Evaluation of Mesenchymal Stem Cells and Osteoblasts’ Adhesion and Proliferation in the Presence of HA-AL Biomaterials
Abstract
:1. Introduction
2. Materials and Methods
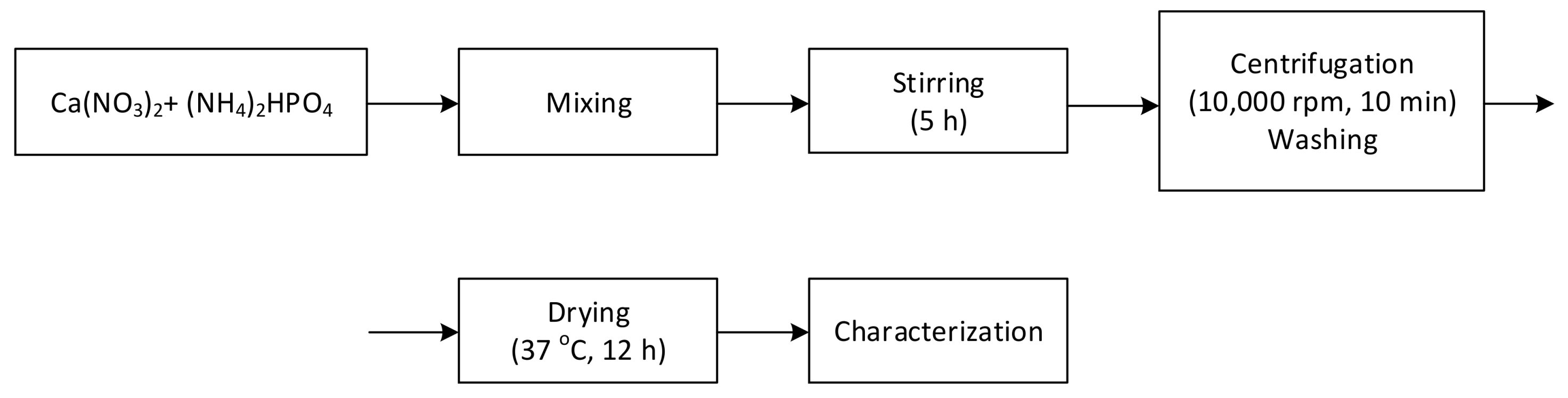
2.1. Hydroxyapatite Synthesis by Wet Precipitation Method
2.2. Hydroxyapatite-Alendronate Synthesis
2.3. Physico-Chemical Characterization of Hydroxyapatite and HA-AL Composite
2.4. MAPLE Deposition
2.5. Spectral Characterization
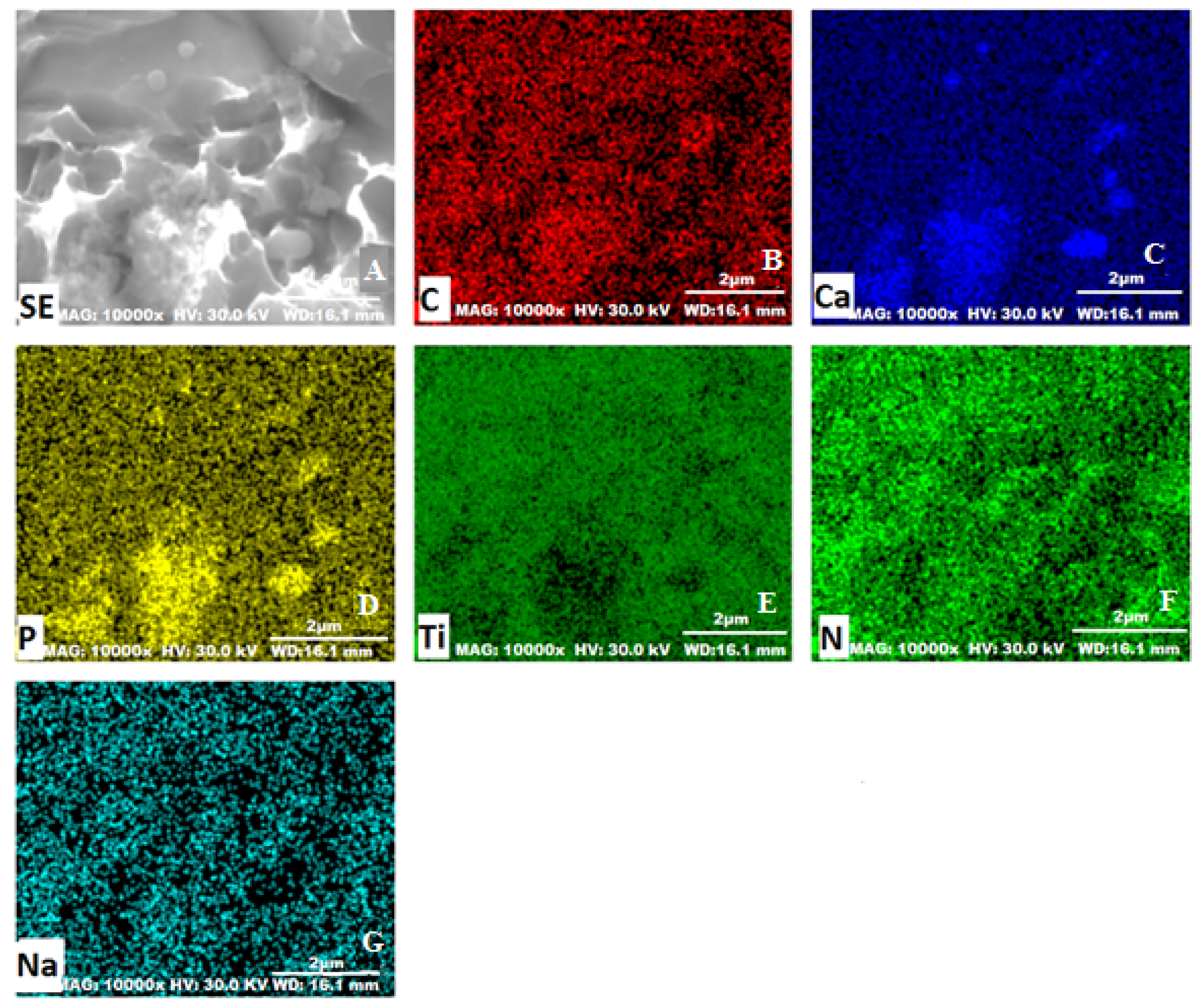
2.6. Scanning Electron Microscopy
2.7. Biocompatibility Assays of HA-AL Biomaterials
2.7.1. Isolation and Characterization of Mesenchymal Stem Cells
2.7.2. In Vitro Cell Culture on Biomaterials
Bone Cell Culture and Seeding Cells
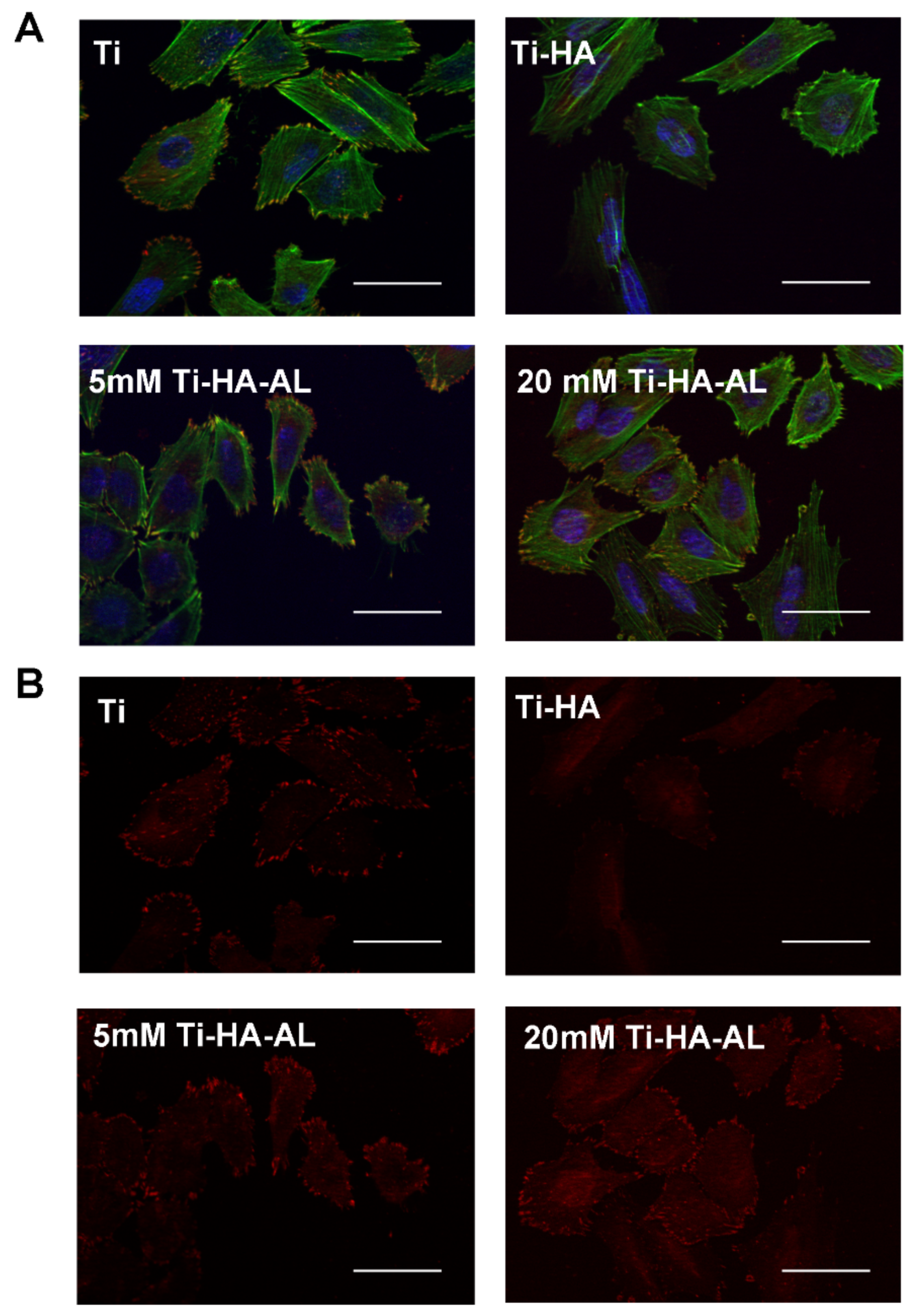
Fluorescence Microscopy
2.7.3. MTS Assay
3. Results
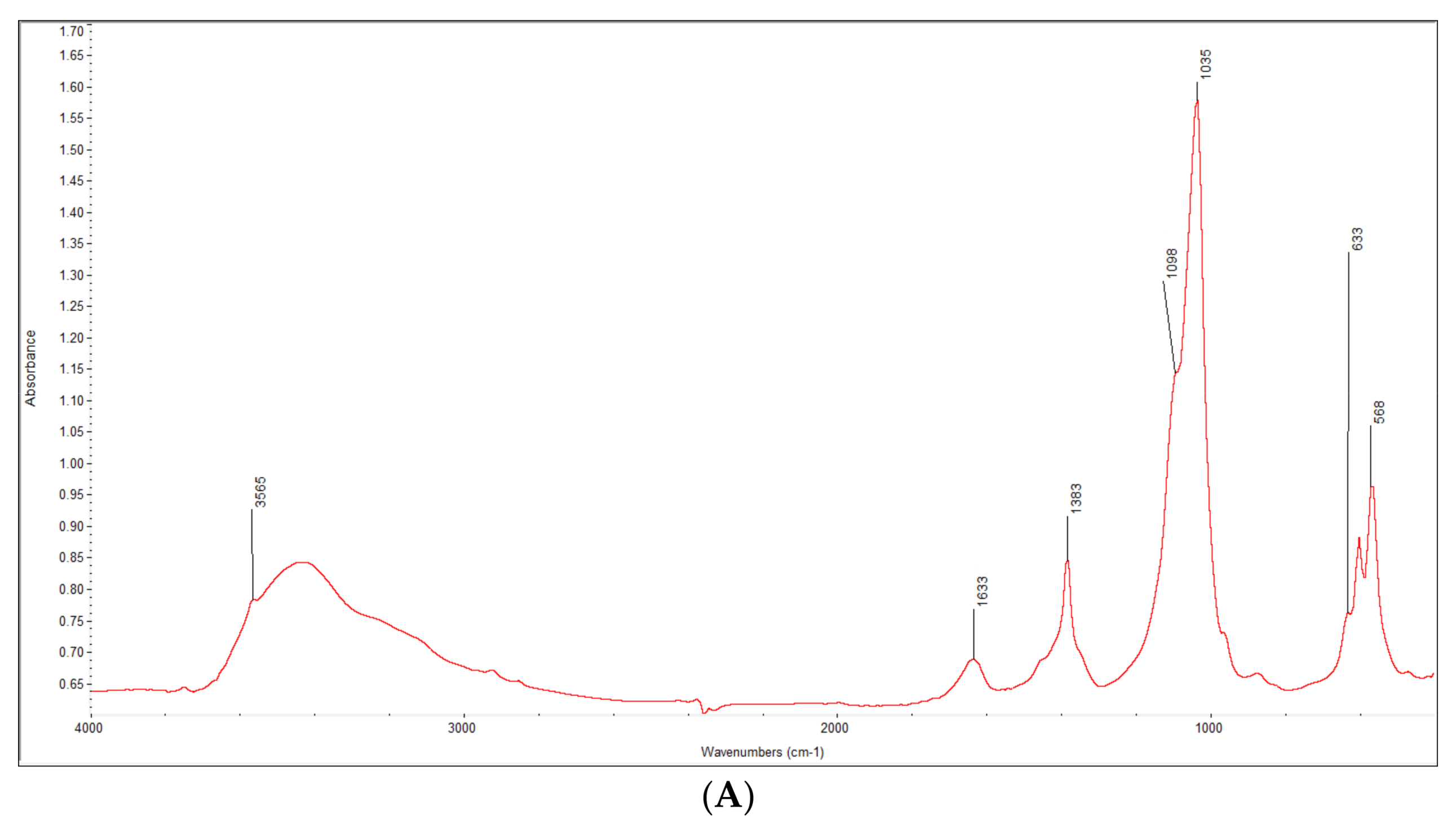
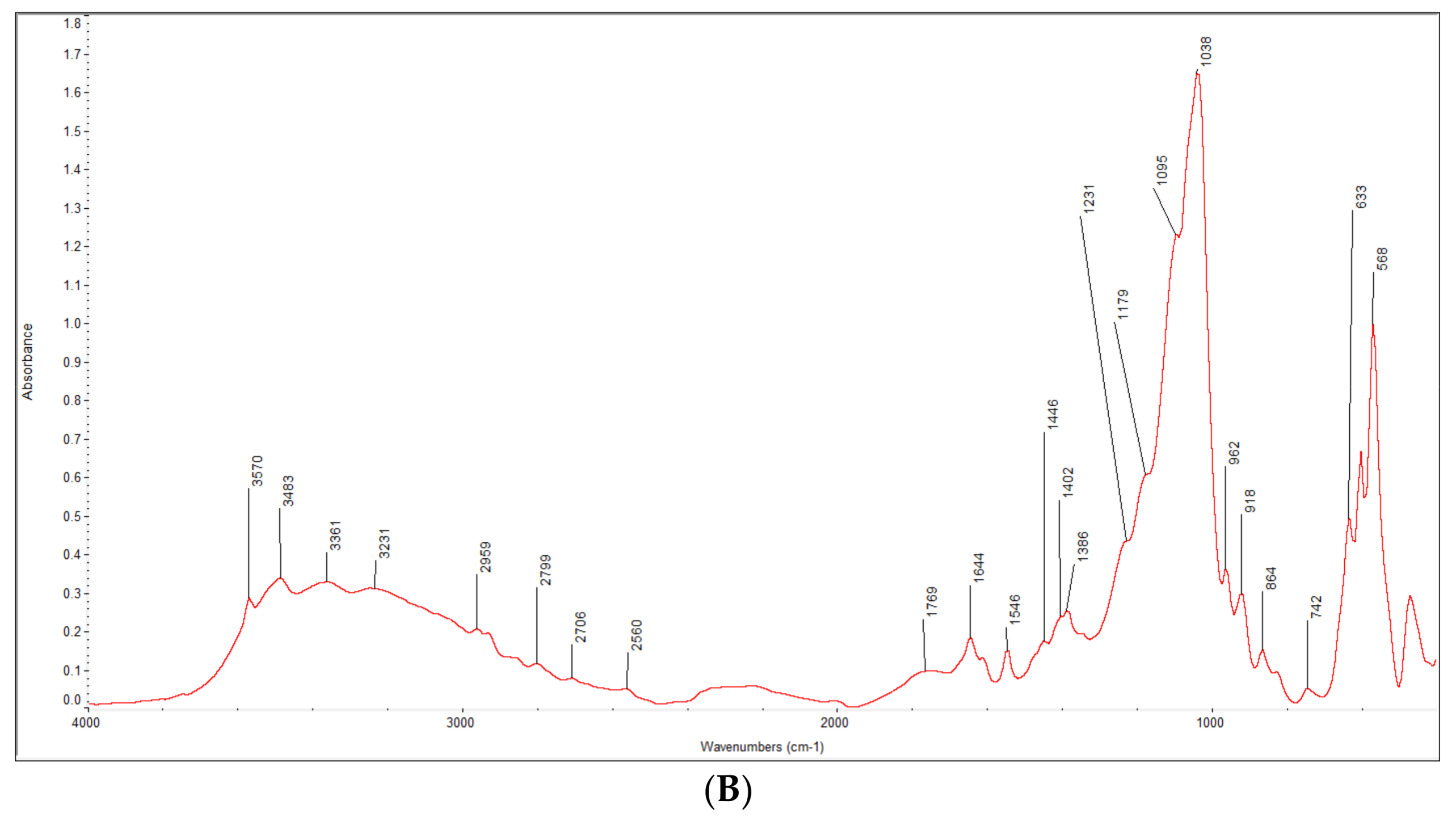
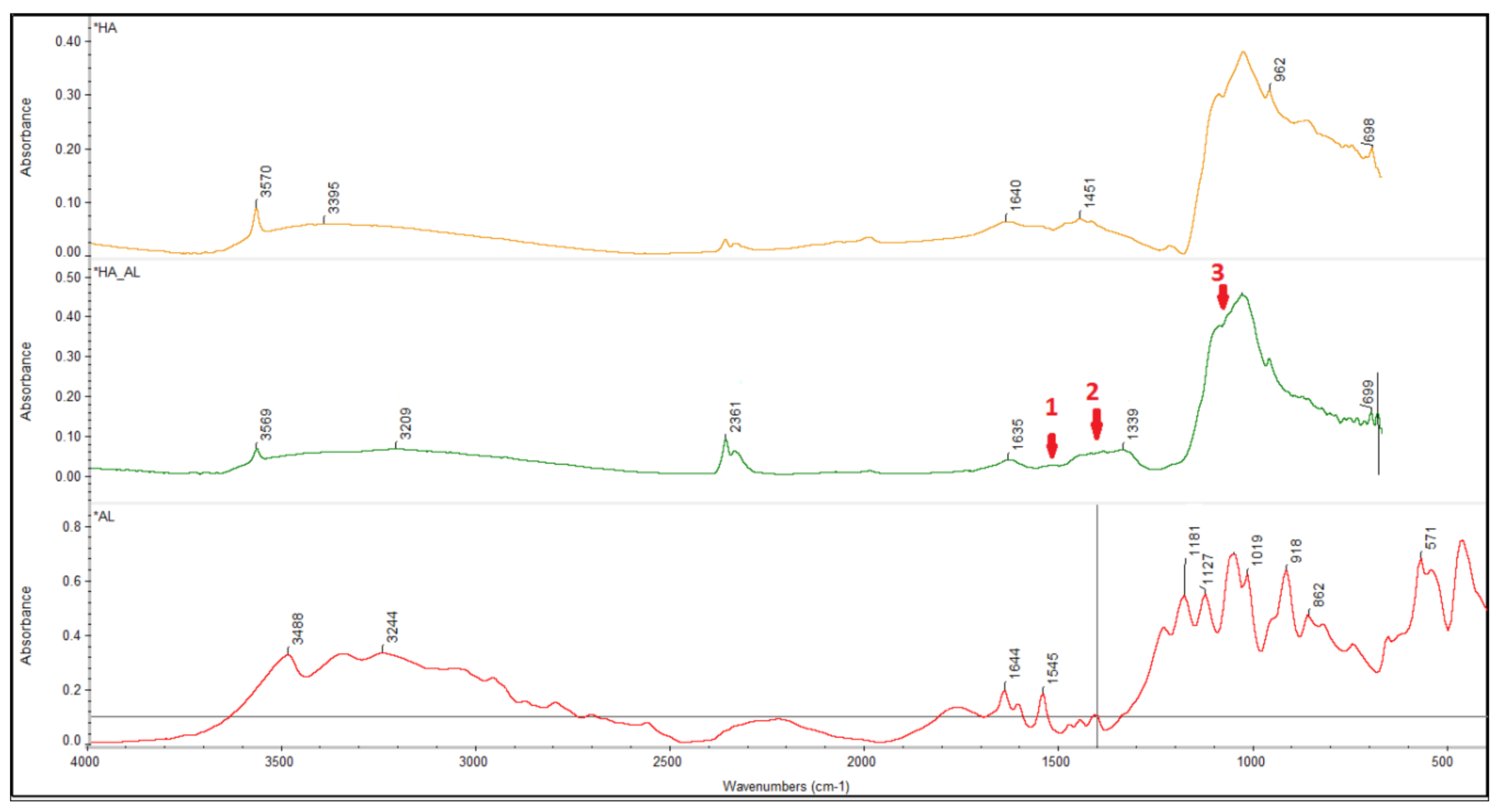
3.1. Characterization of the Synthesized HA-AL Composite
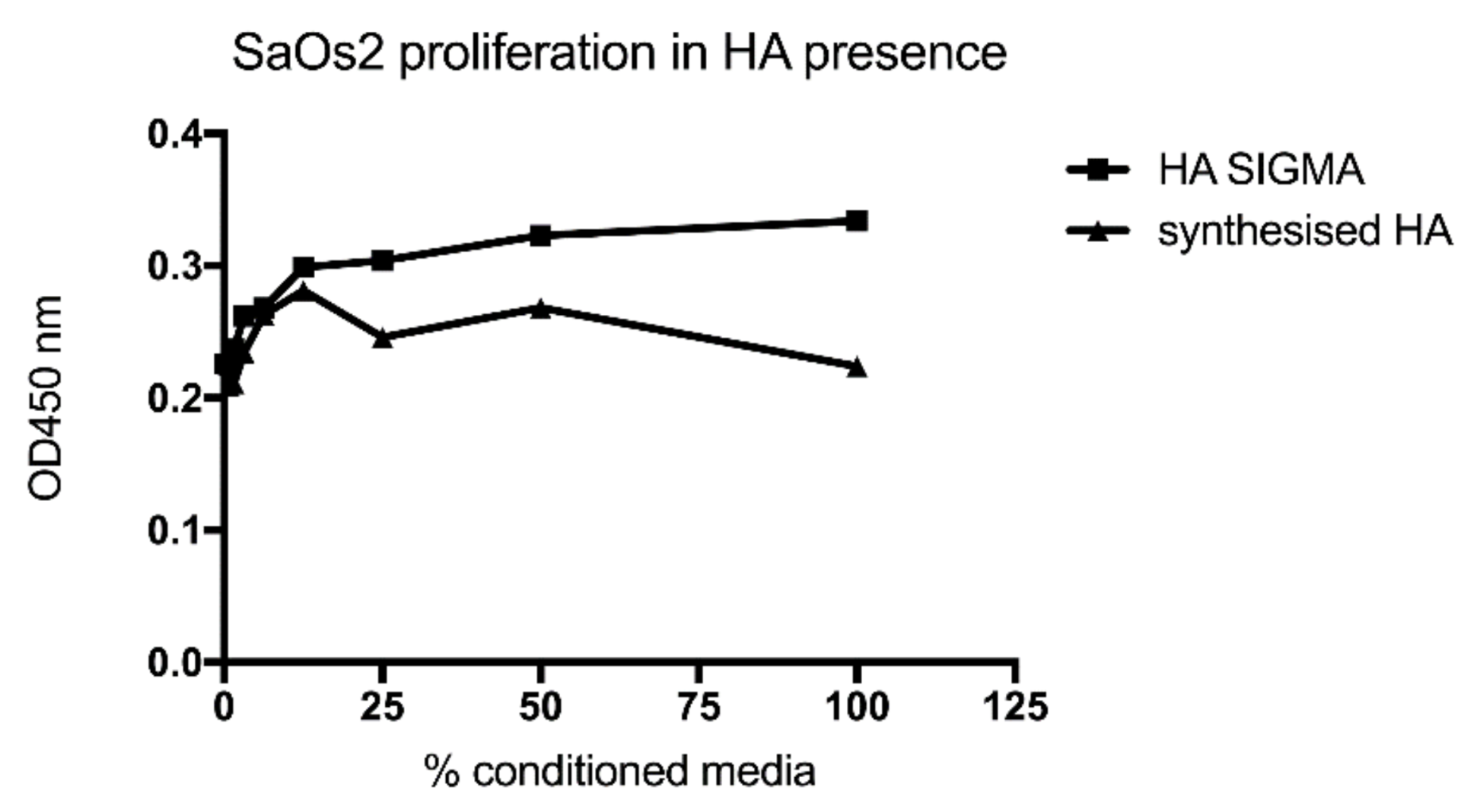
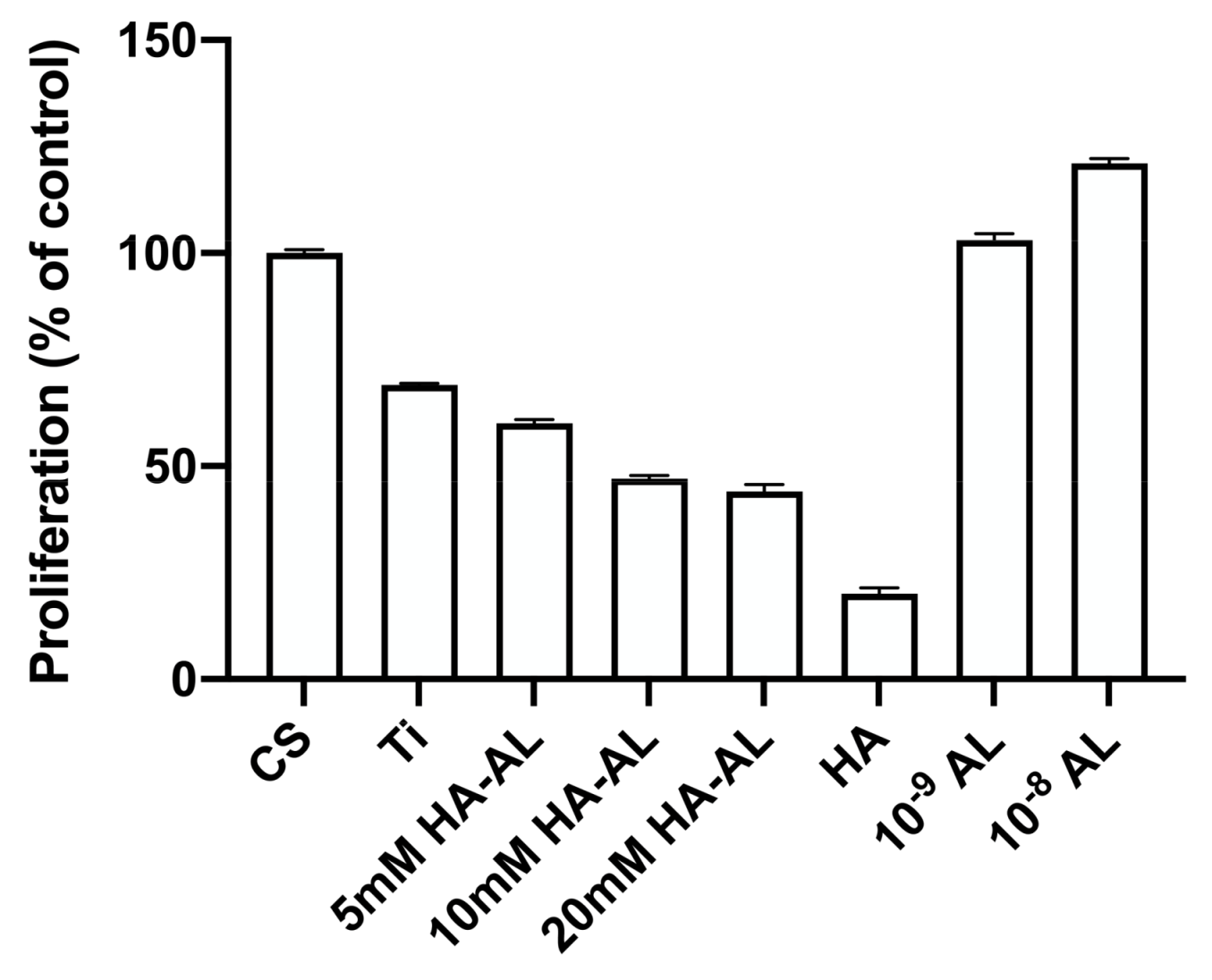
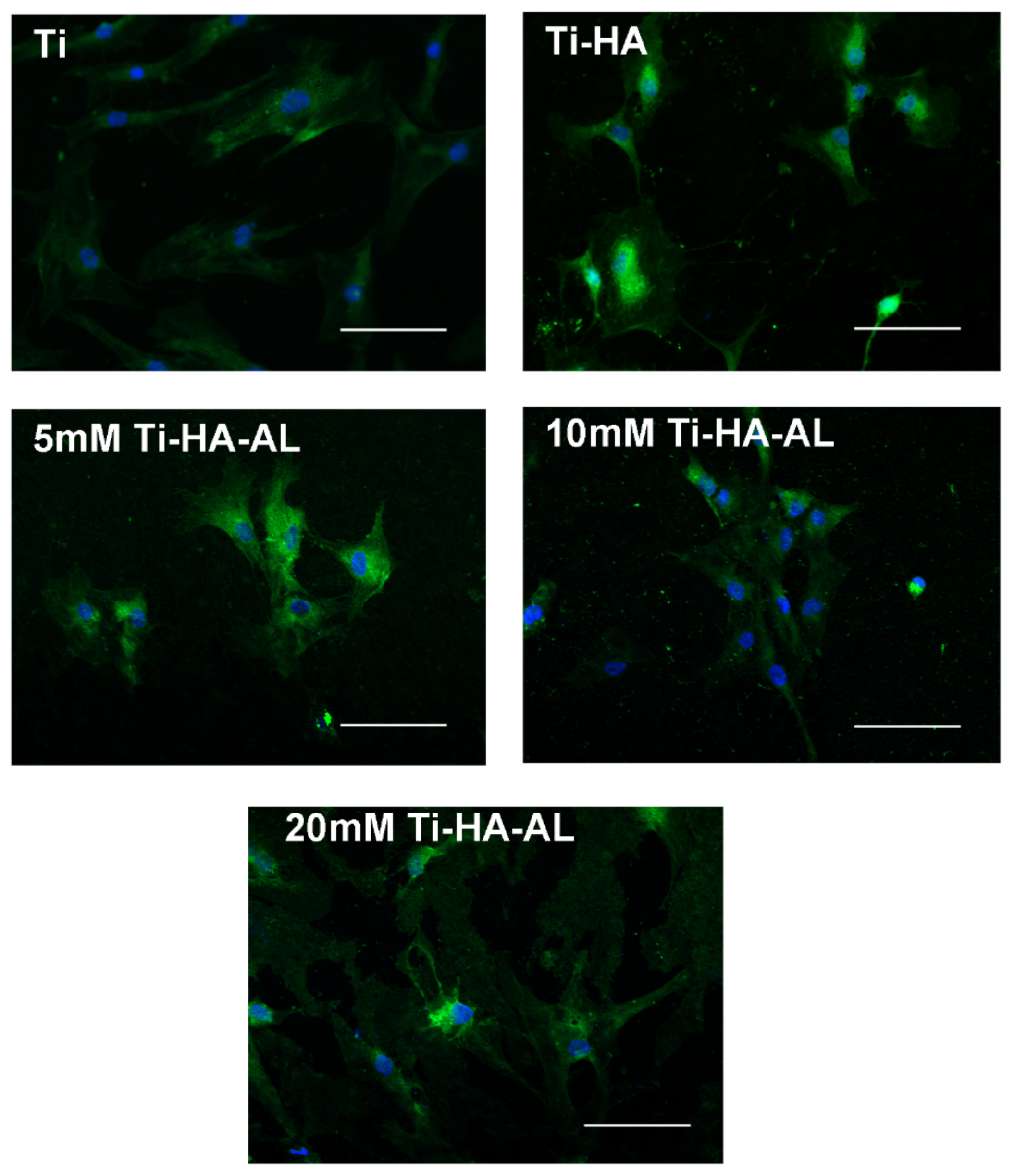
3.2. Testing the Biocompatibility of HA-AL Biomaterials
4. Discussion
4.1. Synthesis and Characterization of the Synthesized Hydroxyapatite and HA-AL
4.2. Human Bone Cell Proliferation in the Presence of HA, Alendronate and HA-AL
4.3. Human Mesenchymal Stem Cells’ Proliferation on HA-AL Films
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jaffe, W.L.; Scott, D.F. Total hip arthroplasty with hydroxyapatite-coated prosthesis. J. Bone Jt. Surg. 1996, 78, A1818–A1934. [Google Scholar] [CrossRef] [PubMed]
- Negrilă, C.C.; Predoi, M.V.; Iconaru, S.L.; Predoi, D.L. Development of zinc-doped hydroxyapatite by sol-gel method for medical applications. Molecules 2018, 23, 2986. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I.; Bianco, A. High thermally stable Mg-substituted tricalcium phosphate via precipitation. Ceram. Int. 2011, 37, 127–137. [Google Scholar] [CrossRef]
- Suratwala, S.J.; Cho, S.K.; Van Raalte, J.J.; Park, S.H.; Seo, S.W.; Chang, S.S.; Gardner, T.R.; Lee, F.Y. Enhancement of periprosthetic bone quality with topical hydroxyapatite-bisphosphonate composite. J. Bone Jt. Surg. Am. 2008, 90, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Puleo, D.A. Encyclopedia of Biomaterials and Biomedical Engineering; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 190–198. [Google Scholar]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef]
- Knoch, F.; Eckhardt, C.; Alabre, C.I.; Schneider, E.; Rubash, H.E.; Shanbhag, A.S. Anabolic effects of bisphosphonates on peri-implant bone stock. Biomaterials 2007, 28, 3549–3559. [Google Scholar] [CrossRef]
- Matthew, T.; Drake, M.D.; Bart, L.; Clarke, M.D.; Sundeep Khosla, M.D. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar]
- Cavalli, L.; Brandi, M.L. Periprosthetic bone loss: Diagnostic and therapeutic approaches. F1000Research 2013, 2, 266. [Google Scholar] [CrossRef]
- Vignoletti, F.; Abrahamsson, I. Quality of reporting of experimental research in implant dentistry. Critical aspects in design, outcome assessment and model validation. J. Clin. Periodontol. 2012, 39, 6–27. [Google Scholar] [CrossRef]
- Chai, C.S.; Ben-Nissan, B. Bioactive nanocrystalline sol-gel hydroxyapatite coatings. J. Mater. Sci. 1999, 10, 465–469. [Google Scholar]
- Manafi, S.A.; Joughehdoust, S. Synthesis of hydroxyapatite nanostructure by hydrothermal condition for biomedical application. Iran. J. Pharm. Sci. 2009, 5, 89–94. [Google Scholar]
- Nayak, A.K. Hydroxyapatite synthesis methodologies: An overview. Int. J. ChemTech Res. 2010, 2, 903–907. [Google Scholar]
- Thamaraiselvi, T.V.; Prabakaran, K.; Rajeswari, S. Synthesis of hydroxyapatite that mimics bone mineralogy. Trends Biomater. Artif. Organs 2006, 19, 81–83. [Google Scholar]
- Shikhanzadeh, M. Direct formation of nanophase hydroxyapatite on cathodically polarized electrodes. J. Mater. Sci. Mater. Med. 1998, 9, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lugo, V.; Karthik, T.V.K.; Mendoza-Anaya, D.; Rubio-Rosas, E.; Villaseñor Cerón, L.S.; Reyes-Valderrama, M.I.; Salinas-Rodríguez, E. Wet chemical synthesis of nanocrystalline hydroxyapatite flakes: Effect of pH and sintering temperature on structural and morphological properties. R. Soc. Open Sci. 2018, 5, 180962. [Google Scholar] [CrossRef] [PubMed]
- Bubb, D.M.; Wu, P.K.; Horwitz, J.S.; Callahan, J.H.; Galicia, M.; Vertes, A. The effect of the matrix on film properties in matrix-assisted pulsed laser evaporation. J. Appl. Phys. 2002, 91, 2055–2058. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Rubini, K.; Bigi, A. Composite nanocrystals provide new insight on alendronate interaction with hydroxyapatite structure. Adv. Mater. 2007, 19, 2499–2502. [Google Scholar] [CrossRef]
- Neamtu, J.; Bubulica, M.V.; Rotaru, A.; Ducu, C.; Balosache, O.E.; Manda, V.C.; Turcu-Stiolica, A.; Nicolicescu, C.; Melinte, R.; Popescu, M.; et al. Hydroxyapatite-alendronate composite systems for biocompatible materials. J. Therm. Anal. Calorim. 2017, 127, 1567–1582. [Google Scholar] [CrossRef]
- Sima, L.E.; Stan, G.E.; Morosanu, C.O.; Melinescu, A.; Ianculescu, A.; Melinte, R.; Neamtu, J.; Petrescu, S.M. Differentiation of mesenchymal stem cells onto highly adherent radio frequency-sputtered carbonated hydroxylapatite thin films. J. Biomed. Mater. Res. A 2010, 95, 1203–1214. [Google Scholar] [CrossRef]
- Meinel, L.; Fajardo, R.; Hofmann, S.; Langer, R.; Chen, J.; Snyder, B. Silk implants for the healing of critical size bone defects. Bone 2005, 37, 688–698. [Google Scholar] [CrossRef]
- Rotaru, L.T.; Varut, R.M.; Nicolaescu, O.; Bubulica, M.; Belu, I. In vitro release studies of alendronate from HA-AL composite deposited on titanium metal substrate. J. Sci. Arts 2019, 19, 443–448. [Google Scholar]
- Ciocilteu, M.V.; Mocanu, A.G.; Mocanu, A.; Ducu, C.; Nicolaescu, O.E.; Manda, V.C.; Turcu-Stiolica, A.; Nicolicescu, C.; Melinte, R.; Balasoiu, M.; et al. Hydroxyapatite-ciprofloxacin delivery system: Synthesis, characterisation and antibacterial activity. Acta Pharm. 2018, 68, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Turcu-Stiolica, A.; Bubulica, M.V.; Nicolaescu, O.E. A design of experiment approach to the synthesis of alendronate-incorporated hydroxyapatite. Rev. Chim. Buchar. 2018, 69, 1944–1948. [Google Scholar]
- Capra, P.; Dorati, R.; Colonna, C.; Bruni, G.; Pavanetto, F.; Genta, I.; Conti, B. A preliminary study on the morphological and release properties of hydroxyapatite-alendronate composite materials. J. Microencapsul. 2011, 28, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Torricelli, P.; Gazzano, M.; Fini, M.; Bigi, A. The effect of zoledronate-hydroxyapatite nanocomposites on osteoclasts and osteoblast-like cells in vitro. Biomaterials 2012, 33, 722–730. [Google Scholar] [CrossRef]
- Colnot, C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J. Bone Miner. Res. 2009, 24, 274–282. [Google Scholar] [CrossRef]
- Haasters, F.; Prall, W.C.; Anz, D.; Bourquin, C.; Pautke, C.; Endres, S.; Mutschler, W.; Docheva, D.; Schieker, M. Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J. Anat. 2009, 214, 759–767. [Google Scholar] [CrossRef]

| Sample | Temperature (oC) | Flow (mL/min) | Stirring (rpm) | pH | Maturation (Days) | AL (mg) in 5 mg HA-AL (HPLC) | Conc. AL (w/w) (%) | Incorporation Efficiency (%) | Size (nm) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 90 | 0.1 | 600 | 10 | 0 | 0.0151 | 0.302 | 9.258565 | 397 |
| Samples | HA | 5 mM HA-AL | 10 mM HA-AL | Ti | Cs |
|---|---|---|---|---|---|
| OD 450 nm | 1.812 ± 0.004 | 1.530 ± 0.008 | 1.421 ± 0.008 | 1.825 ± 0.003 | 1.593 ± 0.006 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolaescu, O.-E.; Turcu-Stiolica, A.; Varut, R.-M.; Mocanu, A.-G.; Belu, I.; Sima, L.E.; Neamtu, J. Evaluation of Mesenchymal Stem Cells and Osteoblasts’ Adhesion and Proliferation in the Presence of HA-AL Biomaterials. Coatings 2019, 9, 782. https://doi.org/10.3390/coatings9120782
Nicolaescu O-E, Turcu-Stiolica A, Varut R-M, Mocanu A-G, Belu I, Sima LE, Neamtu J. Evaluation of Mesenchymal Stem Cells and Osteoblasts’ Adhesion and Proliferation in the Presence of HA-AL Biomaterials. Coatings. 2019; 9(12):782. https://doi.org/10.3390/coatings9120782
Chicago/Turabian StyleNicolaescu, Oana-Elena, Adina Turcu-Stiolica, Renata-Maria Varut, Andreea-Gabriela Mocanu, Ionela Belu, Livia Elena Sima, and Johny Neamtu. 2019. "Evaluation of Mesenchymal Stem Cells and Osteoblasts’ Adhesion and Proliferation in the Presence of HA-AL Biomaterials" Coatings 9, no. 12: 782. https://doi.org/10.3390/coatings9120782
APA StyleNicolaescu, O.-E., Turcu-Stiolica, A., Varut, R.-M., Mocanu, A.-G., Belu, I., Sima, L. E., & Neamtu, J. (2019). Evaluation of Mesenchymal Stem Cells and Osteoblasts’ Adhesion and Proliferation in the Presence of HA-AL Biomaterials. Coatings, 9(12), 782. https://doi.org/10.3390/coatings9120782






