Influence of Sealing Treatment on the Corrosion Resistance of PEO Coated Al-Zn-Mg-Cu Alloy in Various Environments
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Preparation of the PEO Coating
2.3. Sealing Treatment
2.4. Characterization Analysis of the Coatings
2.5. Electrochemical Measurements
3. Results and Discussion
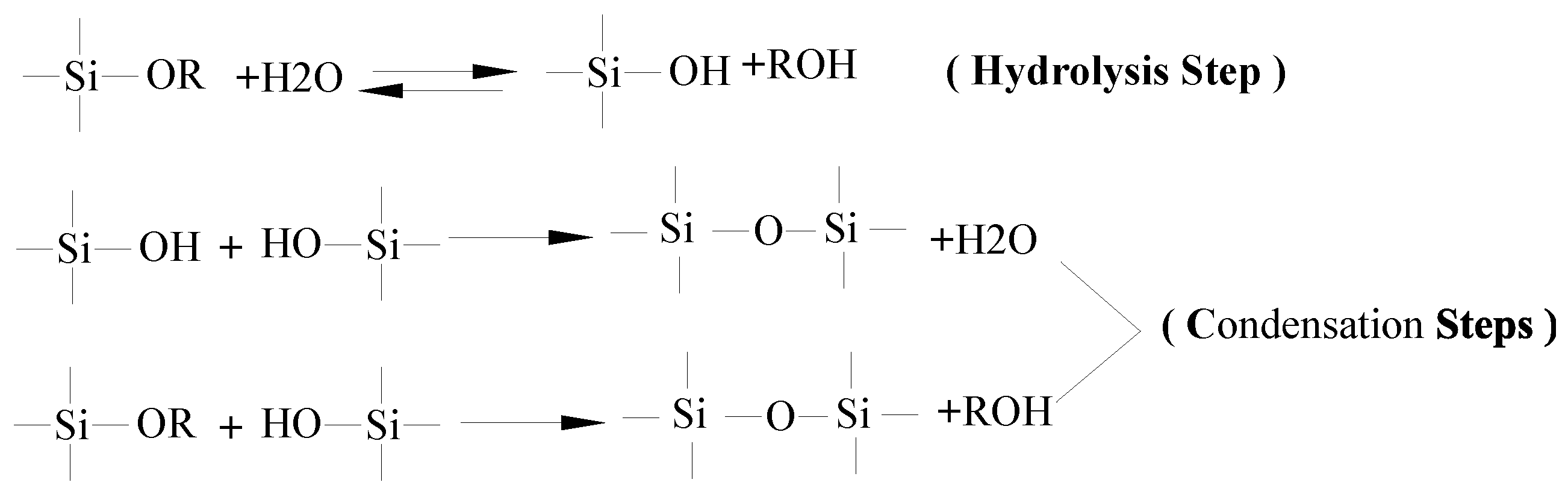
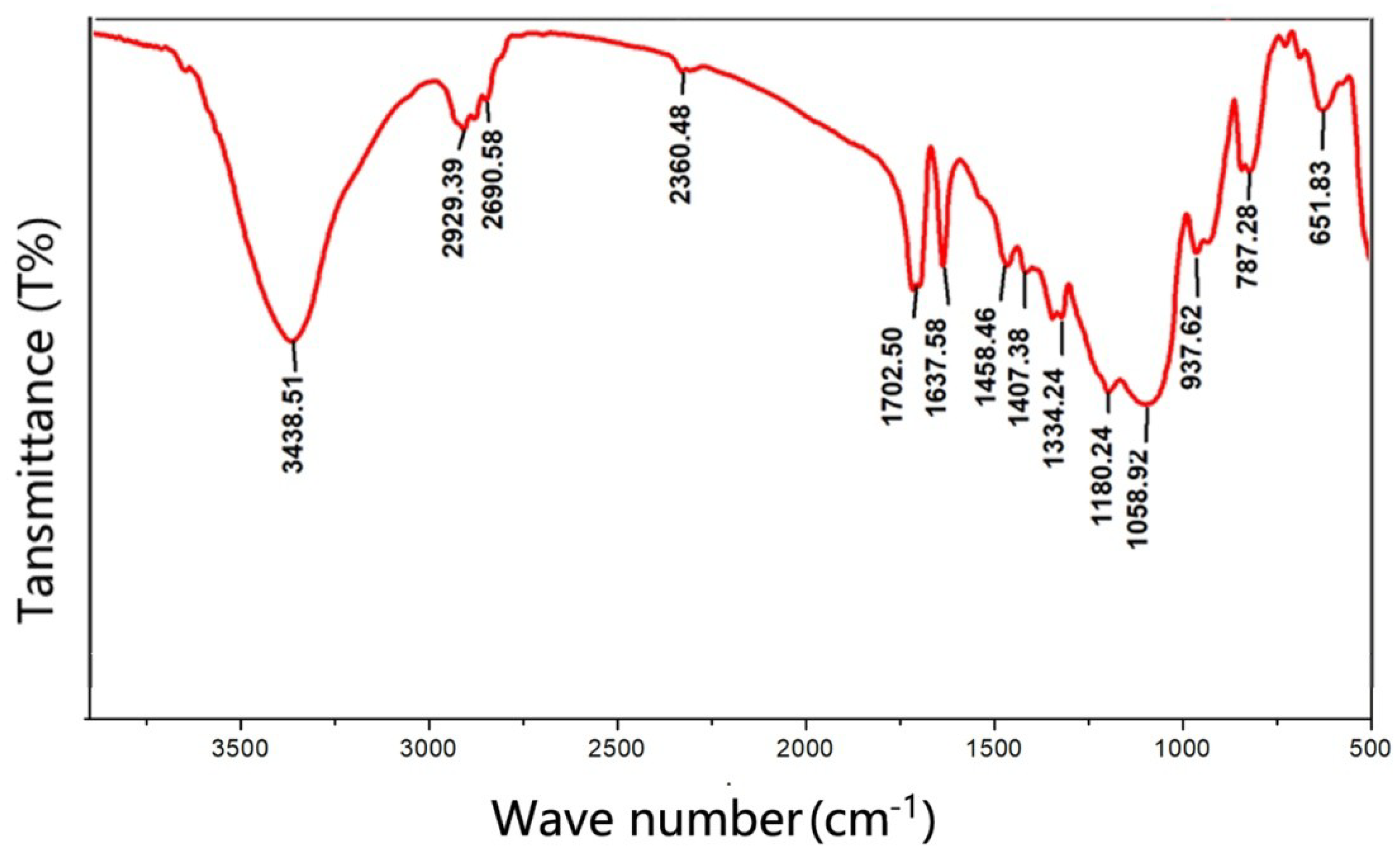
3.1. Formation Mechanism of Siloxane Layer
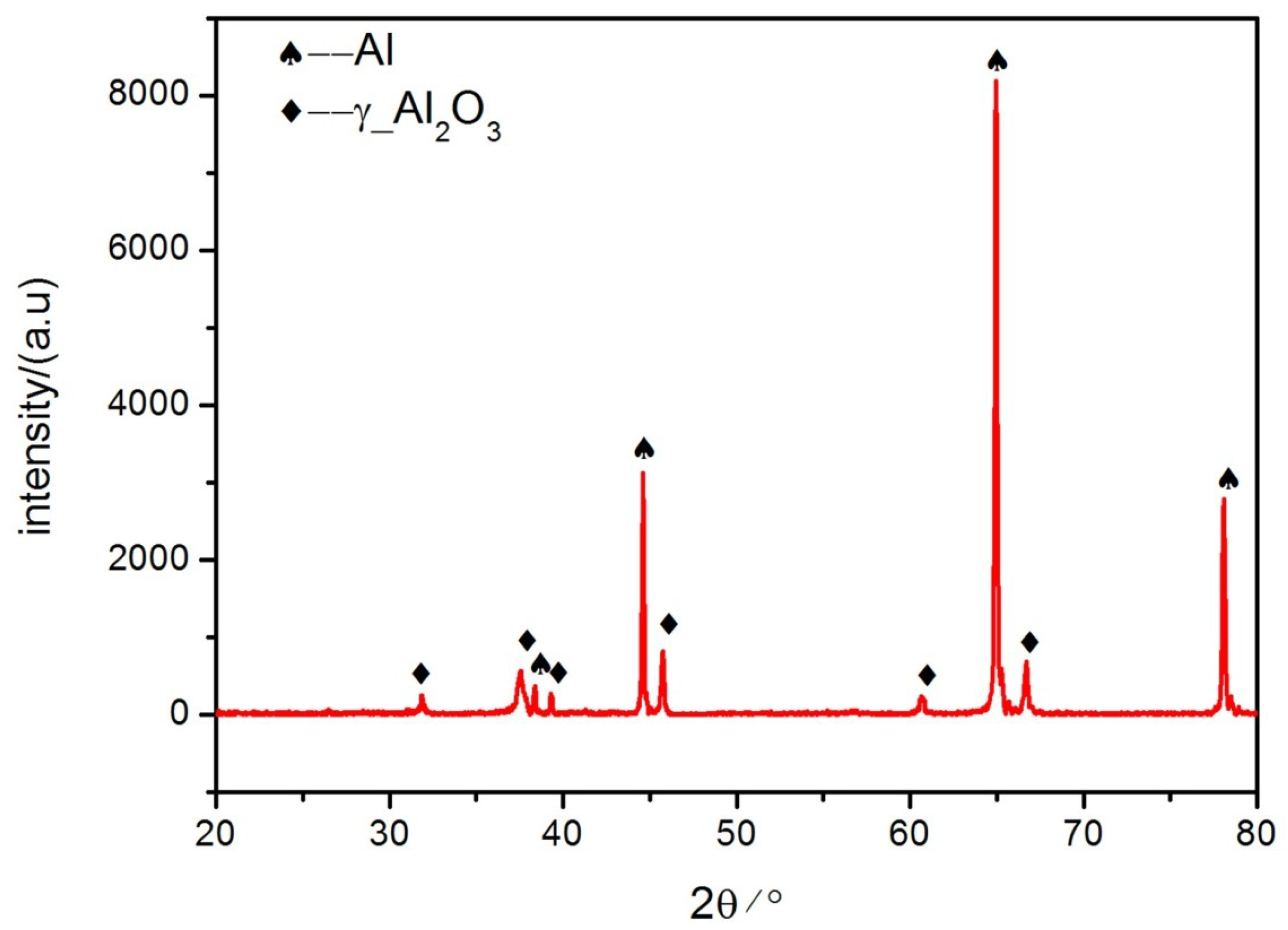
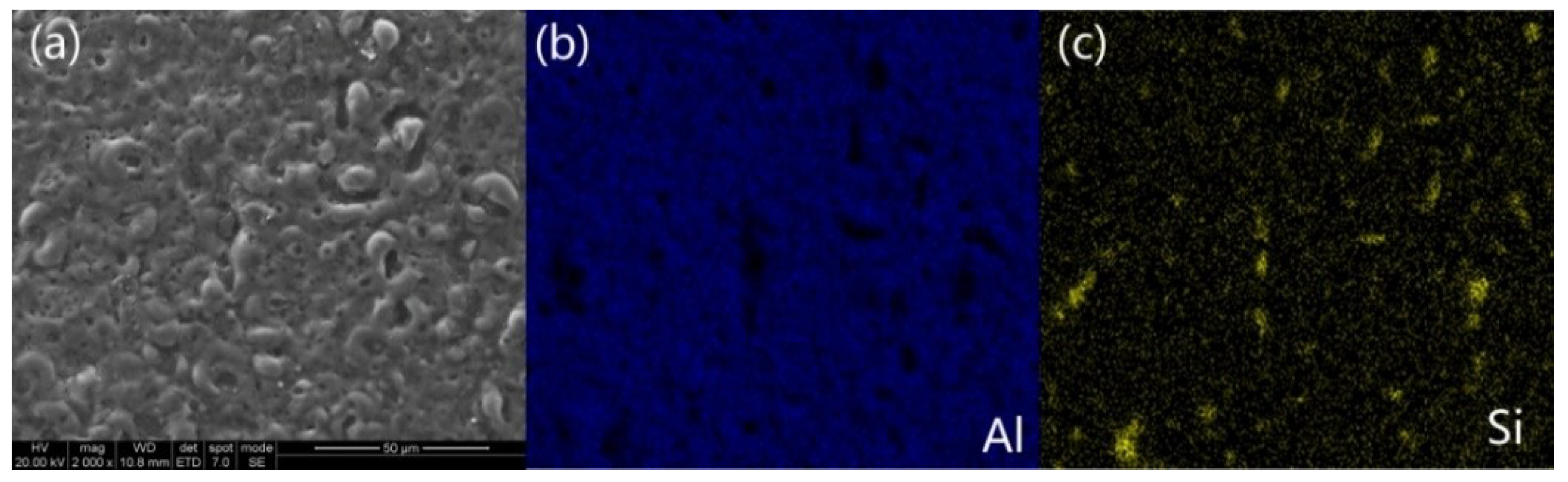
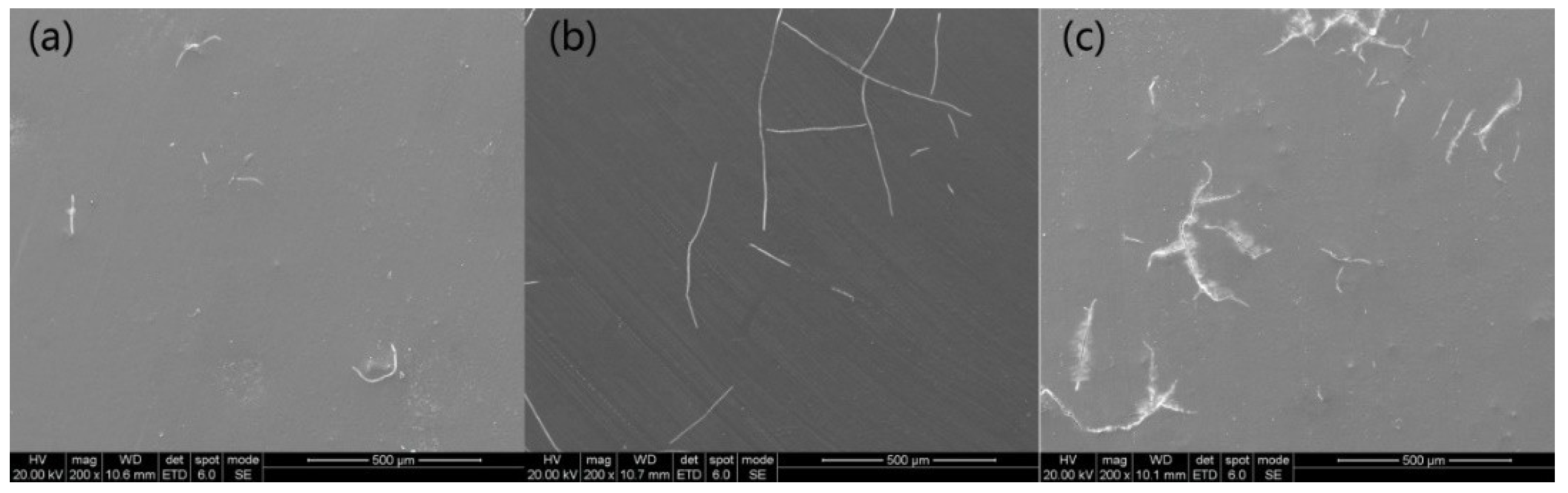
3.2. Morphologies of the Coatings
3.3. Electrochemical Corrosion Behaviour
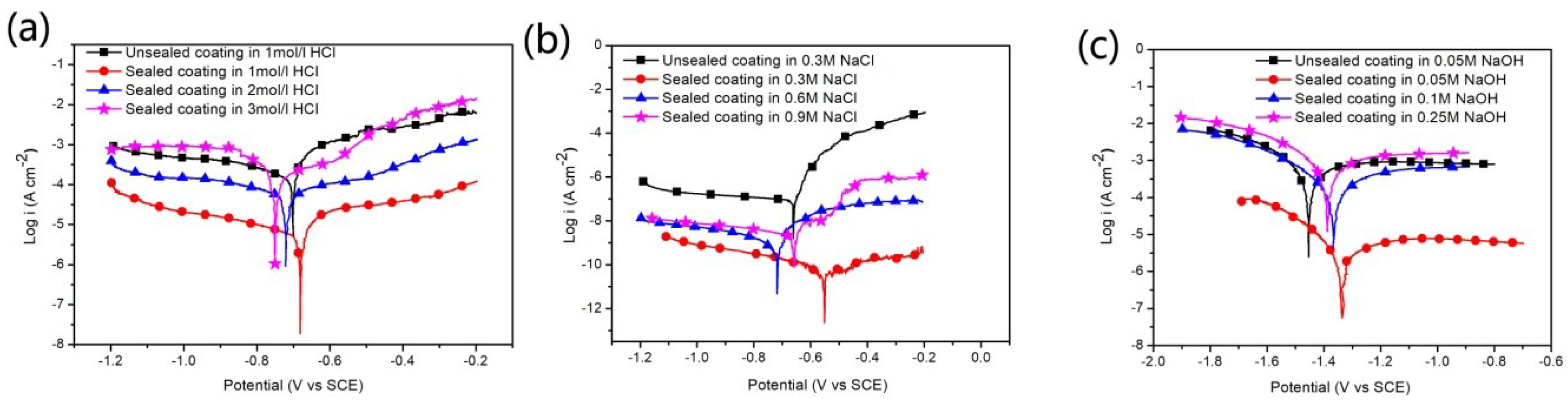
3.3.1. Potentiodynamic Polarization Analysis
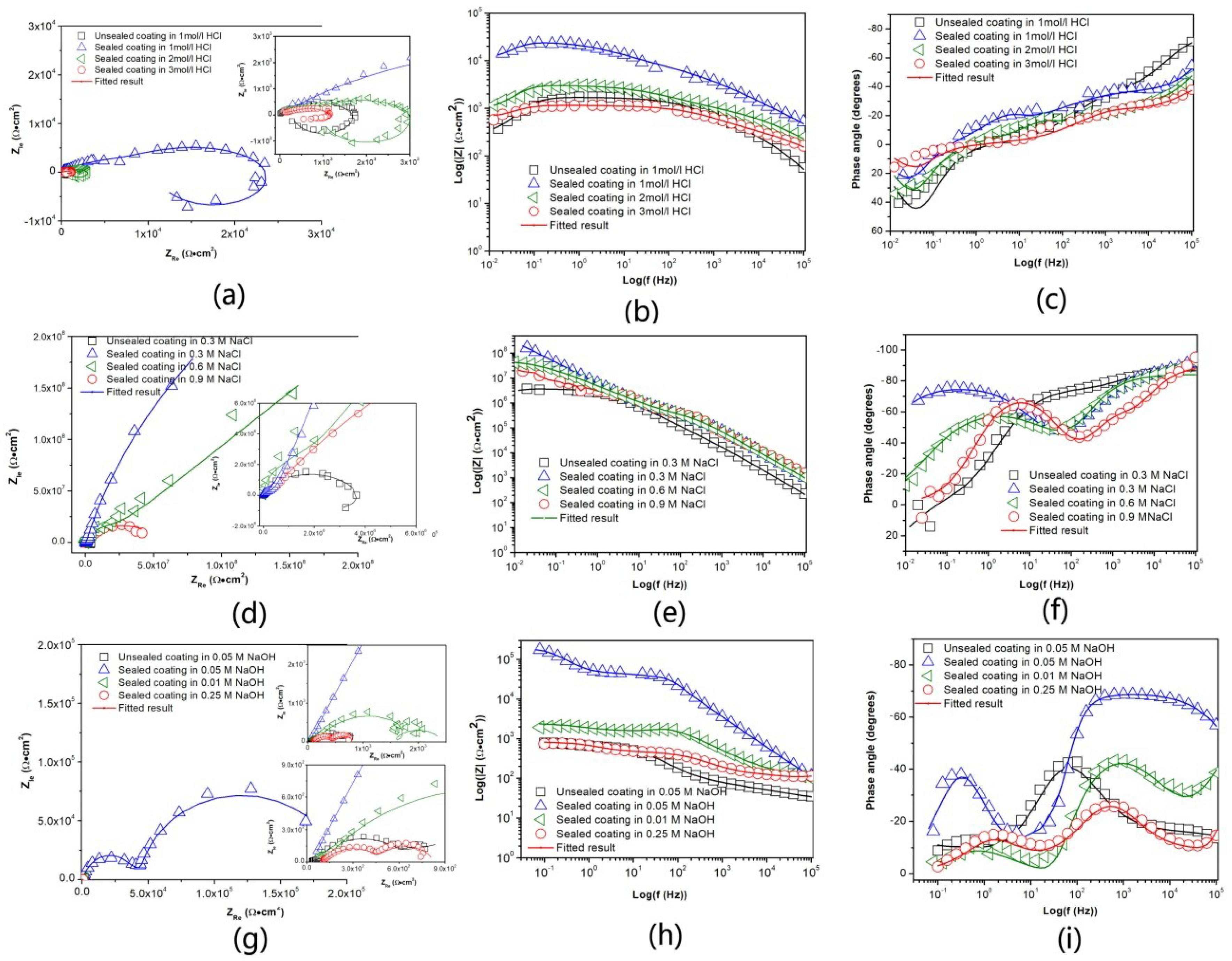
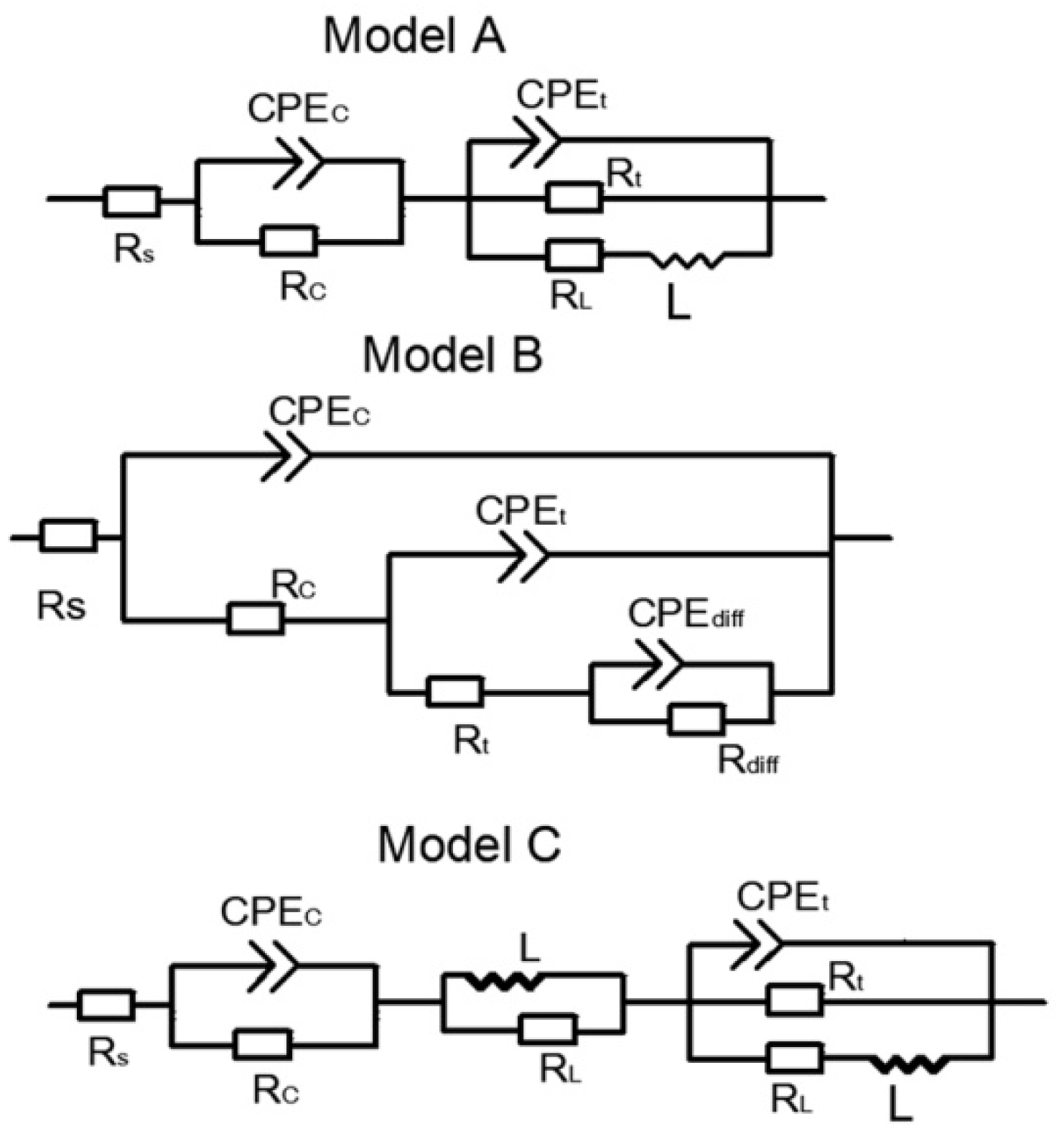
3.3.2. Electrochemical Impedance Spectroscopy (EIS) Analysis
4. Conclusions
- (1)
- The PEO coating formed on an Al–Zn–Mg–Cu alloy had many micro defects, such as pores and cracks. The coating was easily permeated by electrolyte and reduced corrosion resistance.
- (2)
- A silane polymer sealant was synthesized from MPTES and TEOS, effectively filling these micro defects and forming a continuous siloxane layer on the PEO coating by sol-gel technology.
- (3)
- Compared to the unsealed PEO coating, the corrosion resistance of the PEO coating sealed with siloxane was significantly improved in acidic, neutral, and alkaline environments due to the sealing effect of the siloxane layer. The corrosion resistance of the sealed coatings was found to decrease with increasing electrolyte concentration.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Priputen, P.; Palcut, M.; Babinec, M.; Mišík, J.; Černičková, I.; Janovec, J. Correlation between Microstructure and Corrosion Behavior of Near-Equilibrium Al-Co Alloys in Various Environments. J. Mater. Eng. Perform. 2017, 26, 3970–3976. [Google Scholar] [CrossRef]
- Palcut, M.; Priputen, P.; Kusý, M.; Janovec, J. Corrosion behaviour of Al–29at%Co alloy in aqueous NaCl. Corros. Sci. 2013, 75, 461–466. [Google Scholar] [CrossRef]
- Palcut, M.; Ďuriška, L.; Špoták, M.; Vrbovský, M.; Gerhátová, Ž.; Černičková, I.; Janovec, J. Electrochemical corrosion of Al-Pd alloys in HCl and NaOH solution. J. Min. Metall. Sect. B 2017, 53, 333–340. [Google Scholar] [CrossRef]
- Ma, J.; Wen, J.; Li, Q.; Zhang, Q. Electrochemical polarization and corrosion behavior of Al–Zn–In based alloy in acidity and alkalinity solutions. Int. J. Hydrog. Energy 2013, 38, 14896–14902. [Google Scholar] [CrossRef]
- Javidi, M.; Fadaee, H. Plasma electrolytic oxidation of 2024-T3 aluminum alloy and investigation on microstructure and wear behavior. Appl. Surf. Sci. 2013, 286, 212–219. [Google Scholar] [CrossRef]
- Arrabal, R.; Mohedano, M.; Matykina, E.; Pardo, A.; Mingo, B.; Merino, M.C. Characterization and wear behaviour of PEO coatings on 6082-T6 aluminium alloy with incorporated α-Al2O3 particles. Surf. Coat. Technol. 2015, 269, 64–73. [Google Scholar] [CrossRef]
- Dehnavi, V.; Shoesmith, D.W.; Luan, B.L.; Yari, M.; Liu, X.Y.; Rohani, S. Corrosion properties of plasma electrolytic oxidation coatings on an aluminium alloy-The effect of the PEO process stage. Mater. Chem. Phys. 2015, 161, 49–58. [Google Scholar] [CrossRef]
- Fadaee, H.; Javidi, M. Investigation on the corrosion behaviour and microstructure of 2024-T3 Al alloy treated via plasma electrolytic oxidation. J. Alloy. Compd. 2014, 604, 36–42. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Mohamed, A.; Skeldon, P.; Thompson, G.E. Plasma electrolytic oxidation of pre-anodized aluminium. Corros. Sci. 2009, 51, 2897–2905. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O.; Nie, X. Processing microstructure relationships in the plasma electrolytic oxidation (PEO) coating of a magnesium alloy. Mater. Sci. Appl. 2014, 5, 124–139. [Google Scholar] [CrossRef]
- Martin, J.; Leone, P.; Nomine, A.; Veys-Renaux, D.; Henrion, G.; Belmonte, T. Influence of electrolyte ageing on the Plasma Electrolytic Oxidation of aluminium. Surf. Coat. Technol. 2015, 269, 36–46. [Google Scholar] [CrossRef]
- Hu, N.; Dong, X.; He, X.; Browning, J.F.; Schaefer, D.W. Effect of sealing on the morphology of anodized aluminum oxide. Corros. Sci. 2015, 97, 17–24. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Jang, H.; Chung, W. Cr2O3 sealing of anodized aluminum alloy by heat treatment. Surf. Coat. Technol. 2014, 243, 34–38. [Google Scholar] [CrossRef]
- Shahzad, M.; Chaussumier, M.; Chieragatti, R.; Mabru, C.; Rezai-Aria, F. Effect of sealed anodic film on fatigue performance of 2214-T6 aluminum alloy. Surf. Coat. Technol. 2012, 206, 2733–2739. [Google Scholar] [CrossRef]
- Bouali, A.C.; Straumal, E.A.; Serdechnova, M.; Wieland, D.C.F.; Starykevich, M.; Blawert, C.; Hammel, J.U.; Lermontov, S.A.; Ferreira, M.G.S.; Zheludkevich, M.L. Layered double hydroxide based active corrosion protective sealing of plasma electrolytic oxidation/sol-gel composite coating on AA2024. Appl. Surf. Sci. 2019, 494, 829–840. [Google Scholar] [CrossRef]
- Van Phuong, N.; Fazal, B.R.; Moon, S. Cerium- and phosphate-based sealing treatments of PEO coated AZ31 Mg alloy. Surf. Coat. Technol. 2017, 309, 86–95. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Zavidnaya, A.G.; Egorkin, V.S.; Mashtalyar, D.V.; Sergienko, V.I.; Yerokhin, A.L.; Matthews, A. Composite hydroxyapatite–PTFE coatings on Mg–Mn–Ce alloy for resorbable implant applications via a plasma electrolytic oxidation-based route. J. Taiwan. Inst. Chem. E 2014, 45, 3104–3109. [Google Scholar] [CrossRef]
- Whelan, M.; Cassidy, J.; Duffy, B. Sol-gel sealing characteristics for corrosion resistance of anodised aluminium. Surf. Coat. Technol. 2013, 235, 86–96. [Google Scholar] [CrossRef]
- Wojciechowski, J.; Szubert, K.; Peipmann, R.; Fritz, M.; Schmidt, U.; Bund, A.; Lota, G. Anti-corrosive properties of siloxane coatings deposited on anodised aluminium. Electrochim. Acta 2016, 220, 1–10. [Google Scholar] [CrossRef]
- Alyamac, E.; Gu, H.; Soucek, M.D.; Qiub, S.; Buchheit, R.G. Alkoxysi-lane oligomer modified epoxide primers. Prog. Org. Coat. 2012, 74, 67–81. [Google Scholar] [CrossRef]
- Matˇejka, L.; Strachota, A.; Plestil, J.; Whelan, P.; Syeinhart, M.; Slouf, M. Epoxy networks reinforced with polyhedral oligomeric silsesquiox-anes (POSS) Structure and morphology. Macromolecules 2004, 37, 9449–9450. [Google Scholar] [CrossRef]
- Hu, J.M.; Liu, L.; Zhang, J.Q.; Cao, C.N. Electro deposition of siloxane films on aluminum alloys for corrosion protection. Prog.Org. Coat. 2007, 58, 265–271. [Google Scholar] [CrossRef]
- Chruściel, J.J.; Leśniak, E. Modification of epoxy resins with functional siloxanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 41, 67–121. [Google Scholar] [CrossRef]
- Jothi, K.J.; Palanivelu, K. Synergistic effect of siloxane modified nano-composites for active corrosion protection. Ceram. Int. 2013, 39, 7619–7625. [Google Scholar] [CrossRef]
- Pena-Alonso, R.; Rubio, F.; Rubio, J.; Oteo, J.L. Study of the hydrolysis and condensation of γ-aminopropyltriethoxysiloxane by FT-IR spectroscopy. J. Mater. Sci. 2007, 42, 595–603. [Google Scholar] [CrossRef]
- Liu, Q.; Kang, Z.X. One-step electrodeposition process to fabricate superhydrophobic surface with improved anticorrosion property on magnesium alloy. Mater. Lett. 2014, 137, 210–213. [Google Scholar] [CrossRef]
- Han, M.; Go, S.; Ahn, Y. Fabrication of super-hydrophobic surface on magnesium substrate by chemical etching. Bull. Korean Chem. Soc. 2012, 33, 1363–1366. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Jannesari, A.; Ranjbar, Z.; Sobhani, S.; Saeb, M.R. Anti-corrosion hybrid coatings based on epoxy–silicanano-composites: Toward relationship between the morphologyand EIS data. Prog. Org. Coat. 2014, 77, 1169–1183. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, Z.; Wang, X. Kinetic study of methyltriethoxysiloxane (MTES) hydrolysis by FTIR spectroscopy under different temperatures and solvents. Vib. Spectrosc. 2008, 46, 1–7. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Wu, L.K.; Hu, J.M.; Zhang, J.Q. Silane-incorporated epoxy coatings on aluminum alloy (AA2024). Part 1: Improved corrosion performance. Corros. Sci. 2015, 92, 118–126. [Google Scholar]
- Bierwagen, G.; Tallman, D.; Li, J.P.; He, L.Y.; Jeffcoate, C. EIS studies of coated metals in accelerated exposure. Prog. Org. Coat. 2003, 46, 148–157. [Google Scholar] [CrossRef]
- Touzain, S.; le Thu, Q.; Bonnet, G. Evaluation of thick organic coatings degradation in seawater using cathodic protection and thermally accelerated tests. Prog. Org. Coat. 2005, 52, 311–319. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.; Zhou, Y.; Ouyang, J.H.; Guo, L.; Jia, D. Corrosion evaluation of microarc oxidation coatings formed on 2024 aluminum alloy. Corros. Sci. 2010, 52, 2687–2696. [Google Scholar] [CrossRef]
- Shen, D.; He, D.; Liu, F.; Guo, C.; Cai, J.; Li, G.; Ma, H. Effects of ultrasound on the evolution of plasma electrolytic oxidation process on 6061Al alloy. Ultrasonics 2014, 54, 1065–1070. [Google Scholar] [CrossRef]
- Yuan, X.; Yue, Z.F.; Chen, X.; Wen, S.F.; Li, L.; Feng, T. The protective and adhesion properties of silicone-epoxy hybrid coatings on 2024 Al-alloy with a silane film as pretreatment. Corros. Sci. 2016, 104, 84–97. [Google Scholar] [CrossRef]
- Beaufort, L.; Benvenuti, F.; Noels, A.F. Iron (II)–ethylene polymerization catalysts bearing 2,6-bis(imino)pyrazine ligands: Part I. Synthesis and characterization. J. Mol. Catal. A Chem. 2006, 260, 210–214. [Google Scholar] [CrossRef]
- El Rehim, S.S.A.; Hassan, H.H.; Amin, M.A. Corrosion inhibition of aluminum by 1,1 (lauryl amido) propyl ammonium chloride in HCl solution. Mater. Chem. Phys. 2001, 70, 64–72. [Google Scholar] [CrossRef]


| Zn | Mg | Cu | Cr | Fe | Si | Al |
|---|---|---|---|---|---|---|
| 6.28 | 2.19 | 1.6 | 0.15 | 0.4–0.6 | 0.4–0.6 | Bal. |
| Sample | Corrosion Solution | Ecorr (Vvs SCE) | icorr (A/cm²) | βα (mV/decade) | βc (mV/decade) | Rp (Ω) | νcorr (mm/year) |
|---|---|---|---|---|---|---|---|
| In HCl | |||||||
| Unsealed coating | 1 M | −0.70 | 1.36 × 10−4 | 0.07 | 0.42 | 130 | 2.32 |
| Sealed coating | 1 M | −0.68 | 6.30 × 10−6 | 0.11 | 0.42 | 5996 | 0.073 |
| Sealed coating | 2 M | −0.72 | 6.27 × 10−5 | 0.43 | 0.52 | 1636 | 0.72 |
| Sealed coating | 3 M | −0.75 | 2.60 × 10−4 | 0.83 | 0.23 | 304 | 3.02 |
| In NaCl | |||||||
| Unsealed coating | 0.3 M | −0.66 | 8.33 × 10−8 | 0.04 | 1.1 | 2.7 × 105 | 9.7 × 10−4 |
| Sealed coating | 0.3 M | −0.45 | 4.54 × 10−11 | 0.01 | 0.33 | 1.1 × 106 | 2.4 × 10−5 |
| Sealed coating | 0.6 M | −0.71 | 8.41 × 10−10 | 0.02 | 0.45 | 9.7 × 106 | 3.2 × 10−5 |
| Sealed coating | 0.9 M | −0.66 | 2.73 × 10−9 | 0.002 | 0.44 | 3.4 × 105 | 4.4 × 10−5 |
| In NaOH | |||||||
| Unsealed coating | 0.05 M | −1.45 | 3.08 × 10−4 | 0.25 | 0.14 | 125 | 3.58 |
| Sealed coating | 0.05 M | −1.36 | 2.83 × 10−6 | 0.26 | 0.13 | 1.3×104 | 0.033 |
| Sealed coating | 0.1 M | −1.38 | 1.45 × 10−4 | 0.30 | 0.17 | 306 | 1.78 |
| Sealed coating | 0.25 M | −1.40 | 4.35 × 10−3 | 0.75 | 0.26 | 34 | 28.5 |
| Samples | Corrosion Solution | |Zlf0.1Hz| (Ω·cm2) |
|---|---|---|
| In HCl | ||
| Unsealed coating | 1 M | 1113 |
| Sealed coating | 1 M | 2.2 × 105 |
| Sealed coating | 2 M | 2488 |
| Sealed coating | 3 M | 1041 |
| In NaCl | ||
| Unsealed coating | 0.3 M | 3.4 × 106 |
| Sealed coating | 0.3 M | 1.9 × 107 |
| Sealed coating | 0.6 M | 6.2 × 107 |
| Sealed coating | 0.9 M | 4.24 × 107 |
| In NaOH | ||
| Unsealed coating | 0.05 M | 851 |
| Sealed coating | 0.05 M | 1.74 × 105 |
| Sealed coating | 0.1M | 2348 |
| Sealed coating | 0.25 M | 810 |
| Samples | Corrosion Solution | Rc (Ω·cm2) | Cc (F·cm−2) | Rt (Ω·cm2) | Ct (F·cm−2) |
|---|---|---|---|---|---|
| In HCl | |||||
| Unsealed coating | 1 M | 270 | 6.3 × 10−8 | 1449 | 9.6 × 10−5 |
| Sealed coating | 1 M | 1.16 × 104 | 9.4 × 10−10 | 1.36 × 104 | 1.6 × 10−7 |
| Sealed coating | 2 M | 967 | 2.9 × 10−9 | 2118 | 5.4 × 10−7 |
| Sealed coating | 3 M | 561 | 5.5 × 10−8 | 587.6 | 2.8 × 10−6 |
| In NaCl | |||||
| Unsealed coating | 0.3 M | 8.3 × 104 | 6.8 × 10−9 | 3.6 × 106 | 1.4 × 10−10 |
| Sealed coating | 0.3 M | 4.1 × 106 | 1.2 × 10−9 | 2.4 × 108 | 8.8 × 10−12 |
| Sealed coating | 0.6 M | 2.1 × 106 | 2.4 × 10−9 | 1.9 × 108 | 2.7 × 10−10 |
| Sealed coating | 0.9 M | 4.4 × 105 | 8.4 × 10−9 | 5.4 × 107 | 1.32 × 10−9 |
| In NaOH | |||||
| Unsealed coating | 0.05 M | 2.3 × 104 | 6.6 × 10−6 | 401 | 1.7 × 10−5 |
| Sealed coating | 0.05 M | 3.4 × 105 | 9.2 × 10−9 | 3.1 × 104 | 5.3 × 10−8 |
| Sealed coating | 0.1 M | 2468 | 1.9 × 10−8 | 1004 | 7.9 × 10−7 |
| Sealed coating | 0. 25 M | 1175 | 3.4 × 10−7 | 105 | 2.7 × 10−6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, D.; Liu, Z.; Bai, S.; Wang, J. Influence of Sealing Treatment on the Corrosion Resistance of PEO Coated Al-Zn-Mg-Cu Alloy in Various Environments. Coatings 2019, 9, 867. https://doi.org/10.3390/coatings9120867
Zeng D, Liu Z, Bai S, Wang J. Influence of Sealing Treatment on the Corrosion Resistance of PEO Coated Al-Zn-Mg-Cu Alloy in Various Environments. Coatings. 2019; 9(12):867. https://doi.org/10.3390/coatings9120867
Chicago/Turabian StyleZeng, Diping, Zhiyi Liu, Song Bai, and Jian Wang. 2019. "Influence of Sealing Treatment on the Corrosion Resistance of PEO Coated Al-Zn-Mg-Cu Alloy in Various Environments" Coatings 9, no. 12: 867. https://doi.org/10.3390/coatings9120867
APA StyleZeng, D., Liu, Z., Bai, S., & Wang, J. (2019). Influence of Sealing Treatment on the Corrosion Resistance of PEO Coated Al-Zn-Mg-Cu Alloy in Various Environments. Coatings, 9(12), 867. https://doi.org/10.3390/coatings9120867




