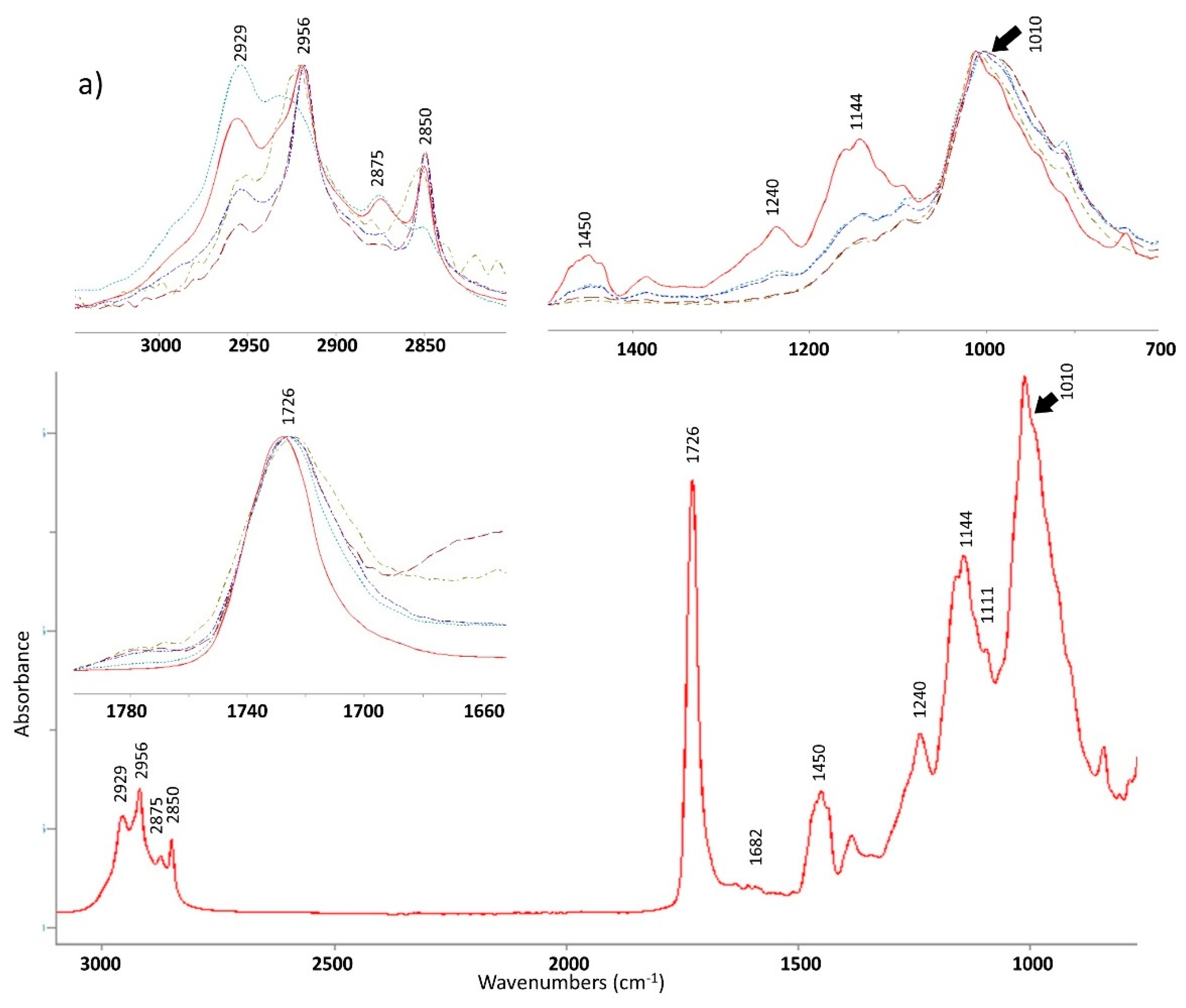
3.1. FTIR-ATR
In order to characterise the chemical modifications in the polymer structure, infrared spectroscopy was performed on samples before and after exposure as well as on the control specimens. There is no difference between the samples before exposure and the control specimens. Spectra of non-exposed and exposed samples are presented in
Figure 1.
Table 3 summarises the main contributions. The three main modifications are increases in absorption from 2800 to 3050 cm
−1, from 1650 to 1800 cm
−1 and from 900 to 1550 cm
−1. The first region corresponds to the bands attributed to methyl and methylene contribution at 2956, 2919, 2875, and 2850 cm
−1 corresponding to δ
as (CH
3), δ
as (CH
2), δ
s (CH
3), and δ
s (CH
2), respectively, with δ
as and δ
s for asymmetric and symmetric vibrational mode.
Photodegradation of acrylic paints produces low molecular weight molecules [
5,
6,
11,
36]. These are generated through Norrish type I or II reactions accompanied by chain scissions in the early steps of the degradation process. In this study, the long exposure times allowed the leaching of the small molecules formed. Consequently, decreases of some peaks can be noticed for both paints. For example, decreases observed for the peaks corresponding to both C–H bend δ
s (CH
2) + δ
as (CH
3) at 1450 cm
−1 and the δ
s (CH
3) at 1682 cm
−1.
For both paints, the contribution at 1144 and 1111 cm−1 attributed to C–O stretching disappears. A noteworthy observation is the degradation of the polymeric side chain which is apparent from decrease of the absorption at 1240 cm−1 attributed to the asymmetric stretching vibration C–O–C bond.
A decrease of the fingerprint region between 900 and 1500 cm
−1 (
Figure 1) can be noticed for all the exposed samples. Chain scissions lead to the generation of products and leaching or evaporation if the products are small enough. In addition to organic compounds found in paints, it is possible to also find clays or minerals such as talc. They act as fillers and their use, makes it possible to reduce the quantity of solvent, improve paint adhesion or avoid surface defect during curing. Due to their inorganic nature, fillers are not prone to photodegradation. The Si–O–Si contribution from the talc is found in the infrared spectrum at 1010 cm
−1 [
37]. No modification of this peak occurred even after samples were exposed in Arizona. Therefore, the gradual degradation of the resin or other organic compounds led to apparently stronger contribution of this peak to the spectra.
After the exposures trials, differences in durability between Paint 1 and Paint 2 were apparent, particularly the contribution of the carbonyl peak at 1726 cm
−1. Carbonyl compounds can be oxidized into new carbonyl groups like ketones at 1710 cm
−1. This phenomenon can be observed by the broadening of this region in the spectrum for Paint 1 and was attributed by Chiantore et al. (2000) [
5] as resulting from a reaction between the resin, oxygen molecules and radical species following β-scission reactions. These new compounds absorb IR in the same region as that of the initial carbonyl ester groups. Their generation counterbalances the initial decrease of the contribution at 1726 cm
−1.
Concerning Paint 2, only a decrease of the peak at 1726 cm−1 is observed reflecting a loss of small molecules after chain scissions. Thus, either there is no compensation by formation of new compounds as seen for Paint 1 or the new compounds are volatized or leached during exposure. A specific component from Paint 1 formulation seems to make possible reactions with new carbonyls and the reaction products are retained within the polymer network.
Another difference is the specific decrease of the δ
as (CH
2) contribution at 2919 cm
−1 for the exposure of samples in the Province of Quebec. This is likely due to the fragmentation of the chain and the generation of unsaturated products. Results from the quantitative analysis of peaks at 2956 and 1726 cm
−1 are presented in
Table 4.
Overall, these results show greater polymer degradation for Paint 1 compared to Paint 2. In addition, for the two paints, greater degradation occurs after samples are exposure in the QUV device. This device is equipped with fluorescent lamps emitting UV rays. From the Planck constant, it can be said that the shorter the wavelength is and the higher the photon energy will be. Consequently, this explains the stronger degradation of the paint. Photons from the visible light are less energetic and contribute less to paint degradation. This confirms the sensitivity of the polymer to UV radiation.
For Paint 1, the values obtained for the ratio at 1726 cm
−1 suggests the generation of new carbonyl compounds as explained above. After exposure in Arizona and in the QUV, percentages of remaining functional groups are still high compared with the two other exposure methods (Florida and Quebec). As indicated in
Tables S4 and S6 of the
Supplementary Materials, strong precipitation was present in Florida and Quebec. New carbonyl compounds are more likely to be leached from the coating compared to samples exposed in Arizona or in the QUV. Both Arizona and QUV exposure led to the same values for the both FTIR ratios. However, differences in the aesthetics of the two coatings between these two methods are strongly different and are described below. This observation confirms the necessity to consider degradation factors other than photodegradation during wood weathering trials.
3.2. Colorimetric Analysis
Colorimetric analyses did not show differences between non exposed samples and control specimens.
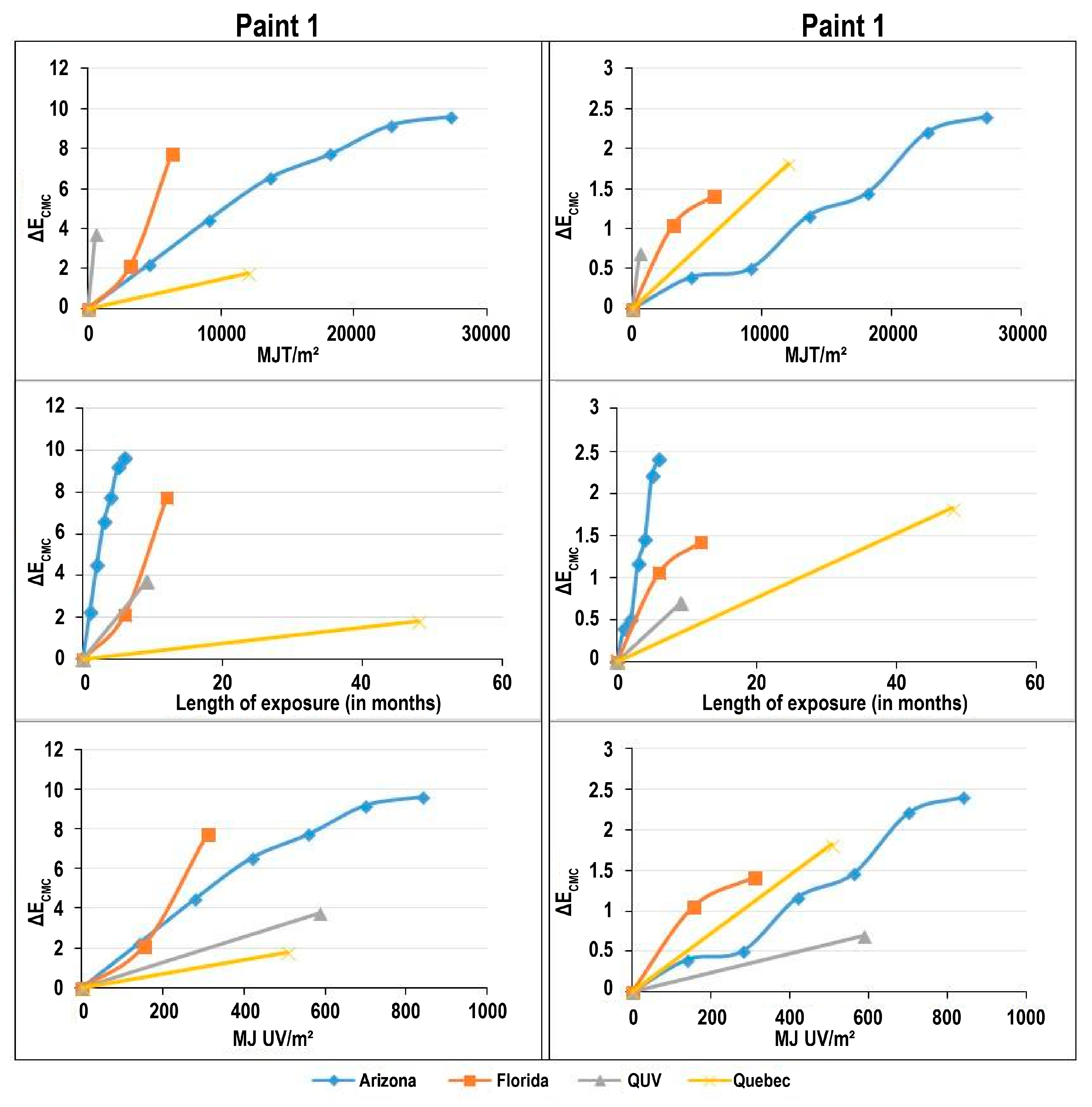
Table 5 summarises the Δ
ECMC values. The higher the Δ
ECMC is, the higher the overall color modification of coatings. Paint 2 presents is more color stabile than Paint 1 except at the Quebec site. This discrepancy will be discussed in the next section. For both paints, the most pronounced changes occurred at the Arizona site with discoloration values for Paint 1 and Paint 2 of 9.62 and 2.41, respectively.
Previous studies attempted to establish a correlation between different weathering methods through the study of degradation after different exposure periods [
21,
38,
39]. During outdoor exposure, severity of degradation depends of geographic area, weather, and timing of the exposure trial. During indoor accelerated weathering exposure, degradation depends of the setup of the device. For the same length of exposure, in terms of the parameters implemented, the degradation will be different. Since photodegradation is the main factor causing surface degradation, modifications due to exposures can be compared using the energy received by the samples, either in MJ T/m
2 or MJ UV/m
2, respectively, megajoule total/m
2 and megajoule/m
2.
Figure 2 shows the color modification values in terms of three kinds of abscissa: MJ UV/m
2, MJ T/m
2 and the length of exposure in days. The global shape of the curves can be visually compared to find either similarities or differences between the exposure methods/sites. Looking at the strong differences between the different curves, it appears that each exposure has a different impact on the paint and, each paint responds differently. This finding suggests that degradation cannot be compared simply in terms of the UV or quantity of energy samples receive or the length of time they are exposed to the weather. Colorimetric analysis captures modifications to the coatings due to weathering and particular features of each weathering method are apparent.
First of all, since the most degrading method varies when results are expressed in terms of length of exposure and the quantum of energy received, which suggests that there are factors other than photodegradation that modify colour of paints.
Table 6 presents the method causing the most pronounced degradation in terms of color change. When expressing colour changes in terms of the total energy received by the samples, the QUV exposure is the most degrading method for both paints. Total energy corresponds here to the summation of the different regions of the electromagnetic spectrum, such as the UV, visible and infrared radiation. This device employed fluorescent lamps producing UV radiation, the most degrading part of the light spectrum.
Consequently, 1 MJ in a QUV is more degrading than 1 MJ from outdoor exposures. Damage will be stronger due to the higher energy of UVs. This observation confirms the photosensitivity of the paints to UV radiation, as well as the efficiency of the accelerated method.
When expressing colour changes in terms of length of exposure, Arizona exposure caused the most severe degradation of both paints. The exposure trial in Arizona used mirrors to amplify light from the sun. Water spray was used as indicated in the ASTM G90 (2010) to provide water for chemical reactions. Moreover, data in
Table S3 of the
Supplementary Materials show abundance of sunshine with high temperature at the Arizona site. These conditions are more severe than those encountered during normal end uses: one day exposure in Arizona is more degrading than one day in a QUV or in Florida or the Province of Quebec. This observation confirms the accelerated aspect of Arizona exposure against the other methods studied.
When expressing colour changes in terms of quantity of MJ UV/m2 received by samples, Florida exposure was the most severe for both paints. Since Florida exposure encountered all the main weathering factors such as wind, fungi, erosion and high humidity, it is the closest to an exposure in the end use environment. Yet, taking into account only the quantity of MJ UV/m2 received by samples limits the interpretation since it does not consider all the above weathering factors and their impacts on samples. This confirms the importance of including the multifactorial aspect of weathering when interpreting colour changes.
Exposure of samples in Quebec did not lead to strong degradation. When the length of exposure is considered, exposure in Quebec causes the least degradation. This observation confirms the acceleration of weathering that can be achieved by exposing samples in Arizona and in Florida, as well as in the QUV.
Values of ΔECMC obtained from measurements on samples exposed in Quebec are almost the same for both paints, in contrast to other results from other trials where the discoloration of Paint 1 was higher than that of Paint 2. This interaction points to the need to expose samples in different climates. In addition, the length of exposures in the Province of Quebec and the others exposures were not the same and this could lead to distortion in the interpretation of results.
Table 2 indicates that red oxide is present in the formulation of the Paint 2. This compound, has strong capacity to absorb in the UV region [
40,
41]. Techniques of accelerated weathering are almost all based on the use of higher intensity UV radiation to accelerate the rate of degradation. In this case, Paint 2 has better resistance against accelerated weathering likely due to the presence of the red pigment in its formulation.
3.3. Overview and Recommendations
Table 7 summarises the parameters as well as the main results obtained from the characterisation of the degradation for each exposure method/trial. The interpretation of these results makes it possible to develop good practice for use of the different weathering methods. These recommendations are relevant for products designed to be used in environmental conditions similar to an oceanic or continental climate. This information will help manufacturers choose the most reliable method according to their needs. It is important to note that degradation occurring in outdoor exposures depends on variables such as geographic area, pollution, weather, years and even days of study, to name only a few. Making comparison between experiments executed in different outdoor sites can be problematic since the variables influence the rates of degradation. This is why, degradation assessments should be performed using the quanta of energy received by samples in addition to length of exposure.
Among the four exposure methods we employed, Arizona exposure resulted in the most rapid degradation. After exposure in such extreme conditions (e.g., high temperature and irradiance), coating failure may not necessarily be due to a poor paint technology. It can also be the result of the unrealistic conditions that samples are exposed to compared to the conditions in expected in service environment. On the other hand, successful performance would indicate that the paint has high resistance to photodegradation. Arizona exposure should be used to test the limits of paint durability against UV radiation rather than to study its performance in service in most environments.
Florida exposure promote fungal growth. Since wood is composed of organic matter it is susceptible to fungal degradation even when it is coated. In oceanic or continental climate where the risk of fungal growth is present, it is important to take this factor into account [
42]. In addition, most of the degradation attributed to weathering in such climates are encountered in Florida although the exposure parameters and the results presented in
Table 7 indicate that degradation is accelerated. However, the rate of acceleration in Florida is not as high as that for Arizona exposure. Florida exposure should be used in order to test paint resistance against synergic degradation between photodegradation and degradation resulting from moisture and fungal growth.
Controlled accelerated exposure, such as the one provided by a QUV device, are convenient due to the possibility of tailoring the degradation parameters. Additionally, the use of fluorescent lamps that generate UV results in accelerated degradation of the paint polymer (acrylic polymer in this paper). This is confirmed here by the values obtained from both colorimetric and infrared analysis. Unlike the other exposure methods, polymer degradation is higher than the discoloration in the QUV device. QUV exposure should be used in order to test the stability of the paint polymer rather than loss of aesthetics of coatings.
Finally, in this experiment, exposure in the Province of Quebec was used to test the paints in the end use environment.
Table 7 shows that even after 48 months of exposure, the quantum of energy received was still below those recorded for the short exposure periods in Arizona and Florida. These results demonstrate the need to use accelerated exposures to provide an indication of the long-term durability of paints in their end use environment. However, even though the amount of energy received was lower, color changes recorded in Province of Quebec are higher than those observed in Arizona when results are expressed using the quantum of energy total received by the samples (
Figure 2). Accelerated exposure methods focus essentially on the role of light as degradation factor. This result emphasises the importance of degradation factors others than light and water in the weathering process. As an example, snow which was only present in Quebec may have influenced color changes. In addition, accelerated exposure provide severe weathering conditions often more severe than those found in the end use environment and thus might lead to degradation that would never occur in service. In the Province of Quebec, panels were exposed at 90° while in others natural weathering sites samples were exposed at 45°. As a consequence, the light energy and the kinetic effect of rain were weaker. Also, exposure at 45° allows the accumulation of by-products or impurities that can protect the coating surface.