Effects of Surface Microstructures on Superhydrophobic Properties and Oil-Water Separation Efficiency
Abstract
1. Introduction
2. Experimental
2.1. Materials
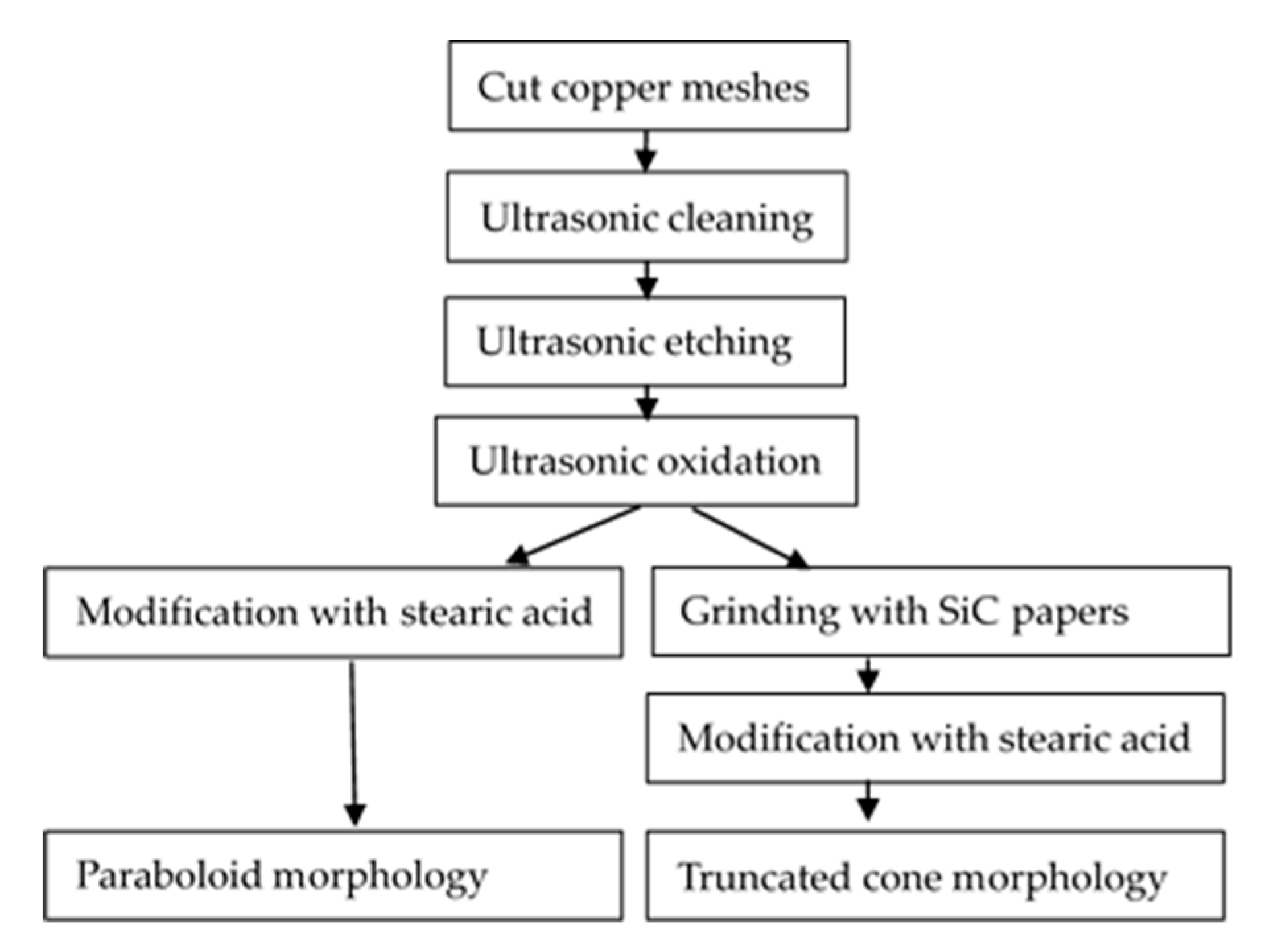
2.2. Fabrication of Superhydrophobic Materials
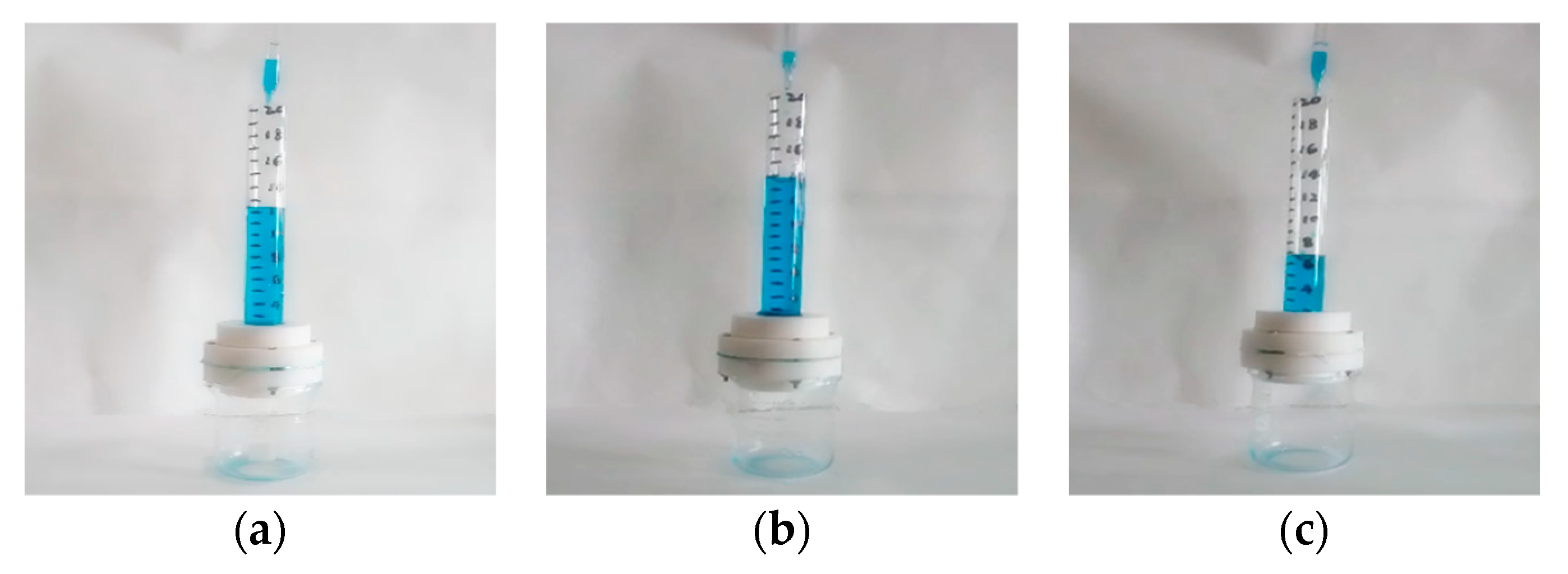
2.3. Oil-Water Separation Test
2.4. Characterization
2.4.1. Surface Morphology Characterization
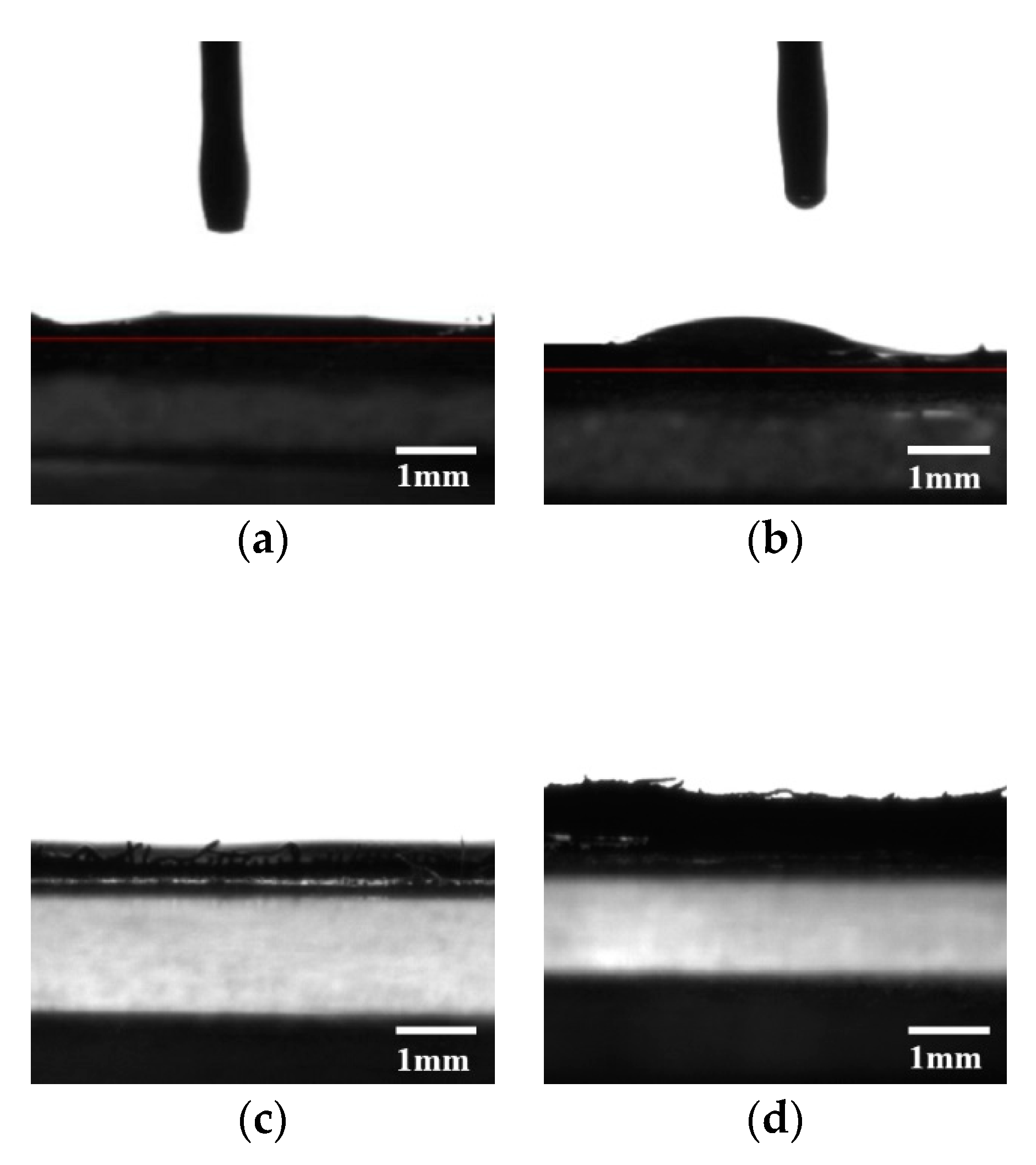
2.4.2. Contact Angle Measurements
3. Results and Discussion
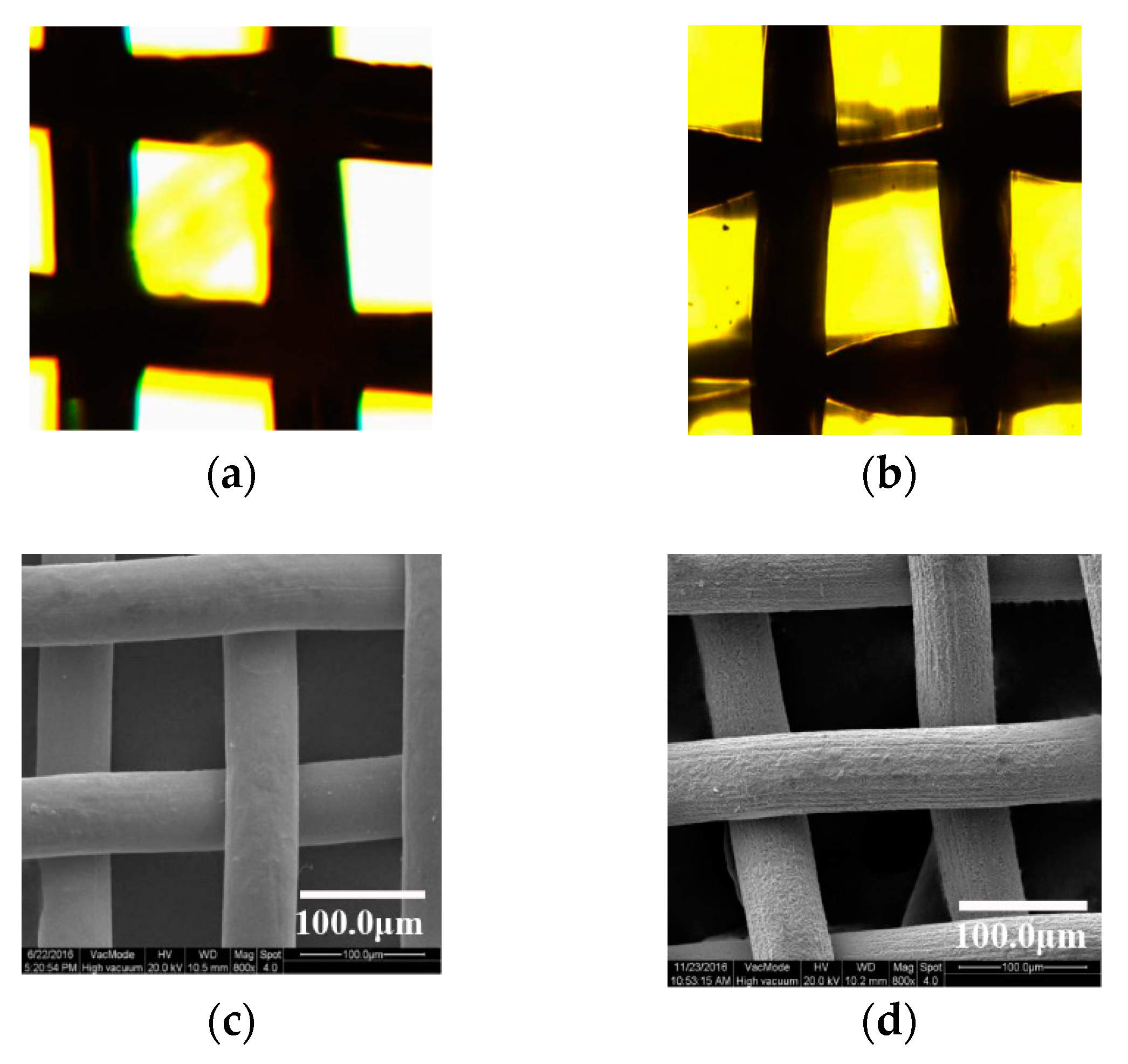
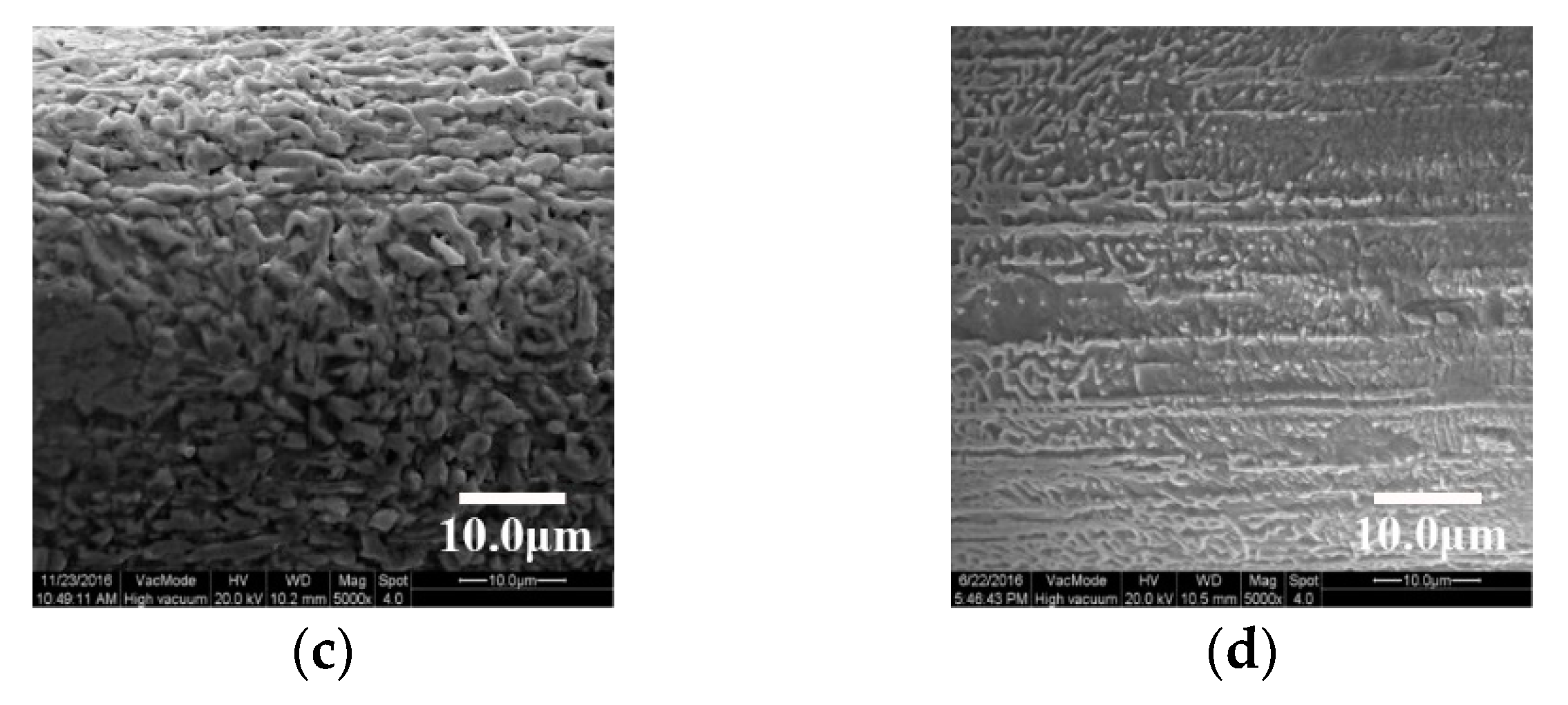
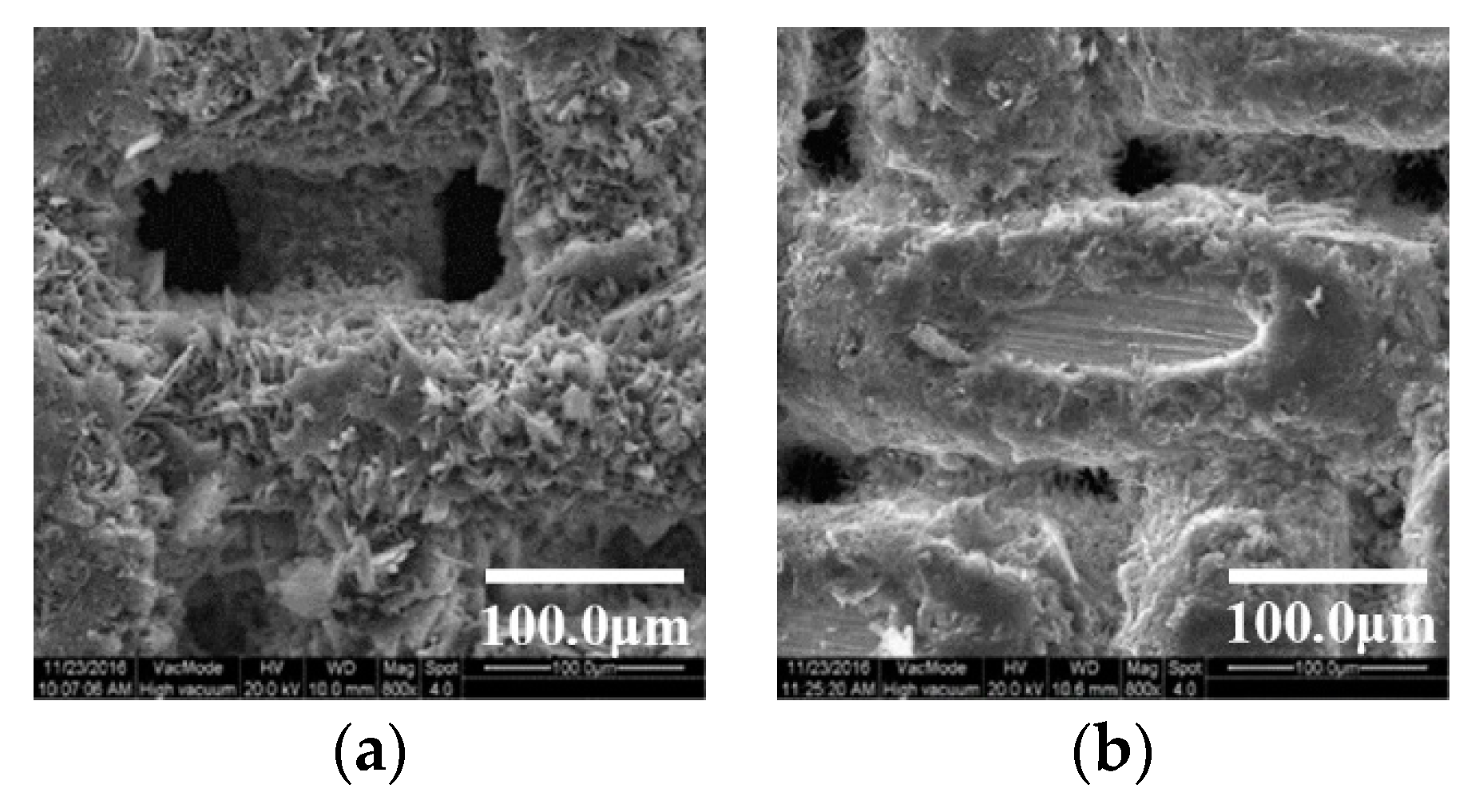
3.1. Surface Microstructure after Ultrasonic Etching and Oxidation
3.2. Surface Microstructure by Grinding with SiC
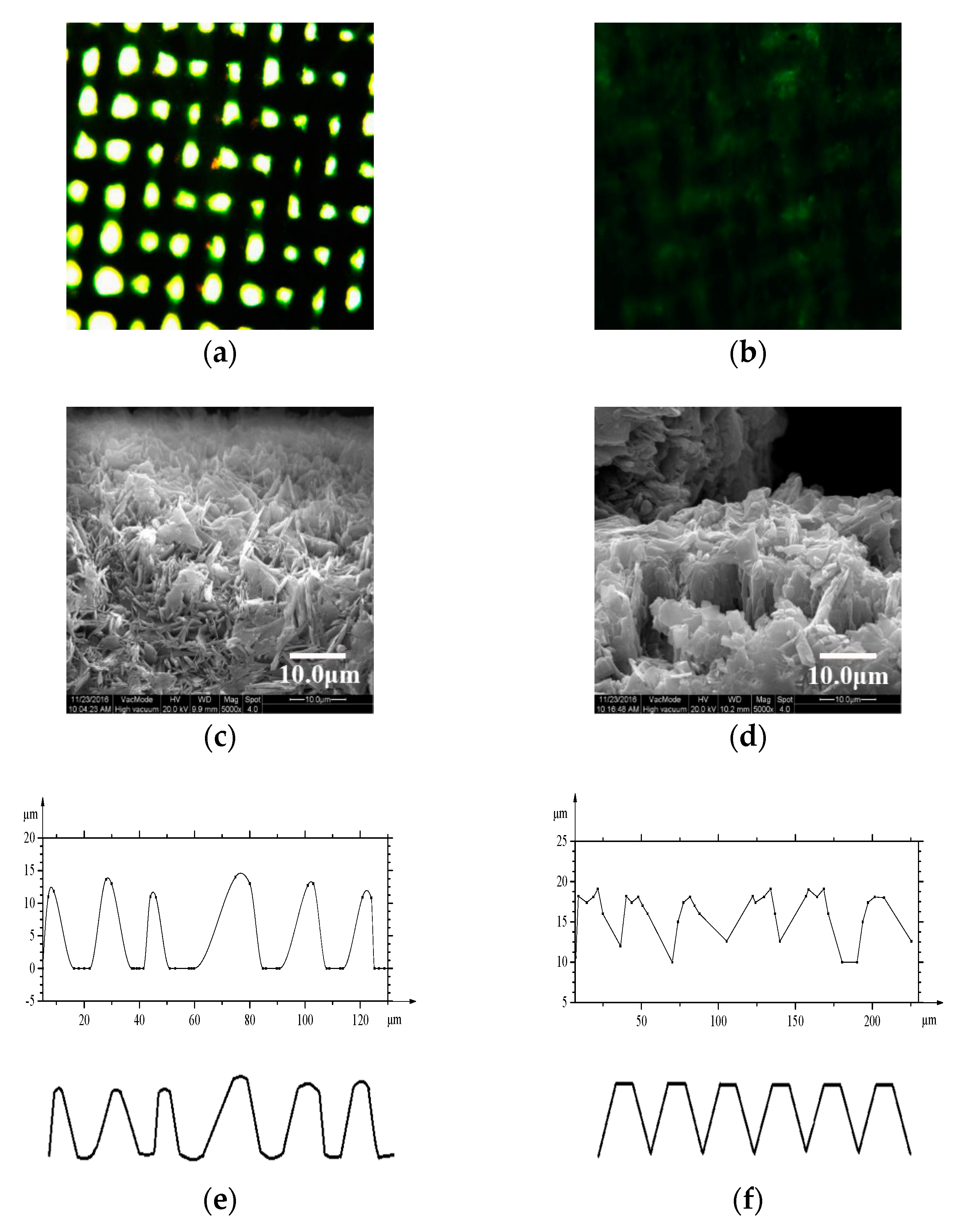
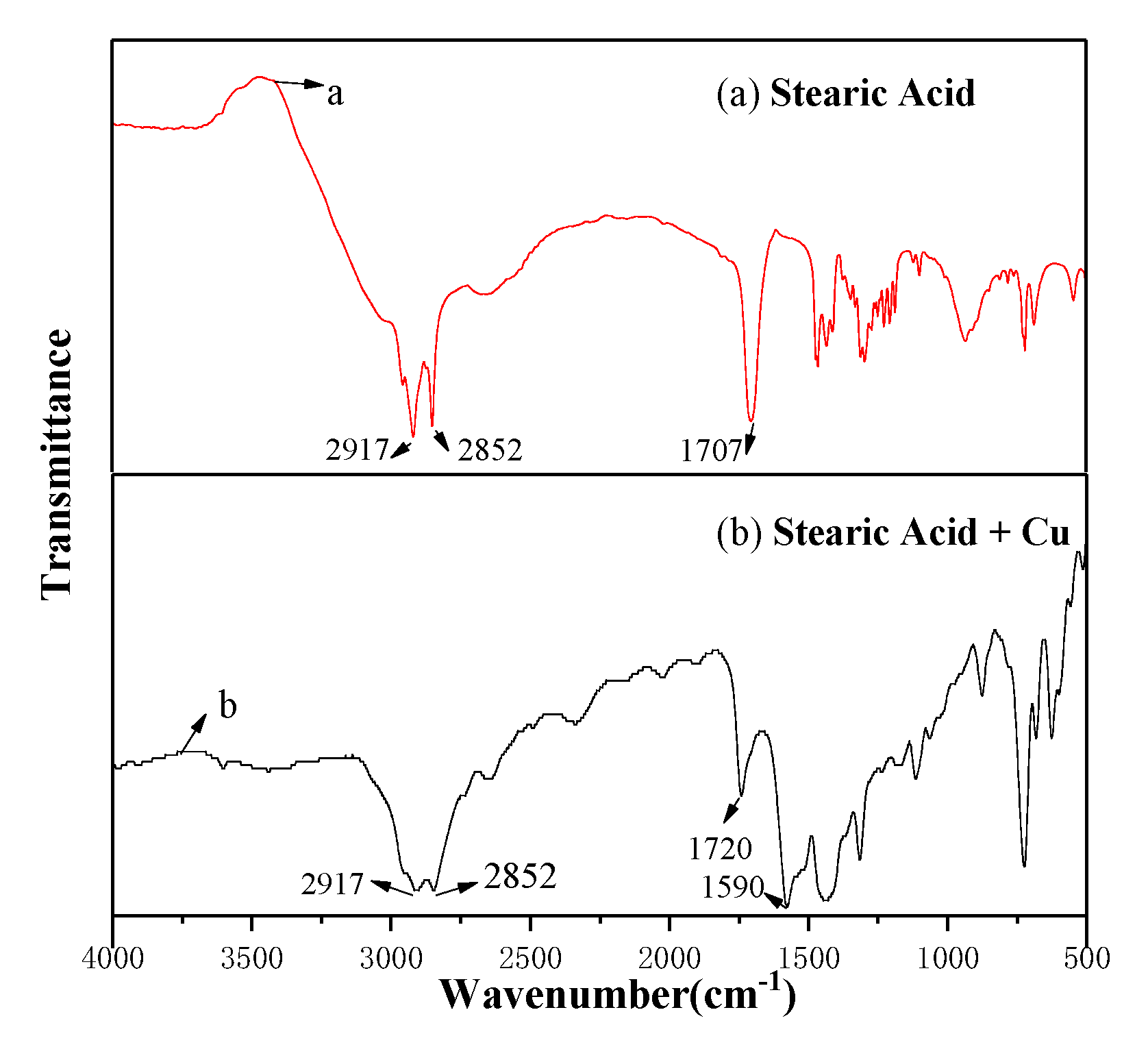
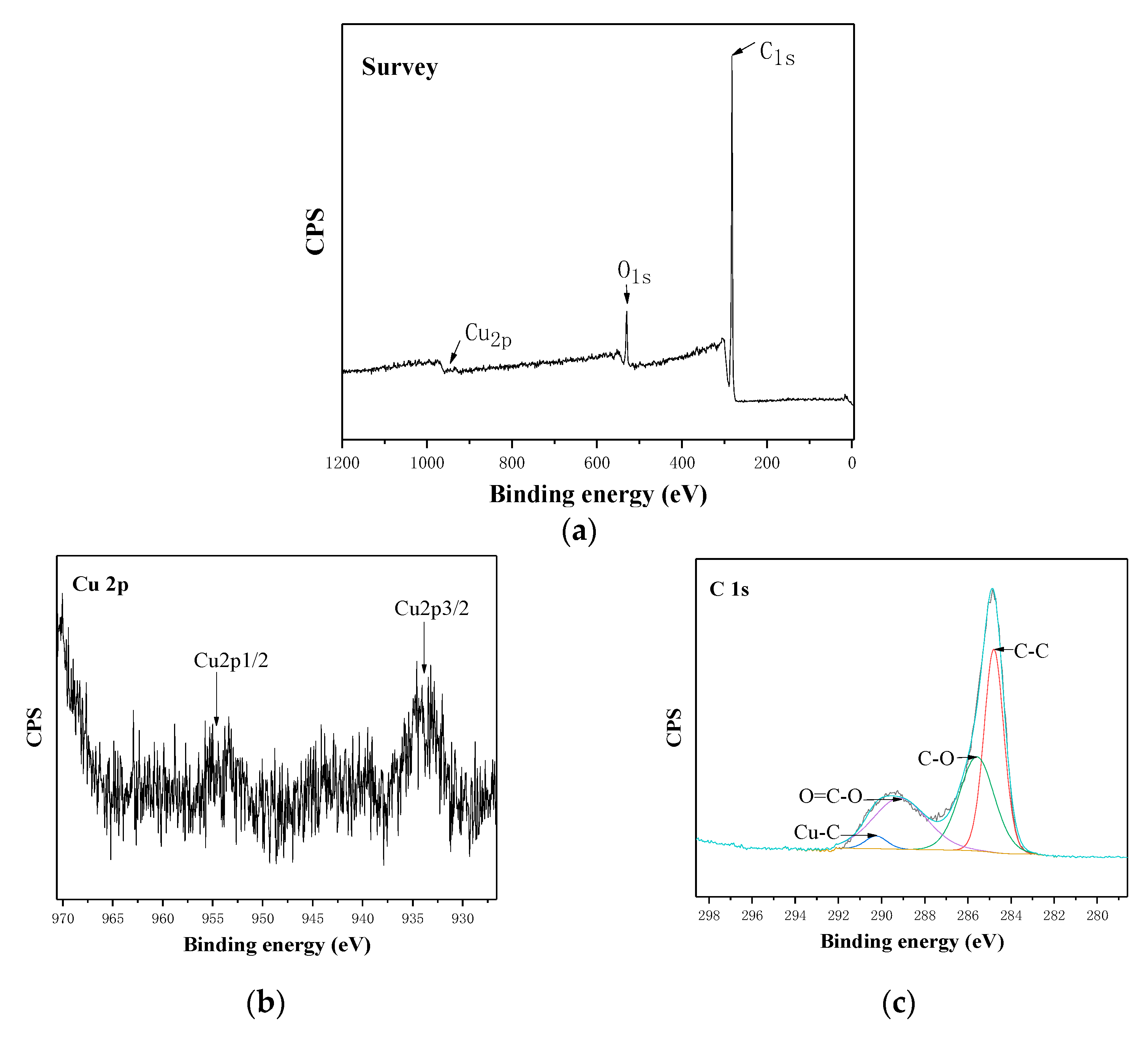
3.3. Surface Microstructure by Modification with SA
3.4. Wettability of the Copper Meshes
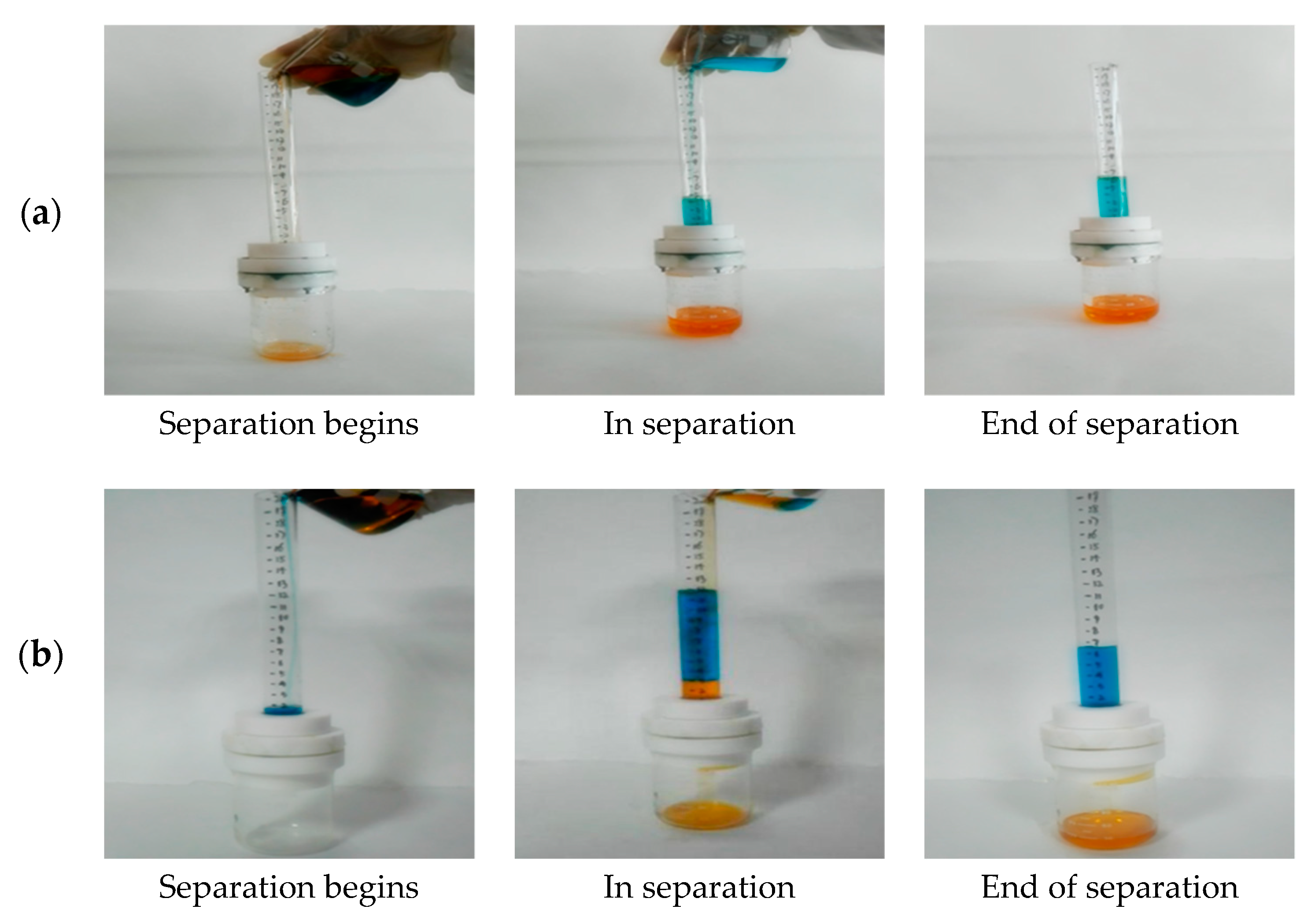
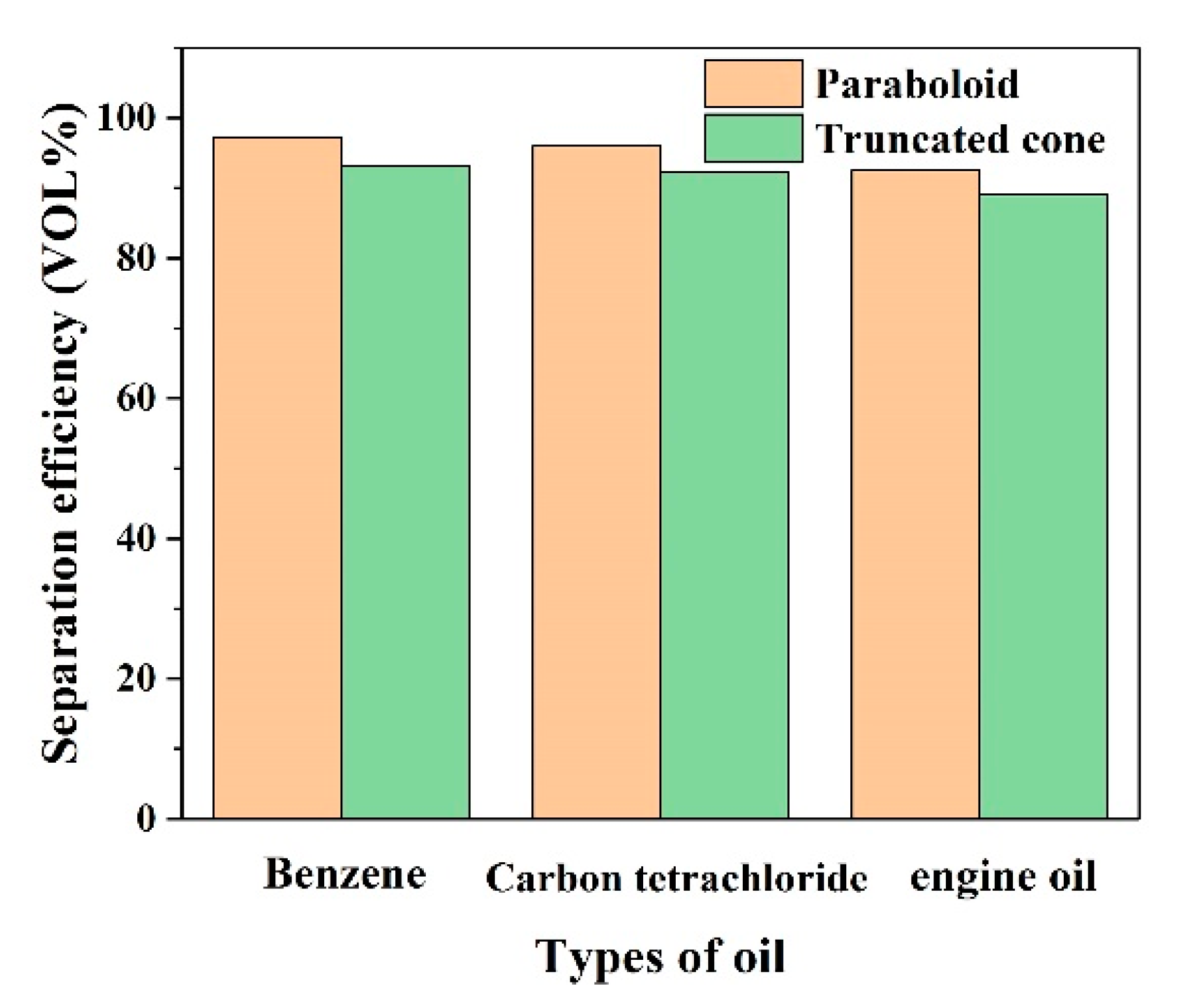
3.5. Oil-Water Separation Performance
3.6. The Height of Pressure Resistance Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kirby, M.F.; Law, R.J. Accidental spills at sea–risk, impact, mitigation and the need for co-ordinated post-incident monitoring. Mar. Pollut. Bull. 2010, 60, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.T.; Lin, B.; Jiang, L.W.; Lin, E.C.; Chen, J.; Zhang, S.J.; Tang, Y.W.; He, F.A.; Li, D.H. Effective preparation of magnetic superhydrophobic Fe3O4/Pu sponge for oil-water separation. Appl. Surf. Sci. 2018, 427, 56–64. [Google Scholar] [CrossRef]
- Kota, A.K.; Kwon, G.; Choi, W.; Mabry, J.M.; Tuteja, A. Hygro-responsive membranes for effective oil-water separation. Nat. Commun. 2012, 3, 1025. [Google Scholar] [CrossRef] [PubMed]
- Crick, C.R.; Bhachu, D.S.; Parkin, I.P. Superhydrophobic silica wool—A facile route to separating oil and hydrophobic solvents from water. Sci. Technol. Adv. Mater. 2014, 15, 065003. [Google Scholar] [CrossRef] [PubMed]
- Crick, C.R.; Ozkan, F.T.; Parkin, I.P. Fabrication of optimized oil–water separation devices through the targeted treatment of silica meshes. Sci. Technol. Adv. Mater. 2015, 16, 055006. [Google Scholar] [CrossRef] [PubMed]
- Velayi, E.; Norouzbeigi, R. Synthesis of hierarchical superhydrophobic zinc oxide nano-structures for oil/water separation. Ceram. Int. 2018, 44, 14202–14208. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, Z. Understanding the separations of oil/water mixtures from immiscible to emulsions on super-wettable surfaces. J. Bionic Eng. 2016, 13, 1–29. [Google Scholar]
- Gao, X.; Yan, X.; Yao, X.; Xu, L.; Zhang, K.; Zhang, J.; Yang, B.; Jiang, L. The dry-style antifogging properties of mosquito compound eyes and artificial analogues prepared by soft lithography. Adv. Mater. 2007, 19, 2213–2217. [Google Scholar] [CrossRef]
- Zhang, Z.; Ge, B.; Men, X.; Li, Y. Mechanically durable, superhydrophobic coatings prepared by dual-layer method for anti-corrosion and self-cleaning. Colloid Surf. A 2016, 490, 182–188. [Google Scholar] [CrossRef]
- Gao, X.; Guo, Z. Mechanical stability, corrosion resistance of superhydrophobic steel and repairable durability of its slippery surface. J. Colloid Interface Sci. 2018, 512, 239–248. [Google Scholar] [CrossRef]
- Shang, Q.; Zhou, Y. Fabrication of transparent superhydrophobic porous silica coating for self-cleaning and anti-fogging. Ceram. Int. 2016, 42, 8706–8712. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Yao, W.; Liu, J.; Han, Z.; Ren, L. Bioinspired structured superhydrophobic and superoleophilic stainless steel mesh for efficient oil-water separation. Colloid Surf. A 2016, 500, 54–63. [Google Scholar] [CrossRef]
- Wu, D.; Wang, P.; Wu, P.; Yang, Q.; Liu, F.; Han, Y.; Xu, F.; Wang, L. Determination of contact angle of droplet on convex and concave spherical surfaces. Chem. Phys. 2015, 457, 63–69. [Google Scholar] [CrossRef]
- Zhao, J.; Su, Z.; Yan, S. Thermodynamic analysis on an anisotropically superhydrophobic surface with a hierarchical structure. Appl. Surf. Sci. 2015, 357, 1625–1633. [Google Scholar] [CrossRef]
- Luo, B.H.; Shum, P.W.; Zhou, Z.F.; Li, K.Y. Surface geometrical model modification and contact angle prediction for the laser patterned steel surface. Surf. Coat. Technol. 2010, 205, 2597–2604. [Google Scholar] [CrossRef]
- Nickelsen, S.; Moghadam, A.D.; Ferguson, J.B.; Rohatgi, P. Modeling and experimental study of oil/water contact angle on biomimetic micro-parallel-patterned self-cleaning surfaces of selected alloys used in water industry. Appl. Surf. Sci. 2015, 353, 781–787. [Google Scholar] [CrossRef]
- Kota, A.K.; Kwon, G.; Tuteja, A. The design and applications of superomniphobic surfaces. NPG Asia Mater. 2014, 6, e109. [Google Scholar] [CrossRef]
- McHale, G.; Shirtcliffe, N.J.; Newton, M.I. Contact-angle hysteresis on super-hydrophobic surfaces. Langmuir 2004, 20, 10146–10149. [Google Scholar] [CrossRef]
- Hoshian, S.; Jokinen, V.; Franssila, S. Robust hybrid elastomer/metal-oxide superhydrophobic surfaces. Soft Matter 2016, 12, 6526–6535. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ogata, S. 3-D thermodynamic analysis of superhydrophobic surfaces. J. Colloid Interface Sci. 2008, 326, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Nosonovsky, M.; Bhushan, B. Roughness optimization for biomimetic superhydrophobic surfaces. Microsyst. Technol. 2005, 11, 535–549. [Google Scholar] [CrossRef]
- Bittoun, E.; Marmur, A. Optimizing super-hydrophobic surfaces: Criteria for comparison of surface topographies. J. Adhes. Sci. Technol. 2009, 23, 401–411. [Google Scholar] [CrossRef]
- Yang, W.; Gao, H.; Zhao, Y.; Bi, K.; Li, X. Facile preparation of nitrogen-doped graphene sponge as a highly efficient oil absorption material. Mater. Lett. 2016, 178, 95–99. [Google Scholar] [CrossRef]
- Rong, J.; Zhang, T.; Qiu, F.; Xu, J.; Zhu, Y.; Yang, D.; Dai, Y. Design and preparation of efficient, stable and superhydrophobic copper foam membrane for selective oil absorption and consecutive oil–water separation. Mater. Des. 2018, 142, 83–92. [Google Scholar] [CrossRef]
- Liu, L.; Singh, R.; Li, G.; Xiao, G.; Webley, P.A.; Zhai, Y. Synthesis of hydrophobic zeolite X@SiO2 core–shell composites. Mater. Chem. Phys. 2012, 133, 1144–1151. [Google Scholar] [CrossRef]
- Hoshian, S.; Jokinen, V.; Somerkivi, V.; Lokanathan, A.R.; Franssila, S. Robust superhydrophobic silicon without a low surface-energy hydrophobic coating. ACS Appl. Mater. Interfaces 2015, 7, 941. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.H.; Zahiri, B.; Sow, P.K.; Mérida, W. On-demand oil-water separation via low-voltage wettability switching of core-shell structures on copper substrates. Appl. Surf. Sci. 2018, 444, 15–27. [Google Scholar] [CrossRef]
- Cao, N.; Yang, B.; Barras, A.; Szunerits, S.; Boukherroub, R. Polyurethane sponge functionalized with superhydrophobic nanodiamond particles for efficient oil/water separation. Chem. Eng. J. 2017, 307, 319–325. [Google Scholar] [CrossRef]
- Guselnikova, O.; Elashnikov, R.; Postnikov, P.; Svorcik, V.; Lyutakov, O. Smart, piezo-responsive polyvinylidenefluoride/polymethylmethacrylate surface with triggerable water/oil wettability and adhesion. ACS Appl. Mater. Interfaces 2018, 10, 37461–37469. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Lai, X.; Su, X.; Liang, T.; Zeng, X. Thiolated graphene-based superhydrophobic sponges for oil-water separation. Chem. Eng. J. 2017, 316, 736–743. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Wang, H.; Wang, C.; Li, H.; Zhang, X.; Yuan, R.; Zhao, Y. Facile preparation of superhydrophobic metal foam for durable and high efficient continuous oil-water separation. Chem. Eng. J. 2017, 322, 157–166. [Google Scholar] [CrossRef]
- Chen, X.; Liang, Y.N.; Tang, X.Z.; Shen, W.; Hu, X. Additive-free poly (vinylidene fluoride) aerogel for oil/water separation and rapid oil absorption. Chem. Eng. J. 2017, 308, 18–26. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, T.; Yang, D.; Qiu, F.; Rong, J.; Xu, J.; Fang, J. The synthesis of hierarchical porous Al2O3/acrylic resin composites as durable, efficient and recyclable absorbents for oil/water separation. Chem. Eng. J. 2017, 309, 522–531. [Google Scholar] [CrossRef]
- Wang, H.; Wang, E.; Liu, Z.; Gao, D.; Yuan, R.; Sun, L.; Zhu, Y. A novel carbon nanotubes reinforced superhydrophobic and superoleophilic polyurethane sponge for selective oil-water separation through a chemical fabrication. J. Mater. Chem. A 2015, 3, 266–273. [Google Scholar] [CrossRef]
- Zang, D.; Liu, F.; Zhang, M.; Niu, X.; Gao, Z.; Wang, C. Superhydrophobic coating on fiberglass cloth for selective removal of oil from water. Chem. Eng. J. 2015, 262, 210–216. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, B.; Bai, J.; Yang, Z.; Guo, S.; Ding, Y.; Yang, L.; Lei, P.; Han, J.; Zhu, J. Synthesis of vertically aligned composite microcone membrane filter for water/oil separation. Mater. Des. 2016, 111, 9–16. [Google Scholar] [CrossRef]
- Cao, C.; Cheng, J. Fabrication of robust surfaces with special wettability on porous copper substrates for various oil/water separations. Chem. Eng. J. 2018, 347, 585–594. [Google Scholar] [CrossRef]
- Qu, M.; Zhang, B.; Song, S.; Chen, L.; Zhang, J.; Cao, X. Fabrication of superhydrophobic surfaces on engineering materials by a solution-immersion process. Adv. Funct. Mater. 2007, 17, 593–596. [Google Scholar] [CrossRef]
- Zhang, W.; Wen, X.; Yang, S. Controlled reactions on a copper surface: Synthesis and characterization of nanostructured copper compound films. Inorg. Chem. 2003, 42, 5005–5014. [Google Scholar] [CrossRef]
- Badge, I.; Sethi, S.; Dhinojwala, A. Carbon nanotube-based robust steamphobic surfaces. Langmuir ACS J. Surf. Colloids 2011, 27, 14726–14731. [Google Scholar] [CrossRef] [PubMed]
- Hardman, S.J.; Muhamad-Sarih, N.; Riggs, H.J.; Thompson, R.L.; Rigby, J.; Bergius, W.N.A.; Hutchings, L.R. Electrospinning superhydrophobic fibers using surface segregating end-functionalized polymer additives. Macromolecules 2011, 44, 6461–6470. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, X.; Li, J.; Liu, J.H.; Huang, X.J. ZnO/CuO hetero-hierarchical nanotrees array: Hydrothermal preparation and self-cleaning properties. Langmuir 2011, 27, 6193–6200. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Liang, S.; Pan, A.; Yuan, Z.; Tang, Y.; Tan, X.P.; Guan, D.K.; Yu, Y. Fabrication of nano-structured super-hydrophobic film on aluminum by controllable immersing method. Appl. Surf. Sci. 2012, 258, 5933–5937. [Google Scholar] [CrossRef]
- Pan, L.; Dong, H.; Bi, P. Facile preparation of superhydrophobic copper surface by HNO3 etching technique with the assistance of CTAB and ultrasonication. Appl. Surf. Sci. 2010, 257, 1707–1711. [Google Scholar] [CrossRef]
- Khosravi, M.; Azizian, S. Preparation of superhydrophobic and superoleophilic nanostructured layer on steel mesh for oil-water separation. Sep. Purif. Technol. 2017, 172, 366–373. [Google Scholar] [CrossRef]
- Heni, W.; Vonna, L.; Fioux, P.; Vidal, L.; Haidara, H. Ultrasonic cavitation test applied to thin metallic films for assessing their adhesion with mercaptosilanes and surface roughness. J. Mater. Sci. 2014, 49, 6750–6761. [Google Scholar] [CrossRef]
- Ma, F.M.; Hao, Q.Y.; Zhang, Y.; Ruan, M.; Yu, Z.L. Fabrication of copper-based superhydrophobic surface by redox etching. Sci. Technol. Eng. 2013, 14, 026. [Google Scholar]
- Zhang, C.L.; Zhang, F.; Song, L.; Zeng, R.C.; Li, S.Q.; Han, E.H. Corrosion resistance of a superhydrophobic surface on micro-arc oxidation coated Mg-Li-Ca alloy. J. Alloys Compd. 2017, 728, 815–826. [Google Scholar] [CrossRef]
- Ma, Q.; Cheng, H.; Fane, A.G.; Wang, R.; Zhang, H. Recent development of advanced materials with special wettability for selective oil/water separation. Small 2016, 12, 2186–2202. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Zhang, Y.L.; Fu, X.Y.; Sun, H.B. Bioinspired underwater superoleophobic membrane based on a graphene oxide coated wire mesh for efficient oil/water separation. ACS Appl. Mater. Interfaces 2015, 7, 20930–20936. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Choi, M.; Heo, J.; Jeong, H.; Park, S.; Hong, J. Cobweb-inspired superhydrophobic multi-scaled gating membrane with embedded network structure for robust water-in-oil emulsion separation. ACS Sustain. Chem. Eng. 2017, 5, 3448–3455. [Google Scholar] [CrossRef]
- Cao, H.; Fu, J.; Liu, Y.; Chen, S. Facile design of superhydrophobic and superoleophilic copper mesh assisted by candle soot for oil water separation. Colloid Surf. A 2017, 537, 294–302. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Wu, Y.; Sun, W.; Wang, J.; Sun, H. One-step method for fabrication of superhydrophobic and superoleophilic surface for water-oil separation. Colloid Surf. A 2018, 552, 32–38. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, X.; Bin, J.; Peng, C.; Xing, S.; Wang, M.; Xiao, J.; Zeng, J.; Xie, Y.; Xiao, X. A novel fabrication of a superhydrophobic surface with highly similar hierarchical structure of the lotus leaf on a copper sheet. Appl. Surf. Sci. 2013, 285, 205–210. [Google Scholar] [CrossRef]
- Li, J.; Huang, R.H.; Zhou, P.; Xu, Y.; Li, Z.P.; Wang, L.F. Influencing factors of splashing caused by engine oil droplets impacting on wall in the crankcase. Trans. CSICE 2017, 6, 548–553. (In Chinese) [Google Scholar]
- Erbil, H.Y.; Demirel, A.L.; Avci, Y.; Mert, O. Transformation of a simple plastic into a superhydrophobic surface. Science 2003, 299, 1377–1380. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, L. Bio-inspired design of multiscale structures for function integration. Nano Today 2011, 6, 155–175. [Google Scholar] [CrossRef]
- Cao, H.; Gu, W.; Fu, J.; Liu, Y.; Chen, S. Preparation of superhydrophobic/oleophilic copper mesh for oil-water separation. Appl. Surf. Sci. 2017, 412, 599–605. [Google Scholar] [CrossRef]
- Jiang, Z.X.; Geng, L.; Huang, Y.D. Design and fabrication of hydrophobic copper mesh with striking loading capacity and pressure resistance. J. Phys. Chem. C 2010, 114, 9370–9378. [Google Scholar] [CrossRef]


| Different Situations | Angel | ||
|---|---|---|---|
| Different Types of Contact Angles | Parabolic | Truncated Cone | |
| water | WCA | 153.6° | 121.8° |
| θA | 154.6° ± 1.1° | 151.5° ± 1.8° | |
| θR | 122.7° ± 1.6° | 119.6° ± 2.7° | |
| Engine oil | – | 5° | 32.9° |
| Carbon tetrachloride | – | 0.1° | 0.1° |
| Different Situations | Height of Pressure Resistance (cm of Water) | |
|---|---|---|
| Parabolic | Truncated Cone | |
| Theoretical value | 12 | 6.9 |
| Actual value | 21.4 | 19.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yang, S.; Zhang, Q.; Zhang, D.; Yang, C.; Wang, Z.; Wang, R.; Song, R.; Wang, W.; Zhao, Y. Effects of Surface Microstructures on Superhydrophobic Properties and Oil-Water Separation Efficiency. Coatings 2019, 9, 69. https://doi.org/10.3390/coatings9020069
Chen Y, Yang S, Zhang Q, Zhang D, Yang C, Wang Z, Wang R, Song R, Wang W, Zhao Y. Effects of Surface Microstructures on Superhydrophobic Properties and Oil-Water Separation Efficiency. Coatings. 2019; 9(2):69. https://doi.org/10.3390/coatings9020069
Chicago/Turabian StyleChen, Yangyang, Shengke Yang, Qian Zhang, Dan Zhang, Chunyan Yang, Zongzhou Wang, Runze Wang, Rong Song, Wenke Wang, and Yaqian Zhao. 2019. "Effects of Surface Microstructures on Superhydrophobic Properties and Oil-Water Separation Efficiency" Coatings 9, no. 2: 69. https://doi.org/10.3390/coatings9020069
APA StyleChen, Y., Yang, S., Zhang, Q., Zhang, D., Yang, C., Wang, Z., Wang, R., Song, R., Wang, W., & Zhao, Y. (2019). Effects of Surface Microstructures on Superhydrophobic Properties and Oil-Water Separation Efficiency. Coatings, 9(2), 69. https://doi.org/10.3390/coatings9020069





