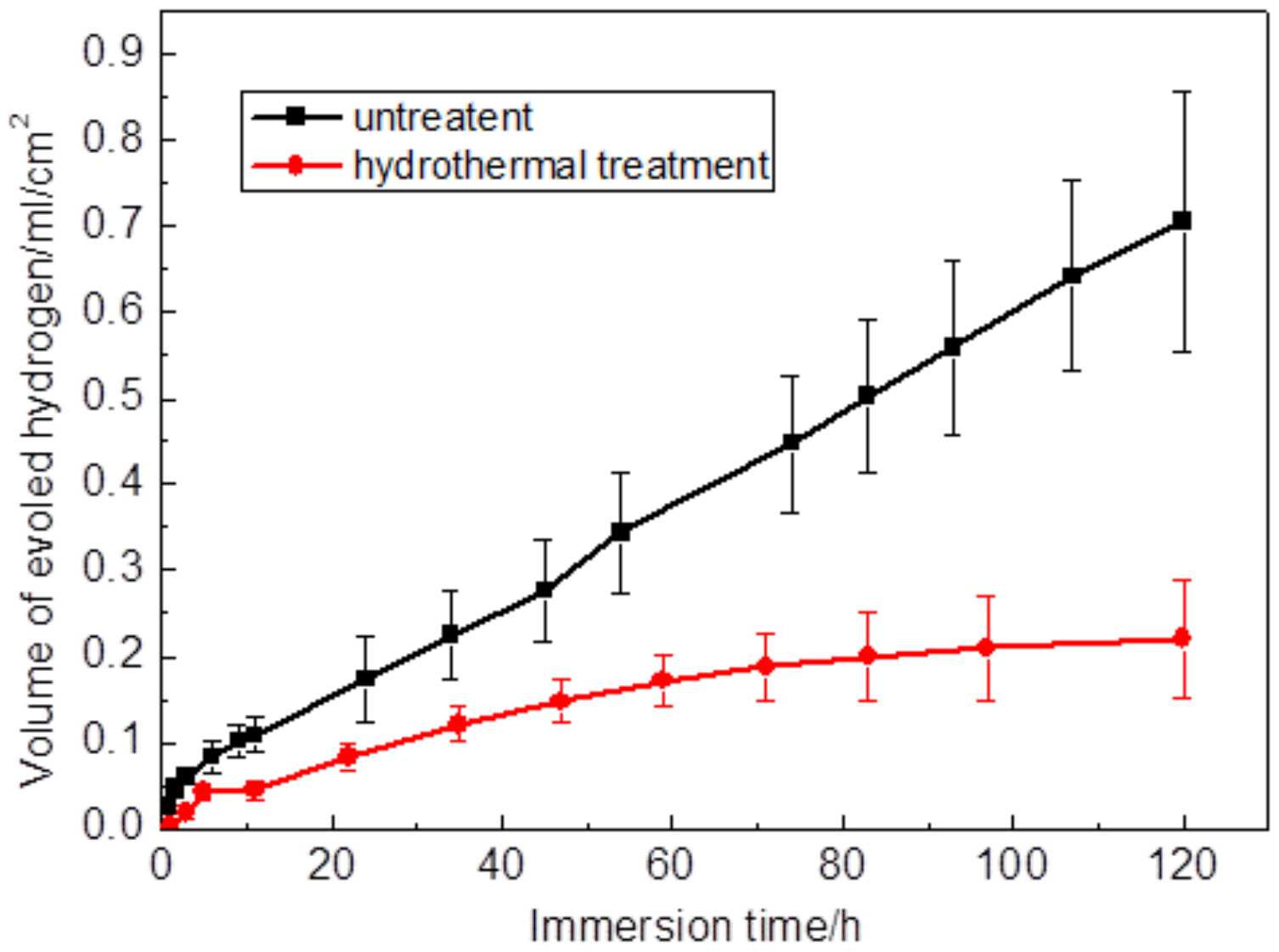
3.5. Immersion Corrosion of Coating
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Husak, Y.; Solodovnyk, O.; Yanovska, A.; Kozik, Y.; Liubchak, I.; Ivchenko, V.; Mishchenko, O.; Zinchenko, Y.; Kuznetsov, V.; Pogorielov, M. Degradation and in vivo response of hydroxyapatite-coated Mg alloy. Coatings 2018, 8, 375. [Google Scholar] [CrossRef]
- Tian, P.; Liu, X. Surface modification of biodegradable magnesium and its alloys for biomedical applications. Regen. Biomater. 2015, 2, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.H.M.; Luthringer, B.J.; Feyerabend, F.; Willumeit, R. Mg and Mg alloys: How comparable are in vitro and in vivo corrosion rates: A review. Acta. Biomater. 2015, 13, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Cheng, P.; Zhang, S.; Zhao, C.; Ni, J.; Zhang, Y.; Zhong, W.; Hou, P.; Zhang, X.; Zheng, Y.; et al. In vitro and in vivo studies on the degradation of high-purity Mg (99.99 wt.%) screw with femoral intracondylar fractured rabbit model. Biomaterials 2015, 64, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Gu, X.N.; Li, S.S.; Li, X.M.; Fan, Y.B. Magnesium based degradable biomaterials: A review. Front. Mater. Sci. 2014, 8, 200–318. [Google Scholar] [CrossRef]
- Pan, C.J.; Pang, L.Q.; Hou, Y.; Lin, Y.B.; Gong, T.; Liu, T.; Ye, W.; Ding, H.Y. Improving corrosion resistance and biocompatibility of magnesium alloy by sodium hydroxide and hydrofluoric acid treatments. Appl. Sci. 2016, 7, 33. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, G.; Tan, L.; Yang, K.; Ai, H. The fluoride coated AZ31B magnesium alloy improves corrosion resistance and stimulates bone formation in rabbit model. Mater. Sci. Eng. C 2016, 63, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.R.; Zhang, S.; Nie, L.L.; Zhu, Z.J.; Zhang, J.Q.; Cao, F.H.; Zhang, J.X. Electrodeposition and corrosion resistance of Ni–P–TiN composite coating on AZ91D magnesium alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 2976–2987. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, J.; Luo, D.; Wang, H.; Gong, X.; Han, Z.; Ren, L. One-step fabrication of biomimetic superhydrophobic surface by electrodeposition on magnesium alloy and its corrosion inhibition. J. Colloid. Interface. Sci. 2017, 491, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Badruddoza Dithi, A.; Nezu, T.; Nagano-Takebe, F.; Hasan, M.; Saito, T.; Endo, K. Application of solution plasma surface modification technology to the formation of thin hydroxyapatite film on titanium implants. Coatings 2019, 9, 3. [Google Scholar] [CrossRef]
- Liu, J.; Xi, T. Enhanced anti-corrosion ability and biocompatibility of PLGA coatings on Mg–Zn–Y–Nd alloy by BTSE-APTES pre-treatment for cardiovascular stent. J. Mater. Sci. Technol. 2016, 32, 845–857. [Google Scholar] [CrossRef]
- Çelik, İ. Structure and surface properties of Al2O3-TiO2 ceramic coated AZ31 magnesium alloy. Ceram. Int. 2016, 42, 13659–13663. [Google Scholar] [CrossRef]
- Ji, M.K.; Oh, G.; Kim, J.W.; Park, S.; Yun, K.D.; Bae, J.C.; Lim, H.P. Effects on antibacterial activity and osteoblast viability of non-thermal atmospheric pressure plasma and heat treatments of TiO2 nanotubes. J. Nanosci. Nanotechnol. 2017, 17, 2312–2315. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wu, G.; Feng, H.; Wang, W.; Zhang, X.; Chu, P.K. Improvement of corrosion resistance and biocompatibility of rare-earth WE43 magnesium alloy by neodymium self-ion implantation. Corros. Sci. 2015, 94, 142–155. [Google Scholar] [CrossRef]
- Cheng, M.; Qiao, Y.; Wang, Q.; Qin, H.; Zhang, X.; Liu, X. Dual ions implantation of zirconium and nitrogen into magnesium alloys for enhanced corrosion resistance, antimicrobial activity and biocompatibility. Colloids. Surf. B Biointerfaces 2016, 148, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Tian, P.; Liu, X.; Zhou, B. In-vitro, degradation, hemolysis, and cytocompatibility of PEO/PLLA composite coating on biodegradable AZ31 alloy. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Xu, D.; Liu, X. Mussel-inspired functionalization of PEO/PCL composite coating on a biodegradable AZ31 magnesium alloy. Colloids. Surf. B Biointerfaces 2016, 141, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Liu, X.; Ding, C. In vitro degradation behavior and cytocompatibility of biodegradable AZ31 alloy with PEO/HT composite coating. Colloids. Surf. B Biointerfaces 2015, 128, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Song, Y.; Shan, D.; Han, E.H. Corrosion behavior of a self-sealing pore micro-arc oxidation film on AM60 magnesium alloy. Corros. Sci. 2015, 100, 275–283. [Google Scholar] [CrossRef]
- Wu, G.; Gong, L.; Feng, K.; Wu, S.; Zhao, Y.; Chu, P.K. Rapid degradation of biomedical magnesium induced by zinc ion implantation. Mater. Lett. 2011, 65, 661–663. [Google Scholar] [CrossRef]
- Amaravathy, P.; Rose, C.; Sathiyanarayanan, S.; Rajendran, N. Evaluation of in vitro bioactivity and MG63 oesteoblast cell response for TiO coated magnesium alloys. J. Sol-Gel. Sci. Technol. 2012, 64, 694–703. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, L.J.; Yu, K.; Chen, C.; Dai, Y.L.; Qiao, X.Y.; Yan, Y.; Yu, Z.M. Effects of chitosan coating on biocompatibility of Mg–6%Zn–10%Ca3(PO4)2 implant. Trans. Nonferrous Met. Soc. China 2015, 25, 824–831. [Google Scholar] [CrossRef]
- Gu, X.N.; Zheng, W.; Cheng, Y.; Zheng, Y.F. A study on alkaline heat treated Mg–Ca alloy for the control of the biocorrosion rate. Acta Biomater. 2009, 5, 2790–2799. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, G.; Zhang, Y.H.; Zhao, Q. Growth and characterization of Mg(OH)2 film on magnesium alloy AZ31. Appl. Surf. Sci. 2011, 257, 6129–6137. [Google Scholar] [CrossRef]
- Peng, F.; Li, H.; Wang, D.; Tian, P.; Tian, Y.; Yuan, G.; Xu, D.; Liu, X. Enhanced corrosion resistance and biocompatibility of magnesium alloy by Mg–Al-layered double hydroxide. ACS. Appl. Mater. Interfaces. 2016, 8, 35033–36044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Xu, C.; Jing, Y.; Zhuang, J.; Wu, R.; Zhang, M. Formation of stacking faults for improving the performance of biodegradable Mg–Ho–Zn alloy. Mater. Lett. 2014, 133, 158–162. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, J.; Kong, P.; Zhang, Z.; Jing, Y.; Zhuang, J.; Wang, W.; Zhang, L.; Xu, C.; Wu, R.; et al. Enhancing the performance of Mg-based implant materials by introducing basal plane stacking faults. J. Mater. Chem. B 2015, 3, 7386–7400. [Google Scholar] [CrossRef]
- Dan, S.; Ma, A.B.; Jiang, J.; Lin, P.; Yang, D.; Fan, J. Corrosion behavior of equal-channel-angular-pressed pure magnesium in NaCl aqueous solution. Corros. Sci. 2010, 52, 481–490. [Google Scholar]
- Yao, C.; Lv, H.; Zhu, T.; Zheng, W.; Yuan, X.; Gao, W. Effect of Mg content on microstructure and corrosion behavior of hot dipped Zn–Al–Mg coatings. J. Alloys. Compd. 2016, 670, 239–248. [Google Scholar] [CrossRef]
- Mosiałek, M.; Mordarski, G.; Nowak, P.; Simka, W.; Nawrat, G.; Hanke, M.; Socha, R.P.; Michalska, J. Phosphate–permanganate conversion coatings on the AZ81 magnesium alloy: SEM, EIS and XPS studies. Surf. Coat. Technol. 2011, 206, 51–62. [Google Scholar] [CrossRef]
- Zhou, W.; Shan, D.; Han, E.H.; Ke, W. Structure and formation mechanism of phosphate conversion coating on die-cast AZ91D magnesium alloy. Corros. Sci. 2008, 50, 329–337. [Google Scholar] [CrossRef]
- Lu, F.; Ma, A.; Jiang, J.; Guo, Y.; Yang, D.; Song, D.; Chen, J. Significantly improved corrosion resistance of heat-treated Mg–Al–Gd alloy containing profuse needle-like precipitates within grains. Corros. Sci. 2015, 94, 171–178. [Google Scholar] [CrossRef]

| Component | Mass | Degree of Purity [%] |
|---|---|---|
| NaCl | 8.035 g/L | 99.5 |
| NaHCO3 | 0.355 g/L | 99.5 |
| KCl | 0.225 g/L | 99.5 |
| K2HPO43H2O | 0.231 g/L | 99.0 |
| MgCl26H2O | 0.311 g/L | 98.0 |
| C(HCl) = 1 M | 39 mL | – |
| CaCl2 | 0.292 g/L | 95.0 |
| Na2SO4 | 0.072 g/L | 99.0 |
| Tris | 6.118 g/L | 99.0 |
| Hydrothermal Time [h] | Jcorr [A/cm2] | Ecorr [V] |
|---|---|---|
| 0 | 2.015 × 10−4 | −1.793 |
| 0.5 | 1.114 × 10−4 | −1.854 |
| 1 | 6.765 × 10−5 | −1.712 |
| 2 | 2.129 × 10−5 | −1.450 |
| 3 | 2.896 × 10−5 | −1.367 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Zhang, J.; Liu, S.; Li, Z.; Zhang, L.; Wu, R.; Hou, L.; Zhang, M. Hydrothermal Synthesis of Protective Coating on Mg Alloy for Degradable Implant Applications. Coatings 2019, 9, 160. https://doi.org/10.3390/coatings9030160
Xie J, Zhang J, Liu S, Li Z, Zhang L, Wu R, Hou L, Zhang M. Hydrothermal Synthesis of Protective Coating on Mg Alloy for Degradable Implant Applications. Coatings. 2019; 9(3):160. https://doi.org/10.3390/coatings9030160
Chicago/Turabian StyleXie, Jinshu, Jinghuai Zhang, Shujuan Liu, Zehua Li, Li Zhang, Ruizhi Wu, Legan Hou, and Milin Zhang. 2019. "Hydrothermal Synthesis of Protective Coating on Mg Alloy for Degradable Implant Applications" Coatings 9, no. 3: 160. https://doi.org/10.3390/coatings9030160
APA StyleXie, J., Zhang, J., Liu, S., Li, Z., Zhang, L., Wu, R., Hou, L., & Zhang, M. (2019). Hydrothermal Synthesis of Protective Coating on Mg Alloy for Degradable Implant Applications. Coatings, 9(3), 160. https://doi.org/10.3390/coatings9030160






