Biocleaning to Remove Graffiti: A Real Possibility? Advances towards a Complete Protocol of Action
Abstract
1. Introduction
2. Materials and Methods
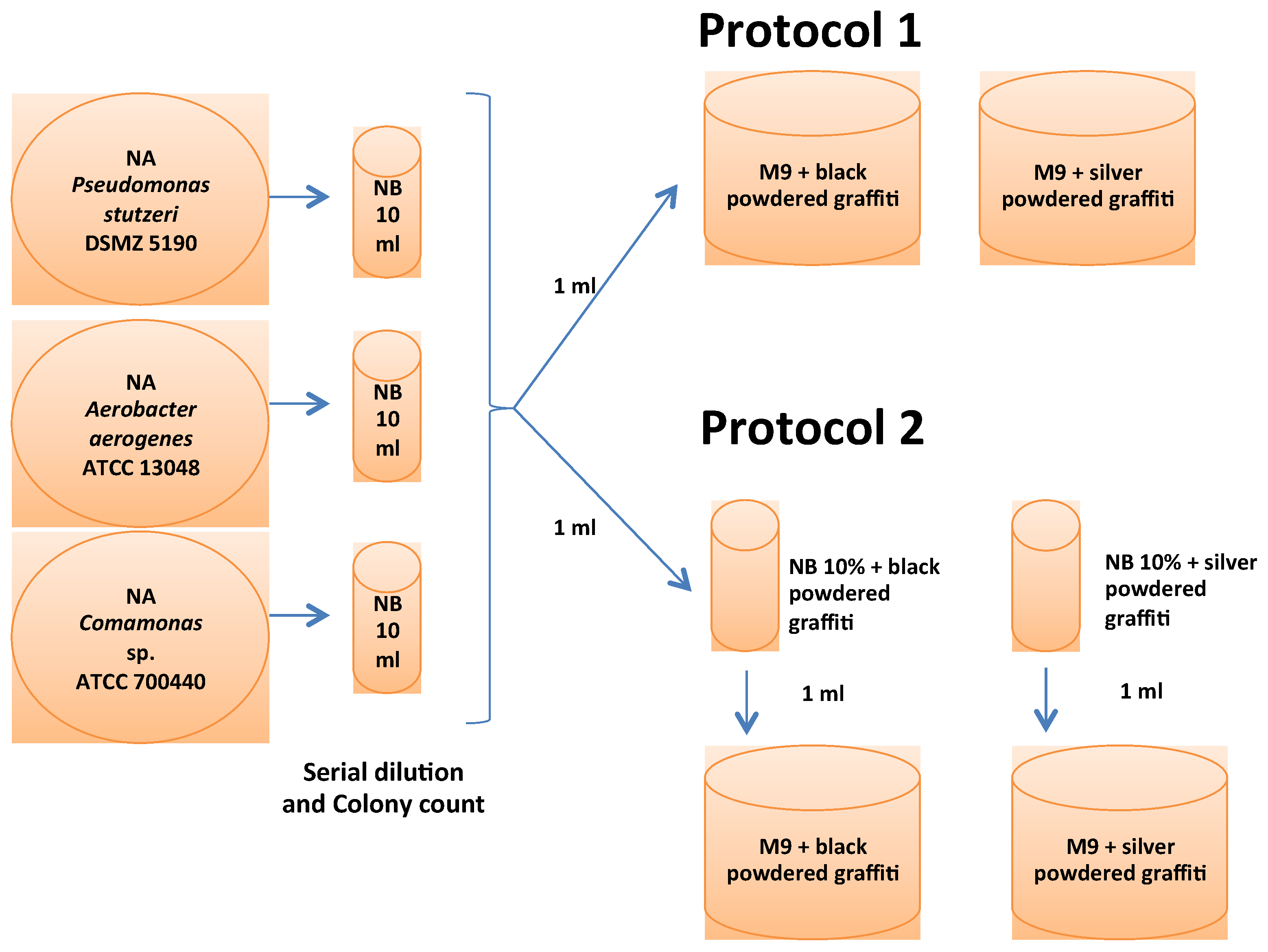
2.1. Bacterial Selection, Media and Culture Protocols for the Adaptation of the Microorganisms
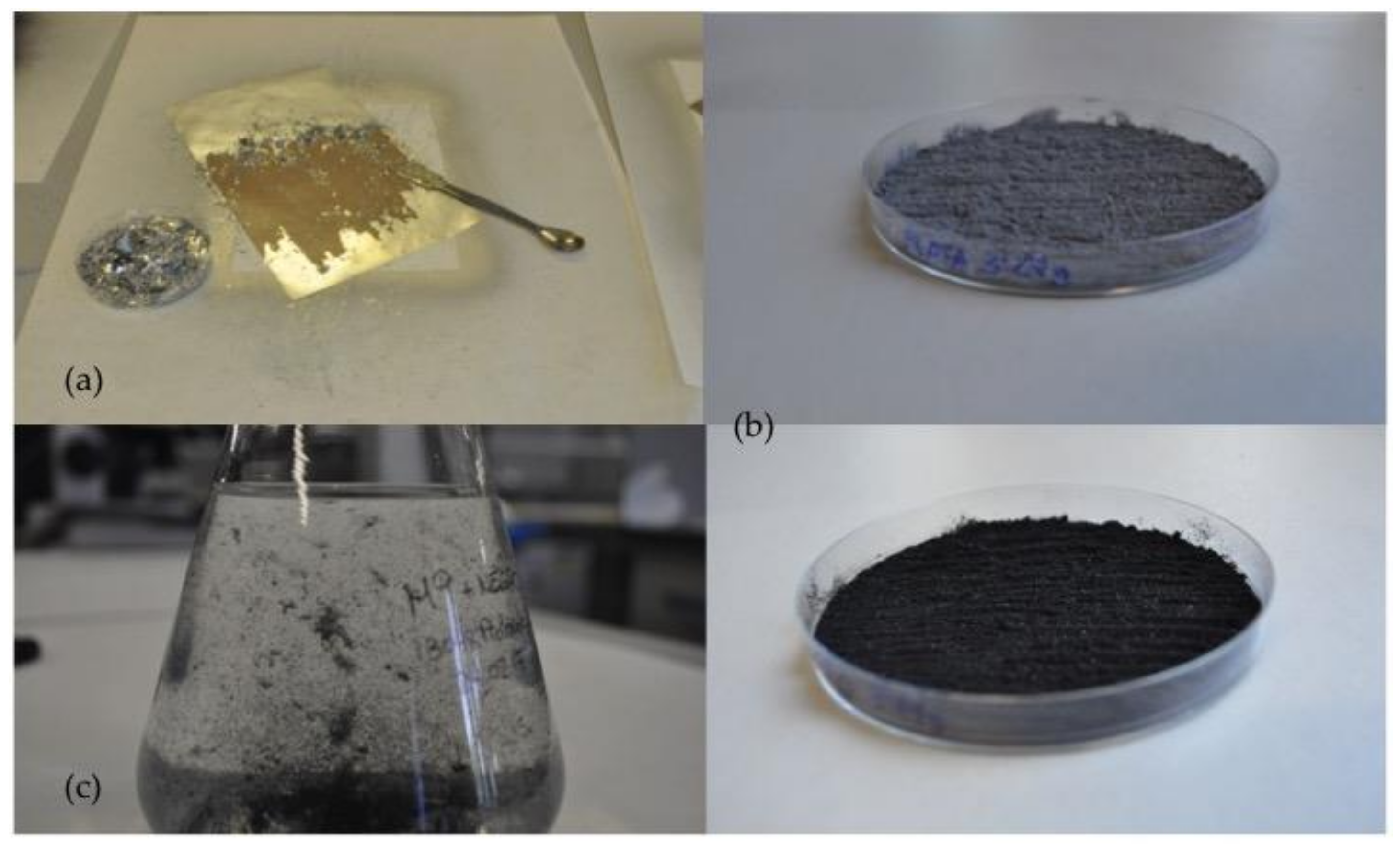
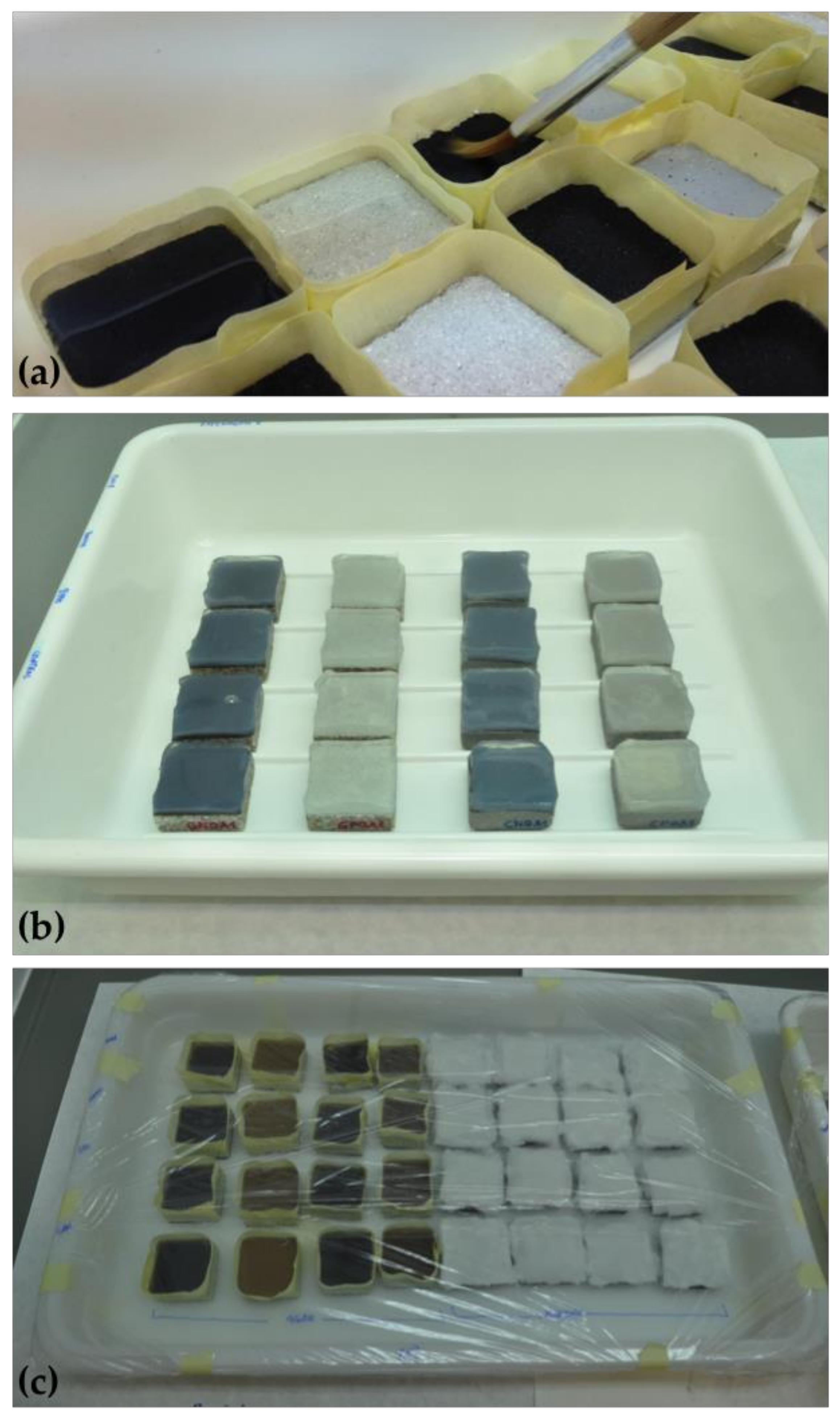
2.2. Preparation of Powdered Graffiti and Painted Natural and Man-Made Stone Specimens
2.3. Biocleaning Assays with the Adapted Microorganisms
2.4. Evaluation of Bacterial Degradation of Powdered Graffiti and Painted Specimens
3. Results
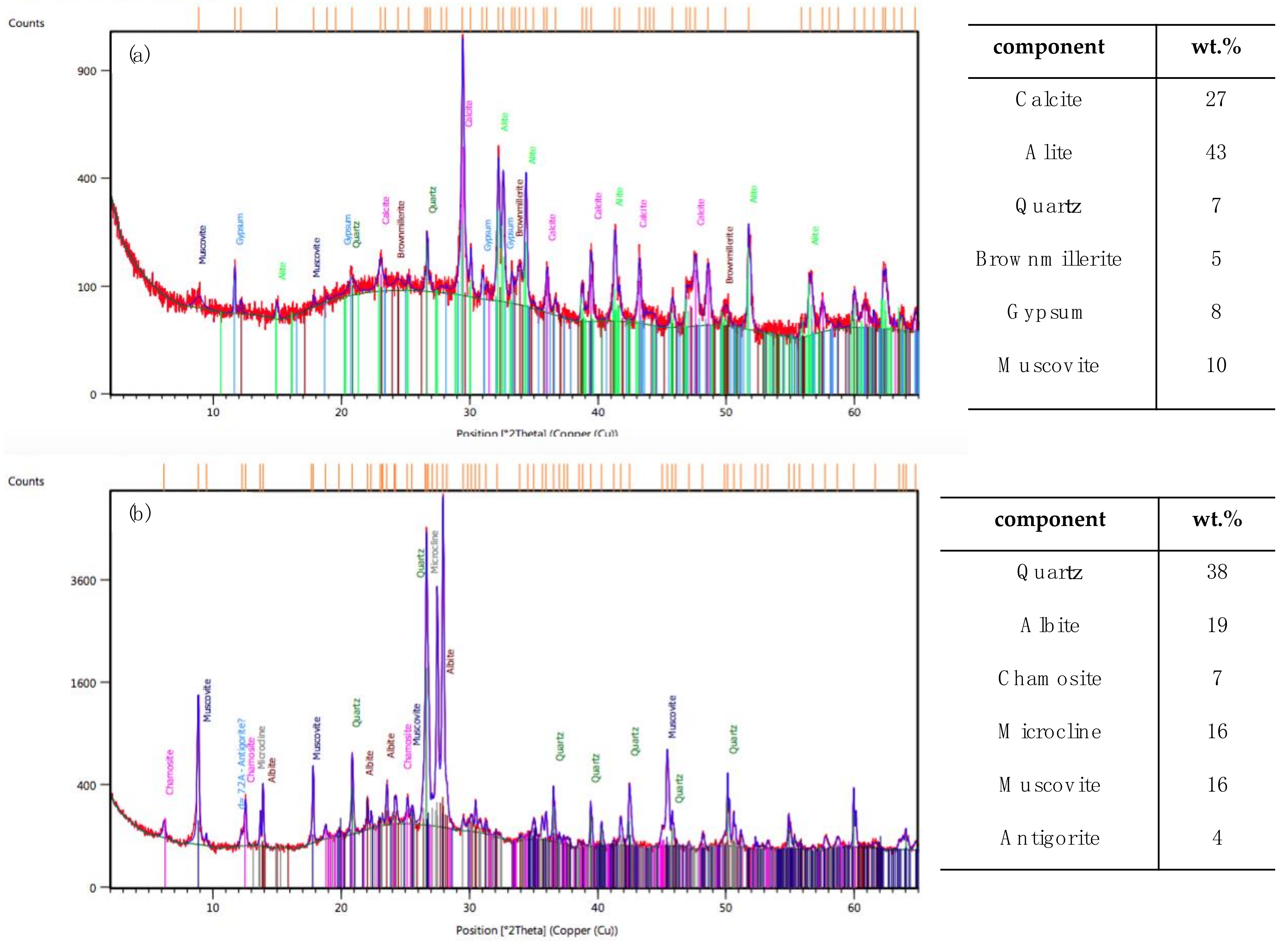
3.1. Powdered Graffiti
3.2. Graffiti Coated Stone and Concrete Specimens
4. Discussion and Final Remarks
- Pseudomonas stutzeri DSMZ 5190 was shown, for the first time, to be able to grow using powdered graffiti as an energy source, and it, therefore, represents a potential candidate for use in the bioremoval of graffiti.
- A liquid culture medium enriched with powdered graffiti, tested here for the first time, enhancing the selection of appropriate bacteria, thus facilitating and accelerating the ability of the microorganisms to degrade the graffiti;
- A protocol for applying the biocleaning treatment to remove graffiti from natural and man-made stone materials—based on the use of agar and water as carrier agents—is proposed.
- Finally, some improvements have been made to the existing methodology, e.g., shortening the time required for adaptation of the microorganisms and the application time, making the method easier to apply on a larger scale.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bosch-Roig, P.; Ranalli, G. Biocleaning of cultural heritage treasures. In Biodeterioration and Preservation in Art, Archaeology and Architecture; Mitchell, R., Clifford, J., Eds.; Archetype Publications: London, UK, 2018; pp. 169–183. [Google Scholar]
- Sanmartín, P.; Cappitelli, F. Evaluation of accelerated ageing tests for metallic and non-metallic graffiti paints applied to stone. Coatings 2017, 7, 180. [Google Scholar] [CrossRef]
- Final Report Summary—GRAFFITAGE (Development of A New Anti-Graffiti System, Based on Traditional Concepts, Preventing Damage of Architectural Heritage Materials); Community Research and Development Information Service, European Comission: Brussel, Belgium, 2011.
- Final Report Summary—EFFACEUR (InnovativE Anti-GraFFiti Product for Application in the Cultural Heritage of EURope); Community Research and Development Information Service, European Comission: Brussel, Belgium, 2013.
- Sanmartín, P.; Cappitelli, F.; Mitchell, R. Current methods of graffiti removal: A review. Constr. Build. Mater. 2014, 71, 363–374. [Google Scholar] [CrossRef]
- Sanmartín, P.; Mitchell, R.; Cappitelli, F. Evaluation of cleaning methods for graffiti removal. In Urban Pollution and Changes to Materials and Building Surfaces; Brimblecombe, P., Ed.; Imperial College Press: River Edge, NJ, USA, 2016; pp. 291–312. [Google Scholar]
- Germinario, G.; van der Werf, I.D.; Palazzo, G.; Regidor Ros, J.L.; Montes-Estelles, R.M.; Sabbatini, L. Bioremoval of marker pen inks by exploiting lipase hydrolysis. Prog. Org. Coat. 2017, 110, 162–171. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Morton, L.H.G.; Loh, K.; Shirakawa, M.A. Biodeterioration of external architectural paint films—A review. Int. Biodeterior. Biodegrad. 2011, 65, 1189–1198. [Google Scholar] [CrossRef]
- Giacomucci, L.; Toja, F.; Sanmartín, P.; Toniolo, L.; Prieto, B.; Villa, F.; Cappitelli, F. Degradation of nitrocellulose-based paint by D. desulfuricans ATCC 13541. Biodegradation 2012, 23, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, P.; DeAraujo, A.; Vasanthakumar, A.; Mitchell, R. Feasibility study involving the search for natural strains of microorganisms capable of degrading graffiti from heritage materials. Int. Biodeterior. Biodegr. 2015, 103, 186–190. [Google Scholar] [CrossRef]
- Sanmartín, P.; Cattò, C.; Bosch-Roig, P.; Troiano, F.; Cappitelli, F. Bioremediation of graffiti using novel commercial bacterial strains. Unpublished work. 2019. [Google Scholar]
- Bosch-Roig, P.; Decorosi, F.; Giovannetti, L.; Ranalli, G.; Viti, C. Connecting phenome to genome in Pseudomonas stutzeri 5190: An artwork biocleaning bacterium. Res. Microbiol. 2016, 167, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Ranalli, G.; Zanardini, E.; Andreotti, A.; Colombini, M.P.; Corti, C.; Bosch-Roig, P.; De Nuntiis, P.; Lustrato, G.; Mandrioli, P.; Rampazzi, L.; et al. Hi-tech restoration by two-steps bio-cleaning process of Triumph of Death fresco at the Camposanto Monumental Cemetery (Pisa, Italy). J. Appl. Microbiol. 2018, 125, 800–812. [Google Scholar] [CrossRef]
- Bosch-Roig, P.; Regidor-Rosi, J.L.; Soriano-Sancho, P.; Montes-Estellés, R.M. Biocleaning of animal glue on wall paintings by Pseudomonas stutzeri. Chim. Oggi–Chem. Today 2013, 31, 50–53. [Google Scholar]
- Ranalli, G.; Alfano, G.; Belli, C.; Lustrato, G.; Colombini, M.P.; Bonaduce, I.; Zanardini, E.; Abbruscato, P.; Cappitelli, F.; Sorlini, C. Biotechnology applied to cultural heritage: Biorestoration of frescoes using viable bacterial cells and enzymes. J. Appl. Microbiol. 2005, 98, 73–83. [Google Scholar] [CrossRef]
- Bosch-Roig, P.; Regidor-Rosi, J.L.; Montes-Estellés, R.M. Biocleaning of nitrate alterations on wall paintings by Pseudomonas stutzeri. Int. Biodeterior. Biodegr. 2013, 84, 266–274. [Google Scholar] [CrossRef]
- Rivas, T.; Pozo, S.; Fiorucci, M.P.; López, A.J.; Ramil, A. Nd:YVO4 laser removal of graffiti from granite. Influence of paint and rock properties on cleaning efficacy. Appl. Surf. Sci. 2012, 263, 563–572. [Google Scholar] [CrossRef]
- Fiorucci, M.P.; Lopez, A.J.; Ramil, A.; Pozo, S.; Rivas, T. Optimization of graffiti removal on natural stone by means of high repetition rate UV laser. Appl. Surf. Sci. 2013, 278, 268–272. [Google Scholar] [CrossRef]
- Sanmartín, P.; Silva, B.; Prieto, B. Effect of surface finish on roughness, color and gloss of ornamental granites. J. Mater. Civ. Eng. 2011, 23, 1239–1248. [Google Scholar] [CrossRef]
- EN 197-1 Composition, Specification and Conformity Criteria for Common Cements; European Committee for Stadardization: Bruxelles, Belgium, 2012.
- UNE-EN 1936:2007 Natural Stone Test Methods—Determination of Real Density and Apparent Density, and of Total and Open Porosity; AENOR: Madrid, Spain, 2007.
- Bosch-Roig, P.; Lustrato, G.; Zanardini, E.; Ranalli, G. Biocleaning of Cultural Heritage stone surfaces and frescoes: Which delivery system can be the most appropriate? Ann. Microbiol. 2015, 65, 1227–1241. [Google Scholar] [CrossRef]
- Bosch-Roig, P.; Regidor Ros, J.L.; Soriano Sancho, M.P.; Montes Estellés, R.; Roig Picazo, P. Biocleaning on wall paintings on uneven surfaces with warm agar gels. In Gels in the Conservation of Art; Archetype Publications: London, UK, 2017; pp. 119–121. [Google Scholar]
- Sanmartín, P.; Silva-Sánchez, N.; Martínez-Cortizas, A.; Prieto, B. Usual and unusual CIELAB color parameters for the study of peat organic matter properties: Tremoal do Pedrido bog (NW Spain). J. Physics. Conf. Ser. 2015, 605, 012014. [Google Scholar] [CrossRef]
- Colorimetry; Central Bureau of the CIE: Vienna, Austria, 1986.
- Wyszecki, G.; Stiles, W.S. Color Science. Concepts and Methods. Quantitative Data and Formulae; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Völz, H.G. Industrial Color Testing; Wiley VCH: Weinheim, Germany, 2001. [Google Scholar]
- Melgosa, M.; Hita, E.; Poza, A.J.; Alman, D.H.; Berns, R.S. Suprathreshold color-difference ellipsoids for surface colors. Color Res. Appl. 1997, 22, 148–155. [Google Scholar] [CrossRef]
- Hardeberg, A.Y. Acquisition and reproduction of color images: Colorimetric and multispectral approaches. Ph.D. Thesis, Ecole Nationale Superieure des Telecommunications, Paris, France, 1999. [Google Scholar]
- Sitole, L.; Steffens, F.; Krüger, T.P.J.; Meyer, D. Mid-ATR-FTIR spectroscopic profiling of HIV/AIDS sera for novel systems diagnostics in global health. OMICS J. Integr. Biol. 2014, 18, 513–523. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Houari, A.; Seyer, D.; Kecili, K.; Heim, V.; Di Martino, P. Kinetic development of biofilm on NF membranes at the Méry-sur-Oise plant, France. Biofouling 2013, 29, 109–118. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Oxford, UK, 2001. [Google Scholar]
- Cappitelli, F.; Toniolo, L.; Sansonetti, A.; Gulotta, D.; Ranalli, G.; Zanardini, E.; Sorlini, C. Advantages of using microbial technology over traditional chemical technology in the removal of black crusts from stone surfaces of historical monuments. Appl. Environ. Microbiol. 2007, 73, 5671–5675. [Google Scholar] [CrossRef] [PubMed]
- Atlas, M.R.; Horowitz, A.; Krichevky, M.; Bej, K.A. Response of microbial population to environmental disturbance. Microbiol. Ecol. 1991, 22, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.C.; Ferrera, R.; Volke, V.; Rodríguez, R.; Fernández, L. Adaptación y Selección de Microorganismos Autóctonos en Medios de Cultivos Enriquecidos con Petróleo Crudo. Terra Latinoamericana 2002, 20, 423–434. (In Spanish) [Google Scholar]

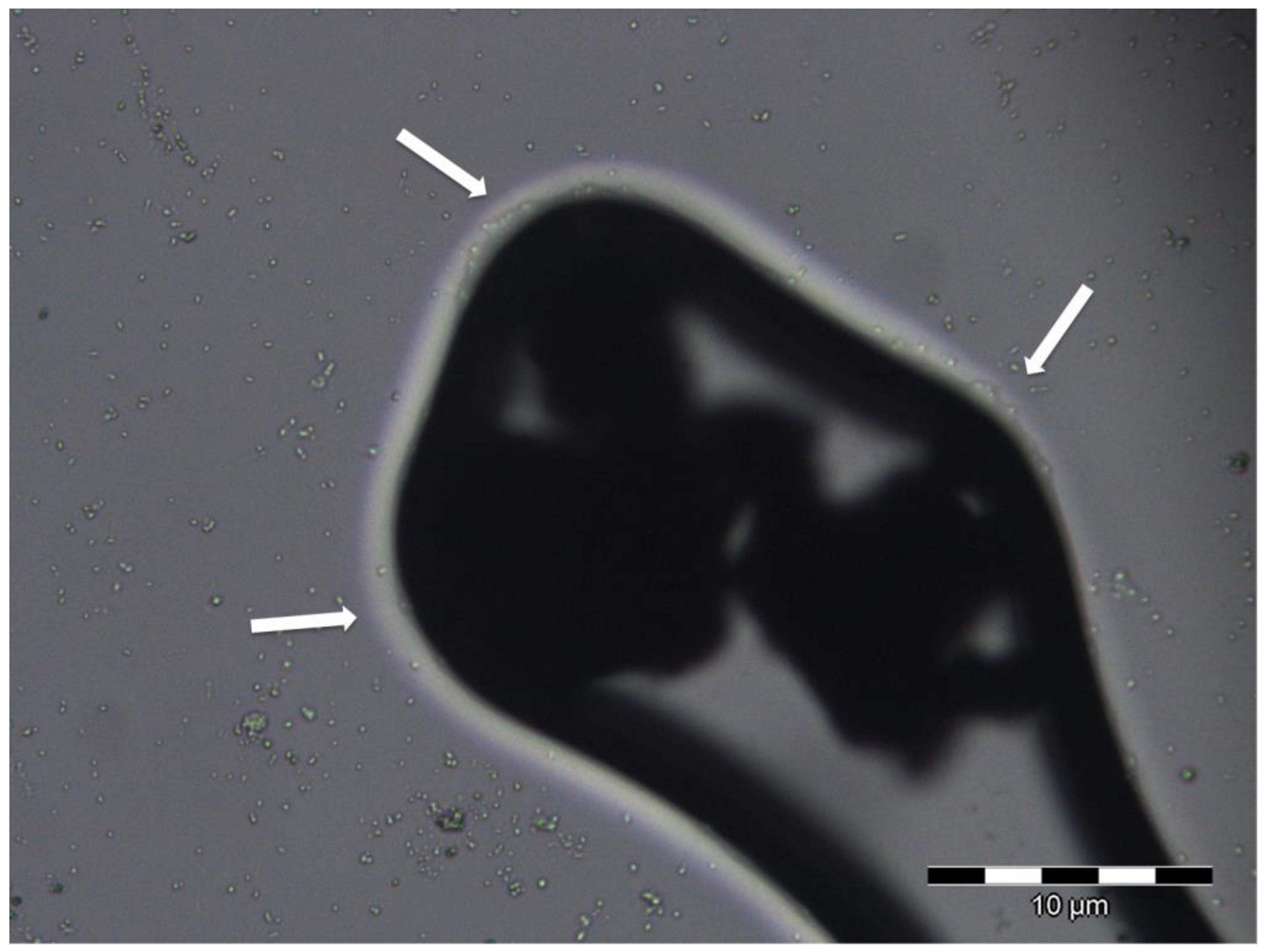
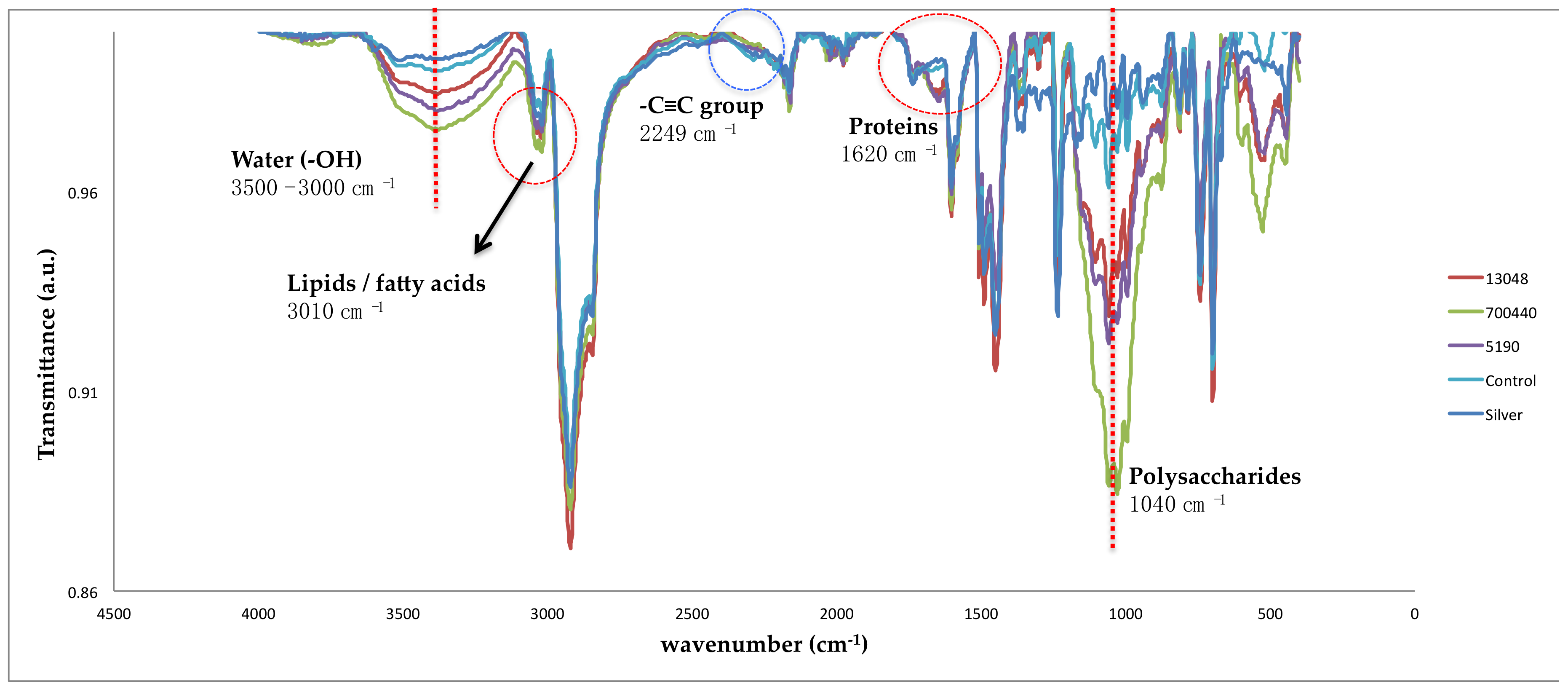
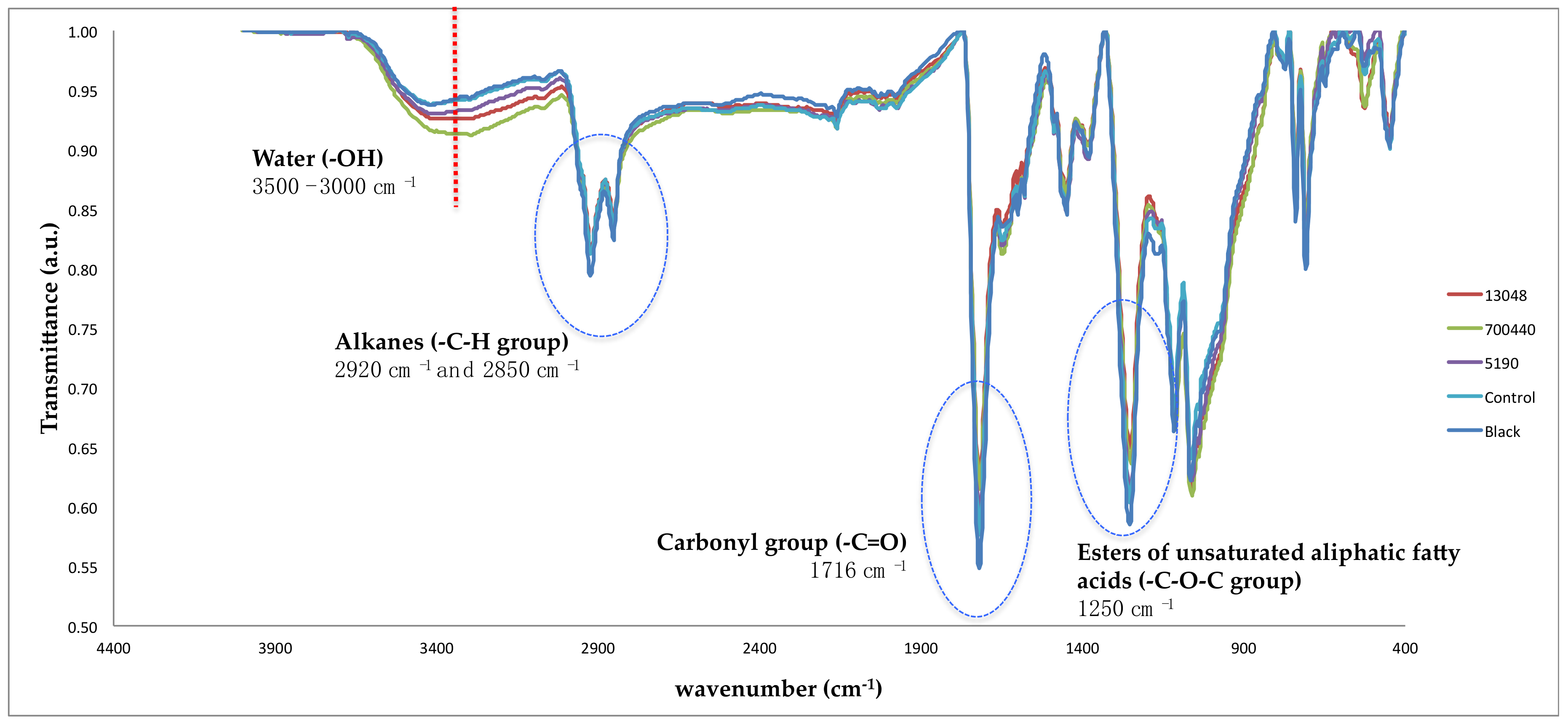
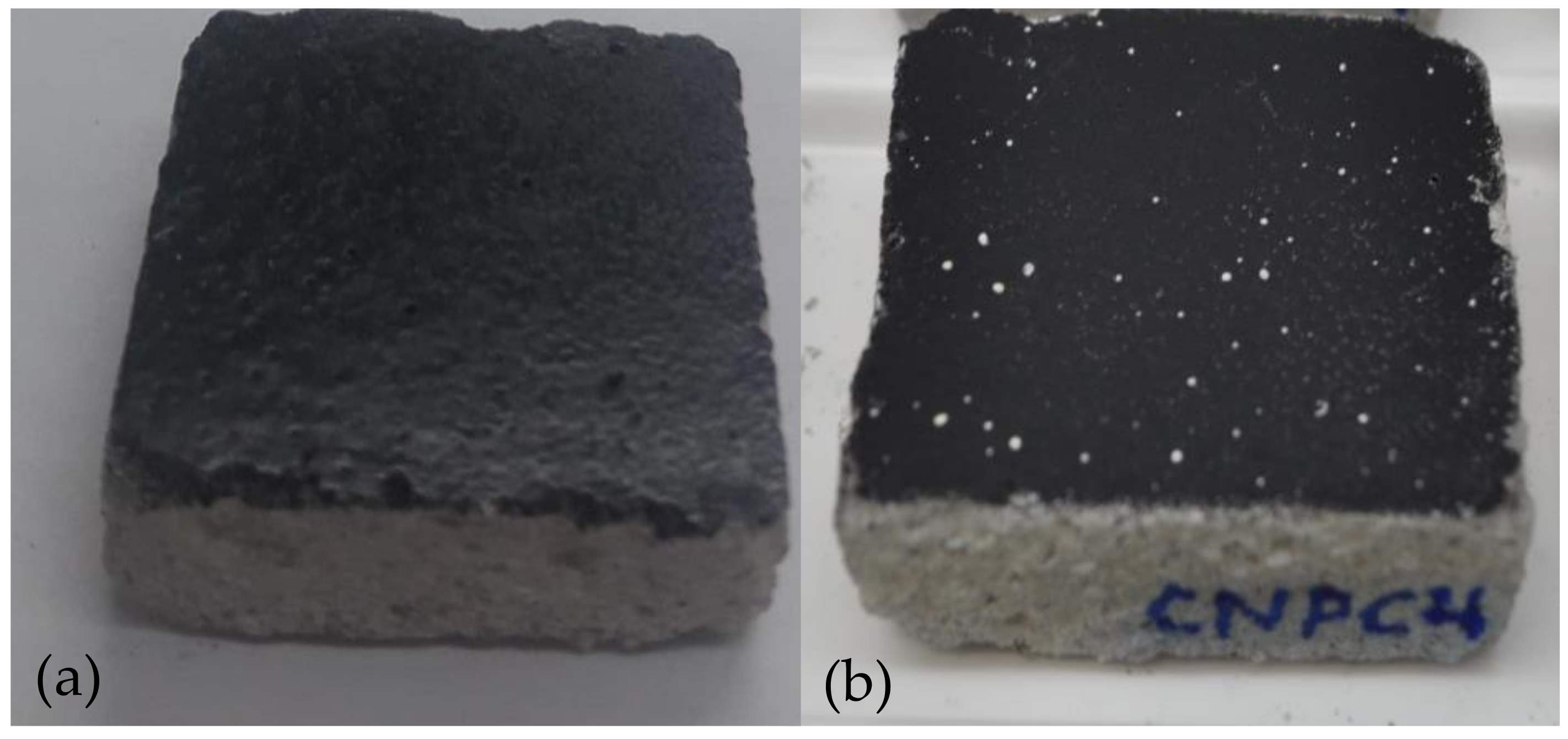
| Silver Graffiti Paint | Control 1-Paint 1 | 5190 1-Paint 1 | 13048 1-Paint 1 | 700440 1-Paint 1 |
| ΔL* | −2.0 | −4.5 | −4.2 | −3.7 |
| Δa* | 0.0 | 0.1 | 0.0 | 0.1 |
| Δb* | 0.8 | 1.3 | 0.5 | 1.1 |
| ΔE*ab | 2.1 | 4.6 | 4.2 | 3.8 |
| Black Graffiti Paint | Control 1-Paint 1 | 5190 1-Paint 1 | 13048 1-Paint 1 | 700440 1-Paint 1 |
| ΔL* | −1.9 | 5.7 | 8.4 | 1.4 |
| Δa* | 0.0 | −0.1 | −0.3 | −0.2 |
| Δb* | −0.5 | −0.5 | −1.6 | −1.0 |
| ΔE*ab | 2.0 | 5.7 | 8.6 | 1.7 |
| Silver Graffiti Paint | Control 1-Paint 1 | 5190 1-Paint 1 | 13048 1-Paint 1 | 700440 1-Paint 1 |
| ΔL* | −15.45 | −7.5 | −14.3 | −5.7 |
| Δa* | −0.1 | −0.0 | 0.1 | −0.1 |
| Δb* | 1.5 | 1.6 | 4.1 | 1.8 |
| ΔE*ab | 15.5 | 7.6 | 15.1 | 6.0 |
| Black Graffiti Paint | Control 1-Paint 1 | 5190 1-Paint 1 | 13048 1-Paint 1 | 700440 1-Paint 1 |
| ΔL* | 0.6 | 1.0 | −0.3 | 0.9 |
| Δa* | −0.1 | −0.0 | −0.1 | −0.1 |
| Δb* | −0.2 | 0.2 | 0.0 | −0.2 |
| ΔE*ab | 0.7 | 1.0 | 0.3 | 1.0 |
| Silver Graffiti Paint | Control 1-Paint 1 | 5190 1-Paint 1 | 13048 1-Paint 1 | 700440 1-Paint 1 |
| ΔL* | −5.3 | −0.6 | 0.8 | 2.8 |
| Δa* | 0.0 | 0.2 | −0.0 | −0.0 |
| Δb* | 1.3 | 4.4 | 4.1 | 3.8 |
| ΔE*ab | 5.5 | 4.4 | 4.2 | 4.7 |
| Black Graffiti Paint | Control 1-Paint 1 | 5190 1-Paint 1 | 13048 1-Paint 1 | 700440 1-Paint 1 |
| ΔL* | 0.9 | −0.7 | −0.5 | −0.3 |
| Δa* | −0.1 | −0.0 | −0.0 | 0.0 |
| Δb* | −0.0 | 0.0 | 0.0 | 0.0 |
| ΔE*ab | 0.9 | 0.7 | 0.5 | 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanmartín, P.; Bosch-Roig, P. Biocleaning to Remove Graffiti: A Real Possibility? Advances towards a Complete Protocol of Action. Coatings 2019, 9, 104. https://doi.org/10.3390/coatings9020104
Sanmartín P, Bosch-Roig P. Biocleaning to Remove Graffiti: A Real Possibility? Advances towards a Complete Protocol of Action. Coatings. 2019; 9(2):104. https://doi.org/10.3390/coatings9020104
Chicago/Turabian StyleSanmartín, Patricia, and Pilar Bosch-Roig. 2019. "Biocleaning to Remove Graffiti: A Real Possibility? Advances towards a Complete Protocol of Action" Coatings 9, no. 2: 104. https://doi.org/10.3390/coatings9020104
APA StyleSanmartín, P., & Bosch-Roig, P. (2019). Biocleaning to Remove Graffiti: A Real Possibility? Advances towards a Complete Protocol of Action. Coatings, 9(2), 104. https://doi.org/10.3390/coatings9020104






