Femtosecond Pulsed Laser Deposition of Chromium Diboride-Rich Thin Films
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
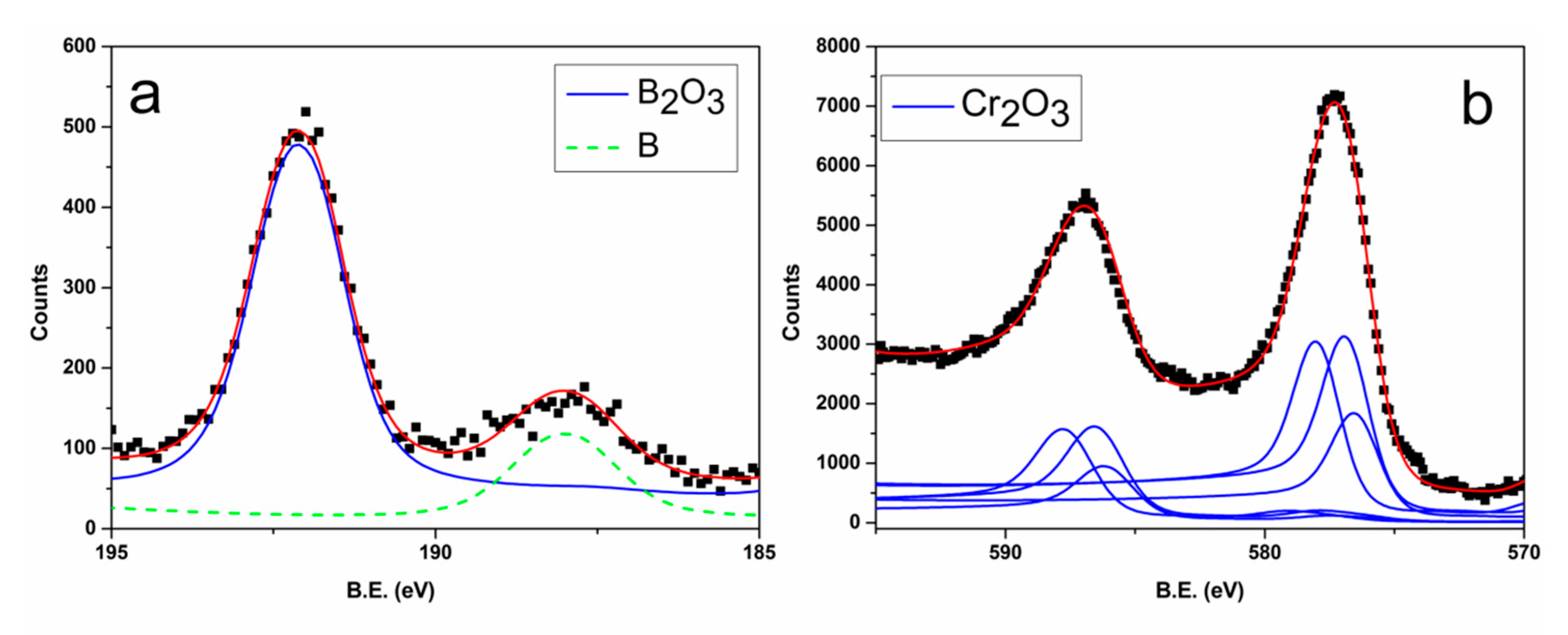
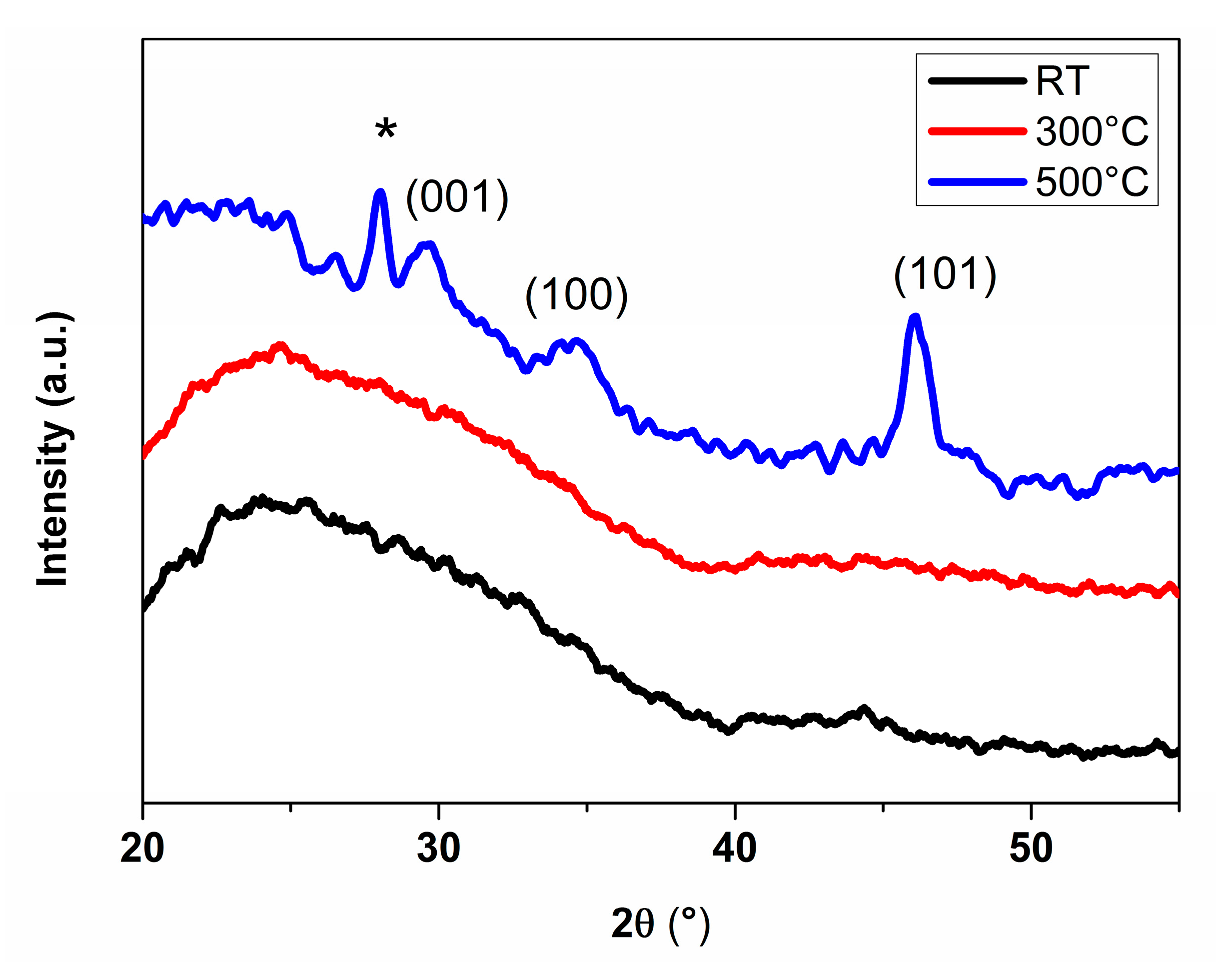
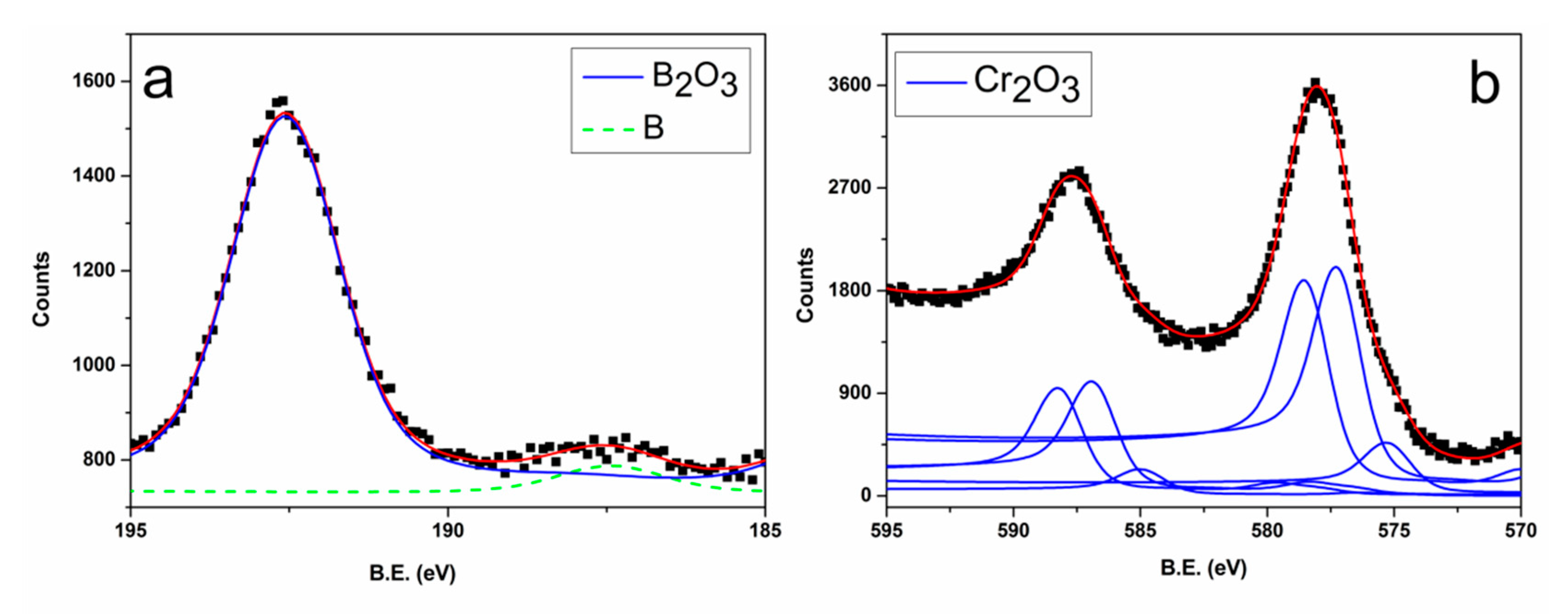
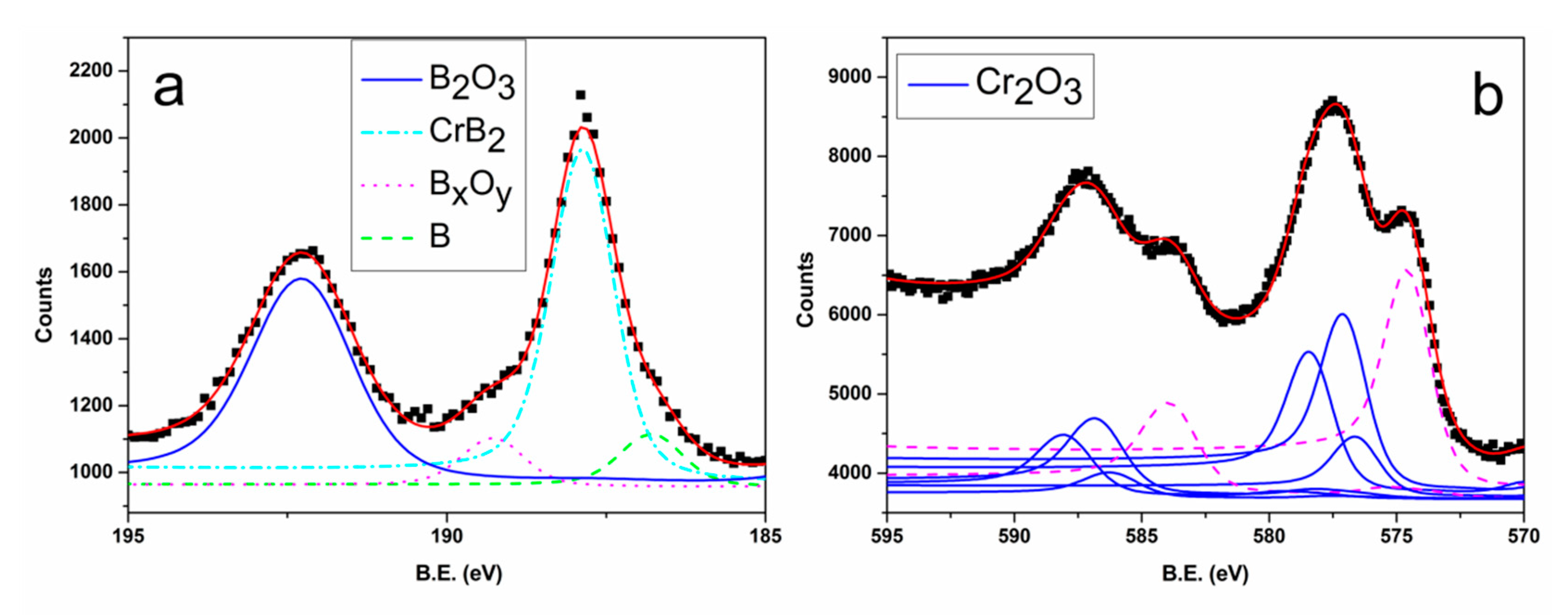
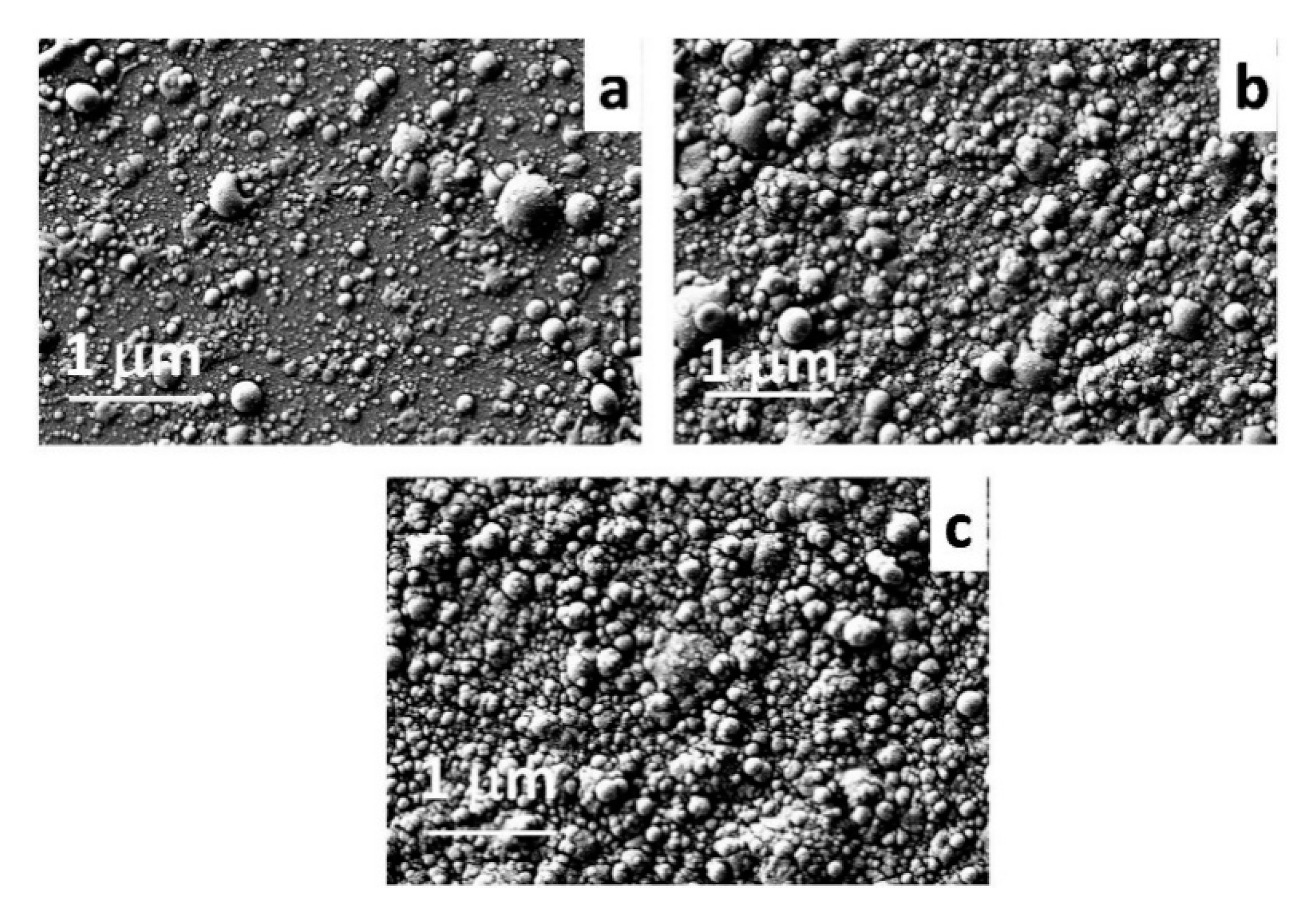
3.1. Films Characterization
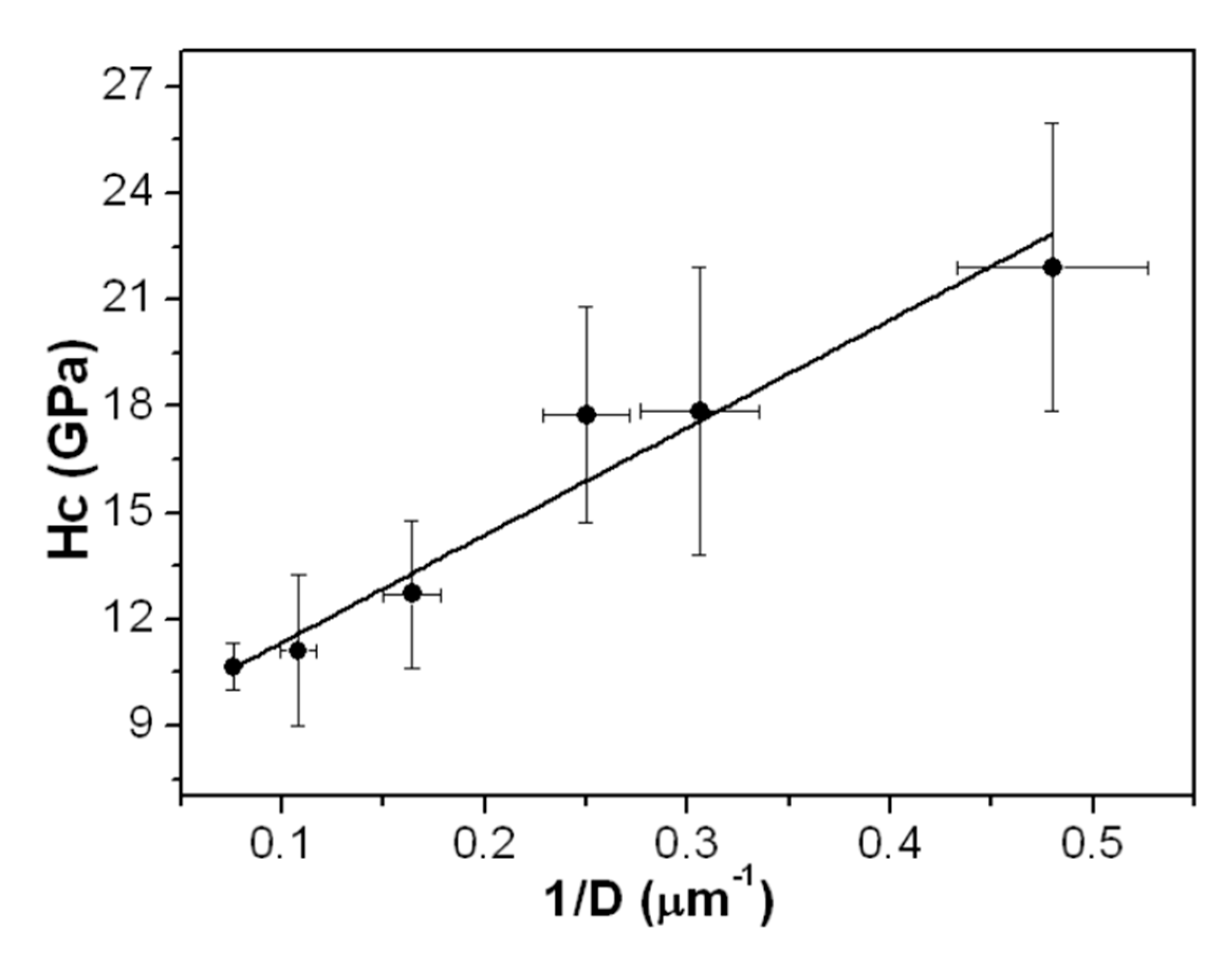
3.2. Films Hardness
3.3. Plasma Analysis
3.4. Ablation-Deposition Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carenco, S.; Portehault, D.; Boissiere, C.; Mezailles, N.; Sanchez, C. Nanoscaled metal borides and phosphides: Recent developments and perspectives. Chem. Rev. 2013, 113, 7981–8065. [Google Scholar] [CrossRef] [PubMed]
- Grechnev, G.E.; Fedorchenko, A.V.; Logosha, A.V.; Panfilov, A.S.; Svechkarev, I.V.; Filippov, V.B.; Lyashchenko, A.B.; Evdokimova, A.V. Electronic structure and magnetic properties of transition metaldi borides. J. Alloy. Compd. 2009, 481, 75–80. [Google Scholar] [CrossRef]
- Wang, S.; Yu, X.; Zhang, J.; Wang, L.; Leinenweber, K.; Xu, H.; Popov, D.; Park, C.; Yang, W.; He, D.; et al. Crystal structures, elastic properties, and hardness of high-pressure synthesized CrB2 and CrB4. J. Superhard Mater. 2014, 36, 279–287. [Google Scholar] [CrossRef]
- Audronis, M.; Kelly, P.J.; Arnell, R.D.; Leyland, A.; Matthews, A. The structure and properties of chromium diboride coatings deposited by pulsed magnetron sputtering of powder targets. Surf. Coat. Technol. 2005, 200, 1366–1371. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, X.; Liang, Y.; Zhang, E.; Gao, H.; Zhang, Z. Preparation and characterization of nanocrystalline chromium boride. J. Mater. Sci. 2006, 41, 7617–7619. [Google Scholar] [CrossRef]
- Dahm, K.L.; Jordan, L.R.; Haase, J.; Dearnley, P.A. Magnetron sputter deposition of chromium diboride coatings. Surf. Coat. Technol. 1998, 108, 413–418. [Google Scholar] [CrossRef]
- Liao, P.K.; Spear, K.E. The B-Cr (boron-chromium) system. Bull. Alloy Phase Diagr. 1986, 7, 232–237. [Google Scholar] [CrossRef]
- Okada, S.; Kudou, K.; Iizumi, K.; Kudaka, K.; Higashi, I.; Lundström, T. Single-crystal growth and properties of CrB, Cr3B4, Cr2B3 and CrB2 from high-temperature aluminum solutions. J. Cryst. Growth 1996, 166, 429–435. [Google Scholar] [CrossRef]
- Nedfors, N.; Primetzhofer, D.; Wang, L.; Lu, J.; Hultman, L.; Jansson, U. Characterization of magnetron sputtered Cr-B and Cr-B-C thin films for electrical contact applications. Surf. Coat. Technol. 2015, 266, 167–176. [Google Scholar] [CrossRef]
- Jordan, L.R.; Betts, A.J.; Dahm, K.L.; Dearnley, P.A.; Wright, G.A. Corrosion and passivation mechanism of chromium diboride coatings on stainless steel. Corros. Sci. 2005, 47, 1085–1096. [Google Scholar] [CrossRef]
- Jha, M.; Marka, S.; Ghanashya, M.; Krishna, M.; Ganguli, A.K. Multifunctional nanocrystalline chromium boride thin films. Mater. Lett. 2012, 73, 220–222. [Google Scholar]
- Wenga, J.; Zuo, X.; Liu, L.; Wang, Z.; Ke, P.; Wei, X.; Wang, A. Dense nanocolumnar structure induced anti-corrosion CrB2 coating with (001) preferred orientation deposited by DC magnetron sputtering. Mater. Lett. 2019, 240, 180–184. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Guo, P.; Ke, P.; Odén, M.; Wang, A. Temperature induced superhard CrB2 coatings with preferred (001) orientation deposited by DC magnetron sputtering technique. In Pulsed Laser Deposition of thin Films; Chrisey, D.B., Hubler, G.K., Eds.; John Wiley & Sons: New York, NY, USA, 1994; pp. 229–264. [Google Scholar]
- Zhou, M.; Nose, M.; Makino, Y.; Nogi, K. New Cr-B hard coatings by r.f.-plasma assisted magnetron sputtering method. Thin Solid Film 1999, 343–344, 234–237. [Google Scholar] [CrossRef]
- Zhou, M.; Nose, M.; Makino, Y.; Nogi, K. Annealing effects on the structure and mechanical properties of r.f.-sputtered Cr-B hard thin films. Thin Solid Film 2000, 359, 165–170. [Google Scholar] [CrossRef]
- Choi, H.S.; Park, B.; Lee, J.J. CrB2 coatings deposited by inductively coupled plasma assisted DC magnetron sputtering. Surf. Coat. Technol. 2007, 202, 982–986. [Google Scholar] [CrossRef]
- Jayaraman, S.; Klein, E.J.; Yang, Y.; Kim, D.Y.; Girolami, G.S.; Abelson, J.R. Chromium diboride thin films by low temperature chemical vapor deposition. J. Vac. Sci. Technol. A 2005, 23, 631–633. [Google Scholar] [CrossRef][Green Version]
- Boubaaya, R.; Benarioua, Y.; Allaoui, O.; Djendel, M. Production of chromium boride layers on carbon steel with conversion treatment: Chromium deposition+diffusion annealing. Acta Phys. Pol. A 2017, 132, 541–543. [Google Scholar] [CrossRef]
- Latini, A.; Rau, J.V.; Ferro, D.; Teghil, R.; Rossi Albertini, V.; Barinov, S.M. Superhard rhenium diboride films: Preparation and characterization. Chem. Mater. 2008, 20, 4507–4511. [Google Scholar] [CrossRef]
- Rau, J.V.; Ferro, D.; Falcone, M.B.; Generosi, A.; Rossi Albertini, V.; Latini, A.; Teghil, R.; Barinov, S.M. Hardness of zirconium diboride films deposited on titanium substrates. Mater. Chem. Phys. 2008, 112, 504–509. [Google Scholar] [CrossRef]
- Rau, J.V.; Latini, A.; Generosi, A.; Rossi Albertini, V.; Ferro, D.; Teghil, R.; Barinov, S.M. Deposition and characterization of superhard biphasic ruthenium borides films. Acta Mater. 2009, 57, 673–681. [Google Scholar] [CrossRef]
- Latini, A.; Rau, J.V.; Teghil, R.; Generosi, A.; Rossi Albertini, V. Superhard properties of rhodium and iridium boride films. ACS Appl. Mater. Interfaces 2010, 2, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Latini, A.; Teghil, R.; DeBonis, A.; Fosca, M.; Caminiti, R.; Rossi Albertini, V. Superhard properties of tungsten tetraboride films. ACS Appl. Mater. Interfaces 2011, 3, 3738–3743. [Google Scholar] [CrossRef] [PubMed]
- De Bonis, A.; Santagata, A.; Rau, J.V.; Latini, A.; Mori, T.; Medici, L.; Teghil, R. Two-phase zirconium boride thin film obtained by ultra-short pulsed laser ablation of a ZrB12 target. App. Surf. Sci. 2013, 283, 715–721. [Google Scholar] [CrossRef]
- Balling, P.; Schou, J. Femtosecond laser ablation dynamics of dielectrics: Basics and applications for thin films. Rep. Prog. Phys. 2013, 76, 036502. [Google Scholar] [CrossRef]
- D’Alessio, L.; Teghil, R.; Zaccagnino, M.; Zaccardo, I.; Ferro, D.; Marotta, V. Pulsed laser ablation and deposition of bioactive glass as coating material for biomedical applications. Appl. Surf. Sci. 1999, 138/139, 527–532. [Google Scholar]
- Joensson, B.; Hogmark, S. Hardness measurements of thin films. Thin Solid Film 1984, 114, 257–269. [Google Scholar] [CrossRef]
- Ferro, D.; Barinov, S.M.; Rau, J.V.; Latini, A.; Scandurra, R.; Brunetti, B. Vickers and Knoop hardness of electron beam deposited ZrC and HfC thin films on titanium. Surf. Coat. Technol. 2006, 200, 4701–4707. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy (XPS) Database; Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012.
- Ennaceur, M.M.; Terreault, B. XPS study of the process of oxygen gettering by thin films of PACVD boron. J. Nucl. Mater. 2000, 280, 33–38. [Google Scholar] [CrossRef]
- Li, R.; Dong, G.; Chen, G. Synthesis, characterization and performance of ternary doped Cu-Ce-B/TiO2 nanotubes on the photocatalytic removal of nitrogen oxides. New J. Chem. 2015, 39, 6854–6863. [Google Scholar] [CrossRef]
- Xu, T.T.; Zheng, J.; Wu, N.; Nicholls, A.W.; Roth, J.R.; Dikin, D.A.; Ruoff, R.S. Crystalline boron nanoribbons: synthesis and characterization. Nano Lett. 2004, 4, 963–968. [Google Scholar] [CrossRef]
- Unveren, E.; Kemnitz, E.; Hutton, S.; Lippitz, A.; Unger, W.E.S. Analysis of highly resolved X-ray photoelectron Cr2p spectra obtained with a Cr2O3 powder sample prepared with adhesive tape. Surf. Interface Anal. 2004, 36, 92–95. [Google Scholar] [CrossRef]
- Payne, B.P.; Biesinger, M.C.; McIntyre, N.S. X-ray photoelectron spectroscopy studies of reactions on chromium metal and chromium oxide surfaces. J. Electron Spectrosc. 2011, 184, 29–37. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Brown, C.; Mycroft, J.R.; Davidson, R.D.; McIntyre, N.S. X-ray photoelectron spectroscopy studies of chromium compounds. Surf. Interface Anal. 2004, 36, 1550–1563. [Google Scholar] [CrossRef]
- Perrière, J.S.; Millon, E.; Craciun, V. ZnO and ZnO-related Compounds. In Pulsed Laser Deposition of Thin Films; Eason, R., Ed.; John Wiley & Sons: Hoboken, NY, USA, 2007; pp. 261–290. [Google Scholar]
- O’Mahony, D.; Lunney, J.G. Group III Nitride Growth. In Pulsed Laser Deposition of Thin Films; Eason, R., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 291–312. [Google Scholar]
- Kabekkodu, S. ICDD (2005), PDF4 (Database); International Centre for Diffraction Data: Newtown Square, PA, USA, 2005. [Google Scholar]
- Salvi, A.M.; Castle, J.E.; Watts, J.F.; Desimoni, E. Peak fitting of the chromium 2p XPS spectrum. Appl. Surf. Sci. 1995, 90, 333–341. [Google Scholar] [CrossRef]
- Steinberger, R.; Duchoslav, J.; Greunz, T.; Arndt, M.; Stifter, D. Investigation of the chemical stability of different Cr(VI) based compounds during regular X-Ray photoelectron spectroscopy measurements. Corros. Sci. 2015, 90, 562–571. [Google Scholar] [CrossRef]
- Kelly, D.; Jung, D.R. Elemental chromium by XPS. Surf. Sci. Spectra 1998, 5, 130–137. [Google Scholar] [CrossRef]
- Mavel, G.; Escard, J.; Costa, P.; Castaing, J. ESCA surface study of metal borides. Surf. Sci. 1973, 35, 109–116. [Google Scholar] [CrossRef]
- Sakamaoto, Y.; Nose, M.; Mae, T.; Honbo, E.; Zhou, M.; Nogi, K. Structure and properties of Cr–B, Cr–B–N and multilayer Cr–B/Cr–B–N thin films prepared by r.f.-sputtering. Surf. Coat. Technol. 2003, 174/175, 444–449. [Google Scholar] [CrossRef]
- De Bonis, A.; Teghil, R.; Rau, J.V.; Galasso, A.; Orlando, S.; Santagata, A. Characterization of the gaseous phase produced by ultra-short Pulsed Laser Ablation of transition metal borides. Appl. Surf. Sci. 2011, 257, 5315–5318. [Google Scholar] [CrossRef]
- De Bonis, A.; Galasso, A.; Santagata, A.; Teghil, R. Ultrashort Pulsed Laser Ablation of magnesium diboride: Plasma characterization and thin films deposition. J. Nanomater. 2015, 2015, 596328. [Google Scholar] [CrossRef]
- Albert, O.; Roger, S.; Glinec, Y.; Loulergue, J.C.; Etchepare, J.; Boulmer-Leborgne, C.; Perrière, J.; Millon, E. Time-resolved spectroscopy measurements of a titanium plasma induced by nanosecond and femtosecond lasers. Appl. Phys. A 2003, 76, 319–323. [Google Scholar] [CrossRef]
- Teghil, R.; D’Alessio, L.; De Bonis, A.; Galasso, A.; Ibris, N.; Salvi, A.M.; Santagata, A.; Villani, P. Nanoparticles and thin film formation in ultrashort pulsed laser deposition of vanadium oxide. J. Phys. Chem. A 2009, 113, 14969–14974. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons: NewYork, NY, USA, 1983; pp. 287–324. [Google Scholar]
- Teghil, R.; Santagata, A.; De Bonis, A.; Galasso, A.; Villani, P. Chromium carbide thin films deposited by ultra-short pulse laser deposition. Appl. Surf. Sci. 2009, 255, 7729–7733. [Google Scholar] [CrossRef]
- Saenger, K.I. Angular distibution of ablated material. In Pulsed Laser Deposition of Thin Films; Chrisey, D.B., Hubler, G.K., Eds.; John Wiley & Sons: New York, NY, USA, 1994; pp. 199–225. [Google Scholar]
- Santagata, A.; Marotta, V.; D’Alessio, L.; Teghil, R.; Ferro, D.; DeMaria, G. Study of the gaseous phase from pulsed laser ablation of titanium carbide. Appl. Surf. Sci. 1997, 109/110, 376–379. [Google Scholar] [CrossRef]
- Boulmer-Leborgne, C.; Benzerga, R.; Perrière, J. Nanoparticle formation by femtosecond laser ablation. In Laser-Surface Interactions for New Materials Production; Miotello, A., Ossi, P.M., Eds.; Springer: Heidelberg, Germany, 2010; pp. 125–140. [Google Scholar]
- Perriere, J.; Boulmer-Leborgne, C.; Benzerga, R.; Tricot, S. Nanoparticle formation by femtosecond laser ablation. J. Phys. D 2007, 40, 7069–7076. [Google Scholar] [CrossRef]
- Schou, J.; Amoruso, S.; Lunney, J.G. Plume dynamics. In Laser Ablation and Its Applications; Phypps, C., Ed.; Springer: NewYork, NY, USA, 2007; pp. 67–95. [Google Scholar]
- O’Connell, G.; Donnelly, T.; Lunney, J.G. Nanoparticle plume dynamics in femtosecond laser ablation of gold. Appl. Phys. A 2014, 117, 289–293. [Google Scholar] [CrossRef]
- Tsakiris, N.; Anoop, K.K.; Ausanio, G.; Gill-Comeau, M.; Bruzzese, R.; Amoruso, S.; Lewis, J. Ultrashort laser ablation of bulk copper argets: Dynamics and size distribution of the generated nanoparticles. J. Appl. Phys. 2014, 115, 243301. [Google Scholar] [CrossRef]
- Teghil, R.; De Bonis, A.; Galasso, A.; Santagata, A.; Villani, P.; Sordelet, D.J. Role and importance of nanoparticles in femtosecond pulsed laser ablation deposition of Al-Cu-Fe quasicrystal. Chem. Phys. Lett. 2007, 438, 85–88. [Google Scholar] [CrossRef]
- Howig, R.E. Vapor pressure data for the elements. In The Characterization of High-Temperature Vapors; Margrave, J.L., Ed.; Wiley & Sons: New York, NY, USA, 1967; pp. 475–494. [Google Scholar]
- Horwitz, J.S.; Sprague, J.A. Film nucleation and growth. In Pulsed Laser Deposition of Thin Films; Chrisey, D.B., Hubler, G.K., Eds.; John Wiley & Sons: New York, NY, USA, 1994; pp. 229–264. [Google Scholar]
- Trelenberg, T.W.; Dinh, L.N.; Stuart, B.C.; Balooch, M. Femtosecond pulsed laser ablation of metal alloy and semiconductor targets. Appl. Surf. Sci. 2004, 229, 268–274. [Google Scholar] [CrossRef]
- Povarnitsyn, M.E.; Itina, T.E.; Sentis, M.; Khishchenko, K.V.; Levashov, P.R. Material decomposition mechanisms in femtosecond laser interactions with metals. Phys. Rev. B 2007, 75, 235414. [Google Scholar] [CrossRef]
- Thornton, J.A. Structure and topography of sputtered coatings. J. Vac. Sci. Technol. 1974, 11, 666–670. [Google Scholar] [CrossRef]
- Hubler, G.K. Comparison of vacuum deposition techniques. In Pulsed Laser Deposition of Thin Films; Chrisey, D.B., Hubler, G.K., Eds.; John Wiley & Sons: New York, NY, USA, 1994; pp. 327–355. [Google Scholar]
- Thornton, J.A. The microstructure of sputter deposited coatings. J. Vac. Sci. Technol. A 1986, 4, 3059–3065. [Google Scholar] [CrossRef]
- Movchan, B.A.; Demchishin, A.V. Structure and properties of thick condensates of nickel, titanium, tungsten, aluminum oxides and zirconium dioxide in vacuum. Fiz. Metal. Metalloved. 1969, 28, 653–660. [Google Scholar]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bonis, A.; Galasso, A.; Latini, A.; Rau, J.V.; Santagata, A.; Curcio, M.; Teghil, R. Femtosecond Pulsed Laser Deposition of Chromium Diboride-Rich Thin Films. Coatings 2019, 9, 777. https://doi.org/10.3390/coatings9120777
De Bonis A, Galasso A, Latini A, Rau JV, Santagata A, Curcio M, Teghil R. Femtosecond Pulsed Laser Deposition of Chromium Diboride-Rich Thin Films. Coatings. 2019; 9(12):777. https://doi.org/10.3390/coatings9120777
Chicago/Turabian StyleDe Bonis, Angela, Agostino Galasso, Alessandro Latini, Julietta V. Rau, Antonio Santagata, Mariangela Curcio, and Roberto Teghil. 2019. "Femtosecond Pulsed Laser Deposition of Chromium Diboride-Rich Thin Films" Coatings 9, no. 12: 777. https://doi.org/10.3390/coatings9120777
APA StyleDe Bonis, A., Galasso, A., Latini, A., Rau, J. V., Santagata, A., Curcio, M., & Teghil, R. (2019). Femtosecond Pulsed Laser Deposition of Chromium Diboride-Rich Thin Films. Coatings, 9(12), 777. https://doi.org/10.3390/coatings9120777










