Applying Baghdadite/PCL/Chitosan Nanocomposite Coating on AZ91 Magnesium Alloy to Improve Corrosion Behavior, Bioactivity, and Biodegradability
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Micro-Arc oxidation (MAO) Coating Process
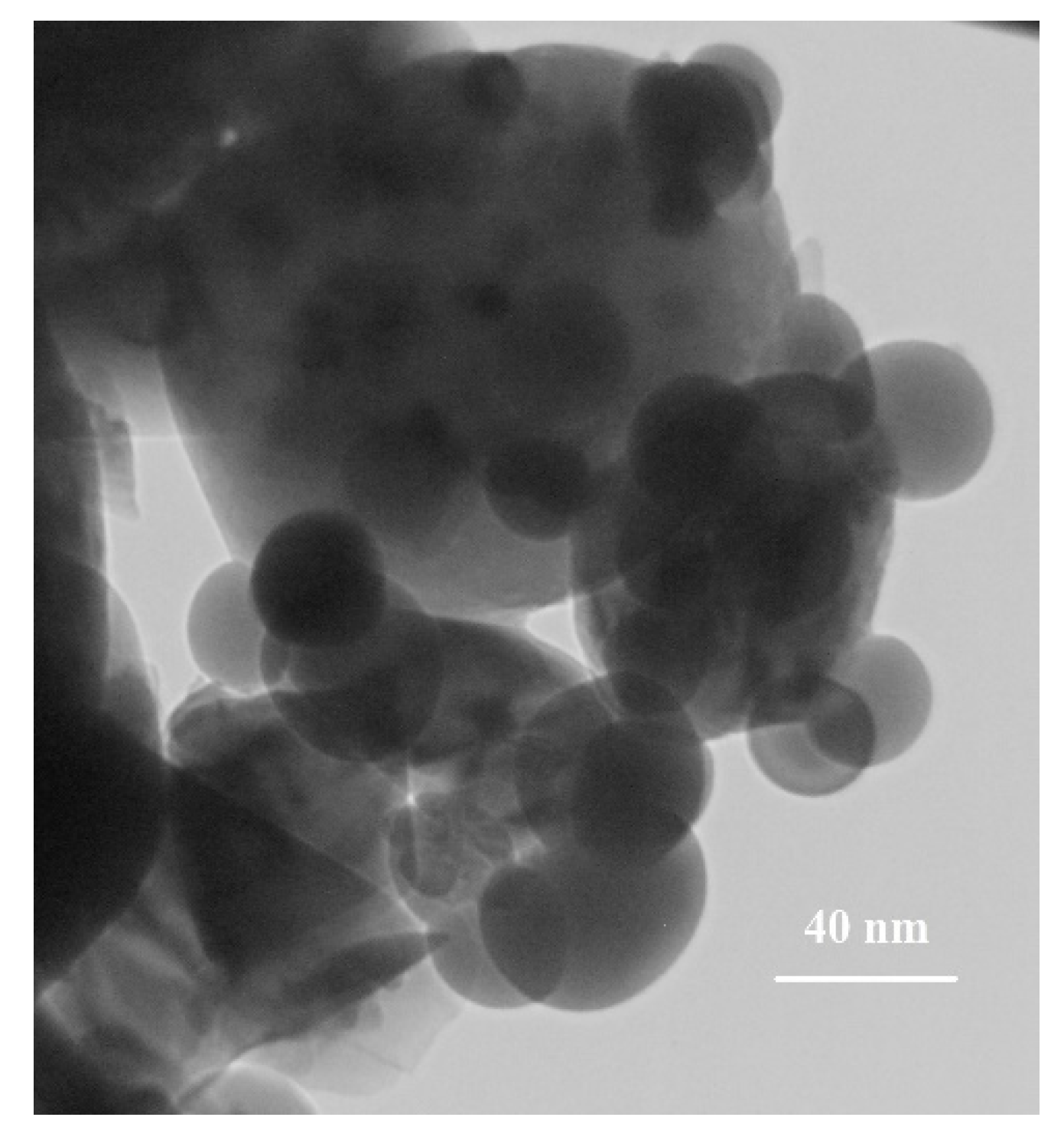
2.3. Preparation of Baghdadite Nanopowder
2.4. Ceramic/Polymer Coating Preparation
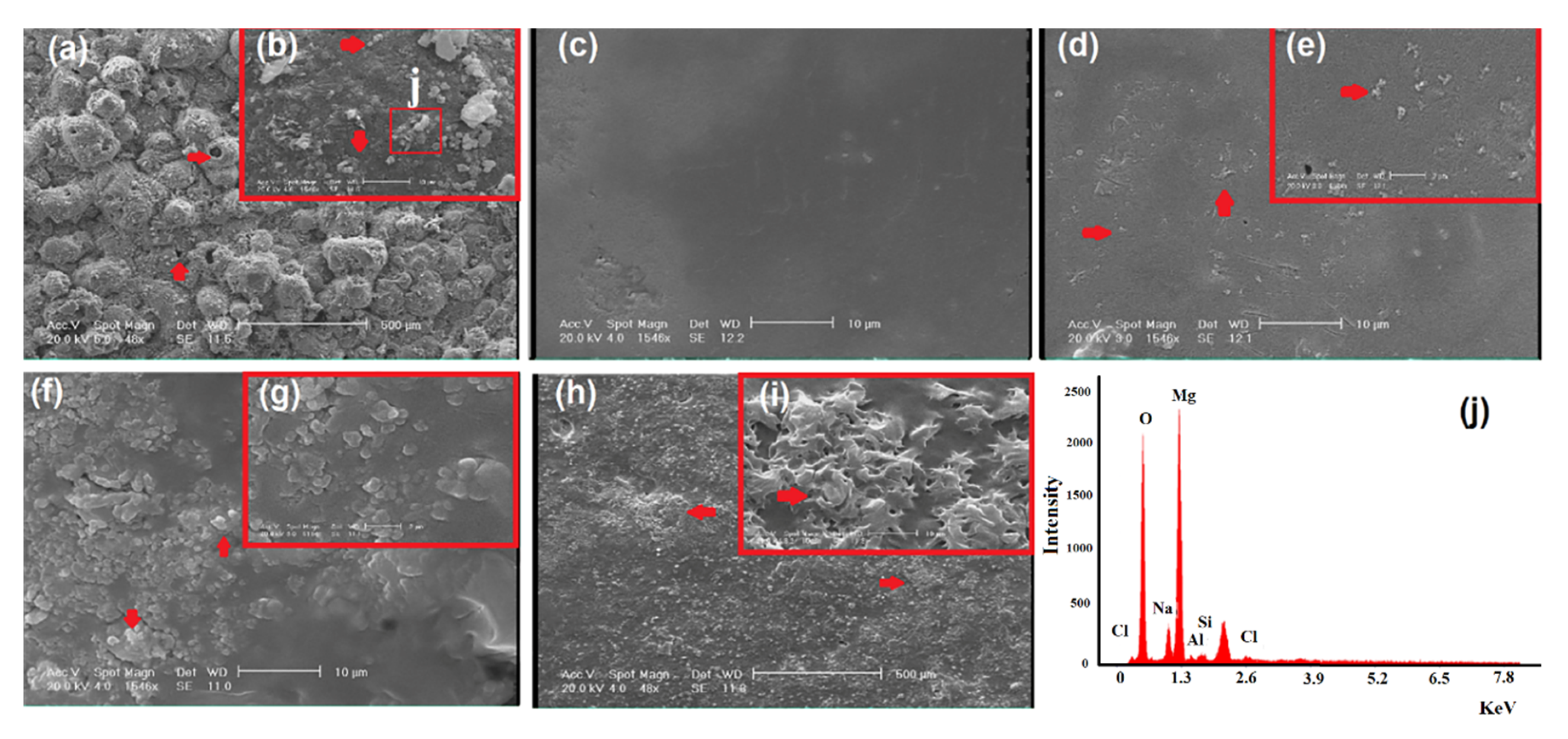
2.5. Characterization of Modified and Unmodified Samples (Coated and Noncoated Samples)
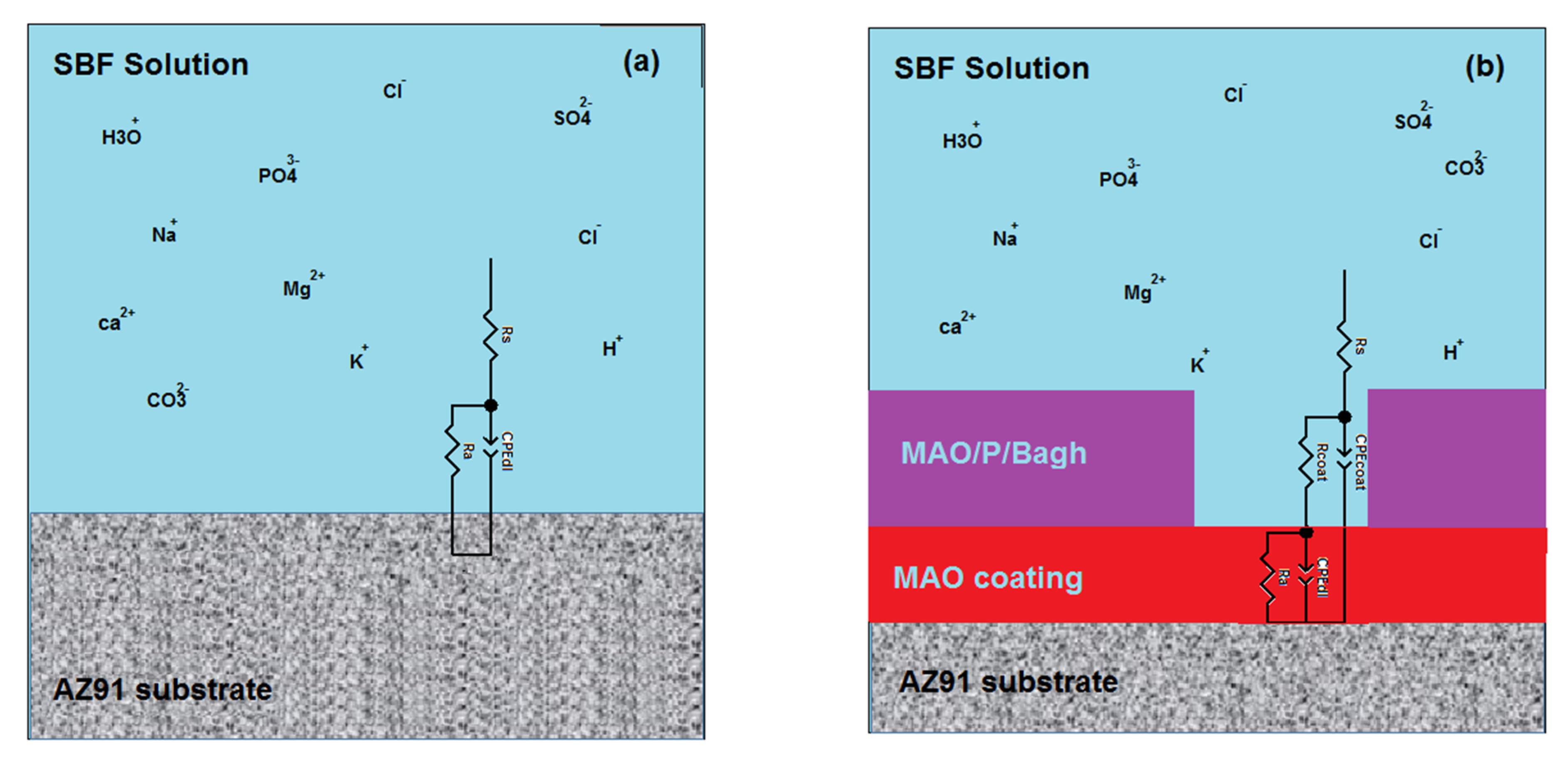
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52. [Google Scholar] [CrossRef] [PubMed]
- Katti, K.S. Biomaterials in total joint replacement. Colloids Surf. B Biointerfaces 2004, 39, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Oldani, C.; Dominguez, A. Titanium as a Biomaterial for Implants. Recent Adv. Arthroplast. 2012, 218, 149–162. [Google Scholar]
- Sing, S.L.; Yeong, W.Y.; Wiria, F.E. Selective laser melting of titanium alloy with 50 wt % tantalum: Microstructure and mechanical properties. J. Alloy. Compd. 2016, 660, 461–470. [Google Scholar] [CrossRef]
- Zhao, X.; Niinomi, M.; Nakai, M.; Hieda, J. Beta type Ti-Mo alloys with changeable Young’s modulus for spinal fixation applications. Acta Biomater. 2012, 8, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzade, S.; Emadi, R.; Tavangarian, F.; Naderi, M. Fabrication and evaluation of silica-based ceramic scaffolds for hard tissue engineering applications. Mater. Sci. Eng. C 2017, 71, 431–438. [Google Scholar] [CrossRef]
- Leda, H. Engineering Materials for Biomedical Applications, 1st ed.; World Scientific: Toh Tuck Link, Singapore, 2004. [Google Scholar]
- Johnson, J. Iliac crest autogenous bone grafting: donor site complications. J. South. Orthop. Assoc. 2000, 9, 91–97. [Google Scholar]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef]
- Song, G.-L. Corrosion Prevention of Magnesium Alloys, 1st ed.; Woodhead Publishing: Oxford, UK, 2013. [Google Scholar]
- Diba, M.; Goudouri, O.-M.; Tapia, F.; Boccaccini, A.R. Magnesium-containing bioactive polycrystalline silicate-based ceramics and glass-ceramics for biomedical applications. Curr. Opin. Solid State Mater. Sci. 2014, 18, 147–167. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Khurshid, Z.; Matinlinna, J.P.; Siddiqui, F.; Nassani, M.Z.; Baroudi, K. Nanomodified peek dental implants: Bioactive composites and surface modification—A review. Int. J. Dent. 2015, 2015, 381759. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.M.; Yeung, K.W.K.; Lam, K.O.; Tam, V.; Chu, P.K.; Luk, K.D.K.; Cheung, K.M.C. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants. Biomaterials 2010, 31, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Arifvianto, B.; Zhou, J. Fabrication of metallic biomedical scaffolds with the space holder method: A review. Materials 2014, 7, 3588–3622. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Fathi, M.; Savabi, O.; Razavi, S.M.; Beni, B.H.; Vashaee, D.; Tayebi, L. Controlling the degradation rate of bioactive magnesium implants by electrophoretic deposition of akermanite coating. Ceram. Int. 2014, 40, 3865–3872. [Google Scholar] [CrossRef]
- Guo, H.F.; An, M.Z.; Huo, H.B.; Xu, S.; Wu, L.J. Microstructure characteristic of ceramic coatings fabricated on magnesium alloys by micro-arc oxidation in alkaline silicate solutions. Appl. Surf. Sci. 2006, 252, 7911–7916. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, C. Development of anodic film on Mg alloy AZ91D. Surf. Coat. Technol. 2006, 201, 2381–2386. [Google Scholar] [CrossRef]
- Gu, X.N.; Zheng, W.; Cheng, Y.; Zheng, Y.F. A study on alkaline heat treated Mg-Ca alloy for the control of the biocorrosion rate. Acta Biomater. 2009, 5, 2790–2799. [Google Scholar] [CrossRef]
- Wu, C.; Wen, Z.; Dai, C.; Lu, Y.; Yang, F. Fabrication of calcium phosphate/chitosan coatings on AZ91D magnesium alloy with a novel method. Surf. Coat. Technol. 2010, 204, 3336–3347. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Soleimani, B.; Tavangarian, F. Two-Step modification process to improve mechanical properties and bioactivity of hydroxyfluorapatite scaffolds. Ceram. Int. 2018, 44, 19756–19763. [Google Scholar] [CrossRef]
- Tiyyagura, H.R.; Fuchs-Godec, R.; Gorgieva, S.; Arthanari, S.; Mohan, M.K.; Kokol, V. Biomimetic gelatine coating for less-corrosive and surface bioactive Mg-9Al-1Zn alloys. J. Mater. Res. 2018, 33, 1449–1462. [Google Scholar] [CrossRef]
- Wang, S.; Yaszemski, M.J.; Knight, A.M.; Gruetzmacher, J.A.; Windebank, A.J.; Lu, L. Photo-Crosslinked poly (ε-caprolactone fumarate) networks for guided peripheral nerve regeneration: Material properties and preliminary biological evaluations. Acta Biomater. 2009, 5, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Jokar, M.; Darvishi, S.; Torkaman, R.; Kharaziha, M.; Karbasi, M. Corrosion and bioactivity evaluation of nanocomposite PCL-forsterite coating applied on 316L stainless steel. Surf. Coat. Technol. 2016, 307, 324–331. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Labbaf, S. Hardystonite-diopside nanocomposite scaffolds for bone tissue engineering applications. Mater. Chem. Phys. 2017, 202, 95–103. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Tavangarian, F. Combustion assisted synthesis of hardystonite nanopowder. Ceram. Int. 2016, 42, 14656–14660. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Tavangarian, F.; Doostmohammadi, A. The influence of polycaporolacton fumarate coating on mechanical properties and in vitro behavior of porous diopside-hardystonite nano-composite scaffold. J. Mech. Behav. Biomed. Mater. 2020, 101, 103445. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Ghomi, H. Mechanical alloying synthesis of forsterite-diopside nanocomposite powder for using in tissue engineering. Ceram. Silikáty 2015, 59, 1–5. [Google Scholar]
- Sadeghzade, S.; Shamoradi, F.; Emadi, R.; Tavangarian, F. Fabrication and characterization of baghdadite nanostructured scaffolds by space holder method. J. Mech. Behav. Biomed. Mater. 2017, 68, 1–7. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Ahmadi, T.; Tavangarian, F. Synthesis, characterization and strengthening mechanism of modified and unmodified porous diopside/baghdadite scaffolds. Mater. Chem. Phys. 2019, 228, 89–97. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Tavangarian, F.; Doostmohammadi, A. In vitro evaluation of diopside/baghdadite bioceramic scaffolds modified by polycaprolactone fumarate polymer coating. Mater. Sci. Eng. C 2020, 106, 110176. [Google Scholar] [CrossRef]
- Roohani-Esfahani, S.I.; Dunstan, C.R.; Davies, B.; Pearce, S.; Williams, R.; Zreiqat, H. Repairing a critical-sized bone defect with highly porous modified and unmodified baghdadite scaffolds. Acta Biomater. 2012, 8, 4162–4172. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, L.D.Y.; da Conceição, T.F.; Spinelli, A.; Scharnagl, N.; Pires, A.T.N. Chitosan coatings crosslinked with genipin for corrosion protection of AZ31 magnesium alloy sheets. Carbohydr. Polym. 2018, 181, 71–77. [Google Scholar] [CrossRef]
- Sowmya, S.; Bumgardener, J.D.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Role of nanostructured biopolymers and bioceramics in enamel, dentin and periodontal tissue regeneration. Prog. Polym. Sci. 2013, 38, 1748–1772. [Google Scholar] [CrossRef]
- Golshirazi, A.; Kharaziha, M.; Golozar, M.A. Polyethylenimine/kappa carrageenan: Micro-arc oxidation coating for passivation of magnesium alloy. Carbohydr. Polym. 2017, 167, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Fathi, M.; Savabi, O.; Vashaee, D.; Tayebi, L. In vitro study of nanostructured diopside coating on Mg alloy orthopedic implants. Mater. Sci. Eng. C 2014, 41, 168–177. [Google Scholar] [CrossRef]
- Joughehdoust, S.; Behnamghader, A.; Imani, M.; Daliri, M.; Doulabi, A.H.; Jabbari, E. A novel foam-like silane modified alumina scaffold coated with nano-hydroxyapatite–poly (ε-caprolactone fumarate) composite layer. Ceram. Int. 2013, 39, 209–218. [Google Scholar] [CrossRef]
- Nassif, N.; Ghayad, I. Corrosion protection and surface treatment of magnesium alloys used for orthopedic applications. Adv. Mater. Sci. Eng. 2013, 2013, 532896. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef]






| Elements | Al | Zn | Mn | Mg |
|---|---|---|---|---|
| wt % | 8.65 | 0.6 | 0.17 | Balanced |
| Designation | Sample |
|---|---|
| M | AZ91 |
| M1 | MAO |
| M2 | Baghdadite powder |
| M3 | MAO-PCL/Ch |
| M4 | MAO-PCL/Ch-1 wt %Bagh |
| M5 | MAO-PCL/Ch-3 wt %Bagh |
| M6 | MAO-PCL/Ch-5 wt %Bagh |
| Samples | Contact Angle (°) | Roughness (µm) |
|---|---|---|
| M | 1 ± 86 | 0.329 ± 0.02 |
| M1 | 33 ± 1.3 | 11.356 ± 0.45 |
| M3 | 51 ± 2 | 4.743 ± 0.23 |
| M4 | 59 ± 1 | 7.792 ± 0.34 |
| M5 | 72 ± 1.2 | 7.026 ± 0.31 |
| M6 | 66 ± 1.3 | 10.610 ± 0.21 |
| Samples | Icorr (A/cm2) | Ecorr (V) |
|---|---|---|
| M | 2 × 10−4 | −1.71 |
| M1 | 0.5 × 10−6 | −1.48 |
| M3 | 9.5 × 10−7 | −1.43 |
| M4 | 4.2 × 10−7 | −1.3 |
| M5 | 5.4 × 10−8 | −1.15 |
| M6 | 9.4 × 10−8 | −1.22 |
| Samples | Impedance in Low Frequency (Ω) |
|---|---|
| M | 2100 |
| M1 | 4500 |
| M3 | 20,000 |
| M4 | 41,000 |
| M5 | 76,000 |
| M6 | 45,000 |
| Samples | Rs | Ra | Rcoat | Rt | CPEcoat | n1 | CPEdl | n2 |
|---|---|---|---|---|---|---|---|---|
| M | 159.5 | 1648 (related to substrate) | – | 1648 | – | – | 2.3055 × 10−5 | 0.921 |
| M1 | 258 | 224.3 | 4223 | 4447.3 | 9.9148 × 10−6 | 0.8726 | 3.5678 × 10−6 | 0.761 |
| M3 | 675.4 | 15284 | 657.1 | 15,941.1 | 4.22221 × 10−7 | 0.827 | 1.3024 × 10−6 | 0.921 |
| M4 | 698 | 32851 | 1771 | 34,622 | 6.13424 × 10−8 | 0.923 | 9.4792 × 10−7 | 0.899 |
| M5 | 110.6 | 81134 | 1991 | 83,125 | 8.1442 × 10−8 | 0.833 | 2.1283 × 10−7 | 0.932 |
| M6 | 79.5 | 45592 | 1951 | 47,543 | 6.5313 × 10−7 | 0.942 | 1.288 × 10−6 | 0.894 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soleymani, F.; Emadi, R.; Sadeghzade, S.; Tavangarian, F. Applying Baghdadite/PCL/Chitosan Nanocomposite Coating on AZ91 Magnesium Alloy to Improve Corrosion Behavior, Bioactivity, and Biodegradability. Coatings 2019, 9, 789. https://doi.org/10.3390/coatings9120789
Soleymani F, Emadi R, Sadeghzade S, Tavangarian F. Applying Baghdadite/PCL/Chitosan Nanocomposite Coating on AZ91 Magnesium Alloy to Improve Corrosion Behavior, Bioactivity, and Biodegradability. Coatings. 2019; 9(12):789. https://doi.org/10.3390/coatings9120789
Chicago/Turabian StyleSoleymani, Farzad, Rahmatollah Emadi, Sorour Sadeghzade, and Fariborz Tavangarian. 2019. "Applying Baghdadite/PCL/Chitosan Nanocomposite Coating on AZ91 Magnesium Alloy to Improve Corrosion Behavior, Bioactivity, and Biodegradability" Coatings 9, no. 12: 789. https://doi.org/10.3390/coatings9120789
APA StyleSoleymani, F., Emadi, R., Sadeghzade, S., & Tavangarian, F. (2019). Applying Baghdadite/PCL/Chitosan Nanocomposite Coating on AZ91 Magnesium Alloy to Improve Corrosion Behavior, Bioactivity, and Biodegradability. Coatings, 9(12), 789. https://doi.org/10.3390/coatings9120789





