One-Step Method for Preparing Dispersive Tea Polyphenol/Graphene Nanosheets Enhanced with Anticorrosion Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
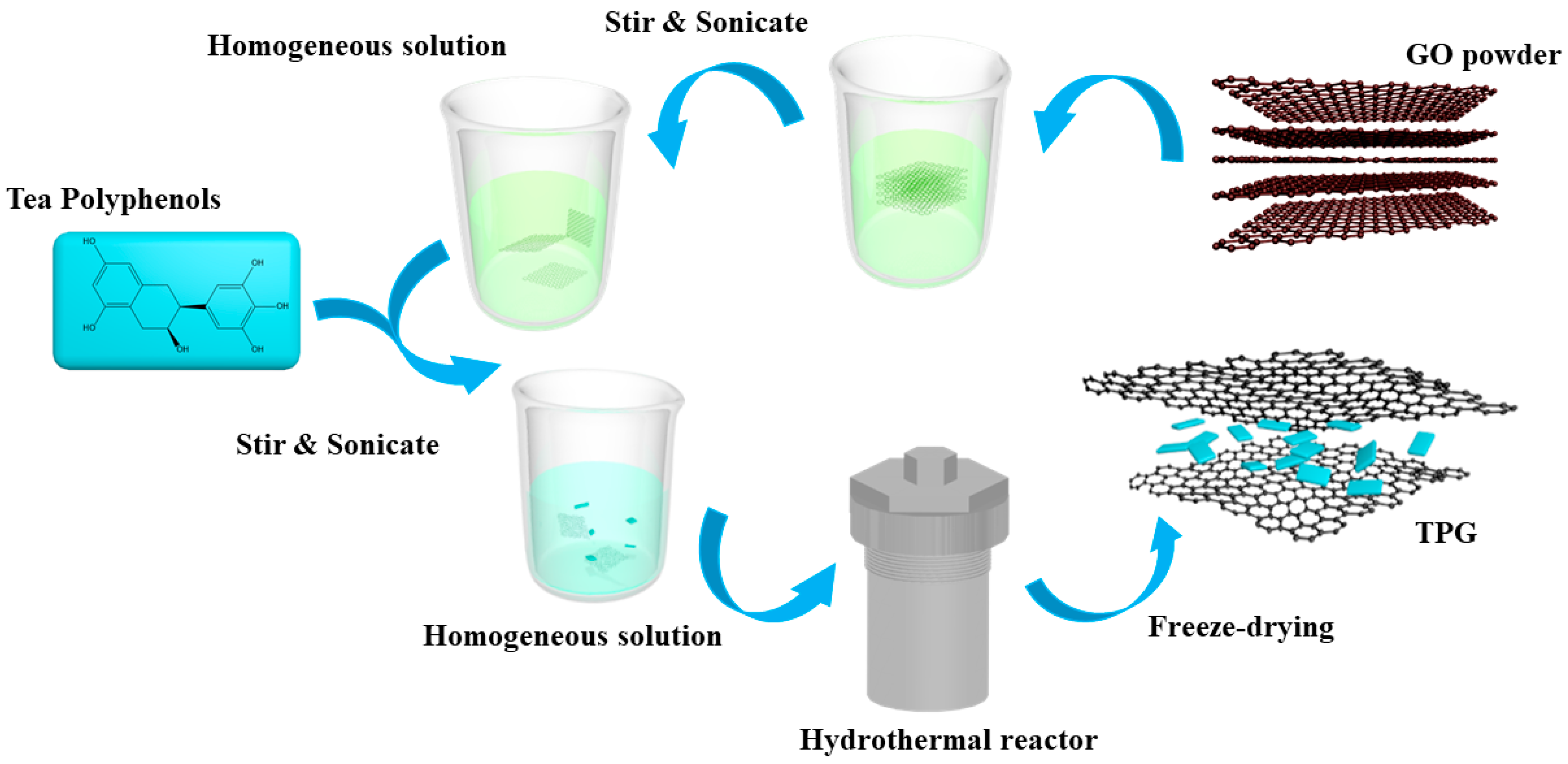
2.2. Synthesis of TP/GE (Graphene) Nanocomposites
2.3. Composite-Coating Manufacture
2.4. Material Characterization
2.5. Electrochemical Experiment
3. Results and Discussion
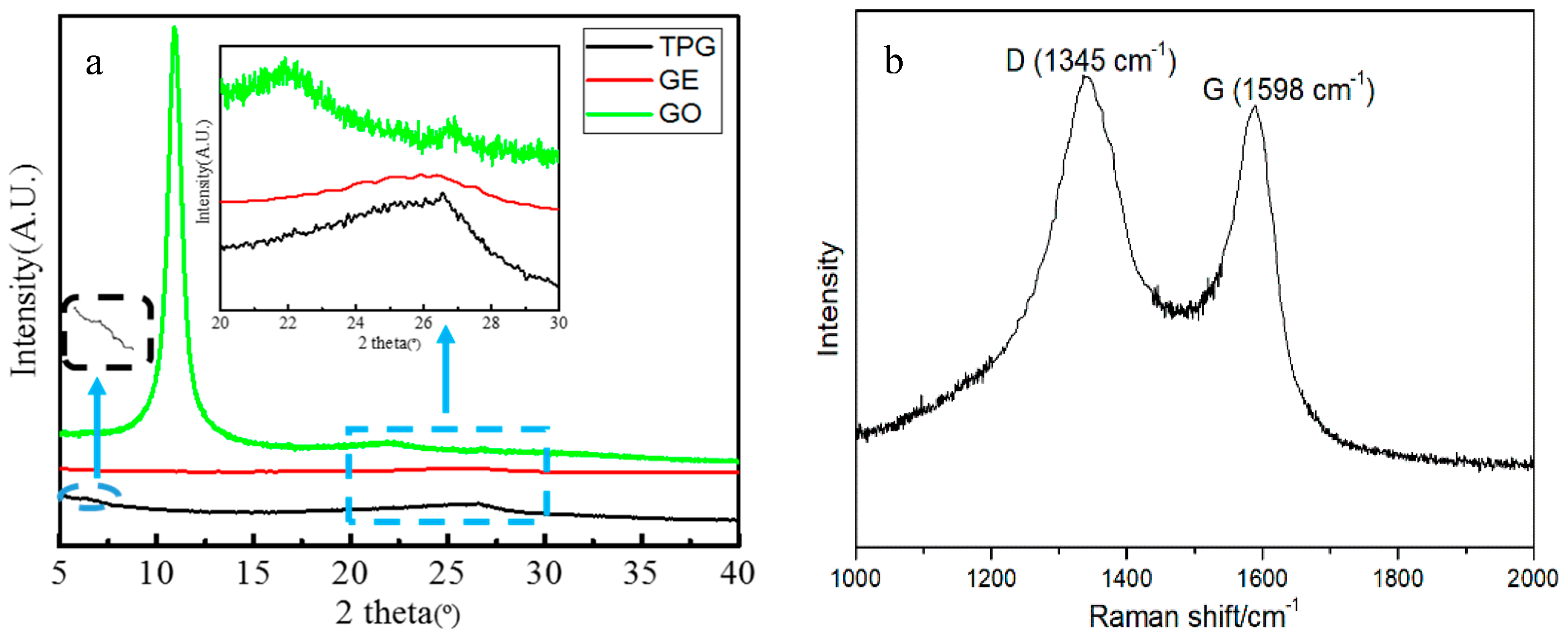
3.1. Structural Analysis of the TP/GE Composite Material
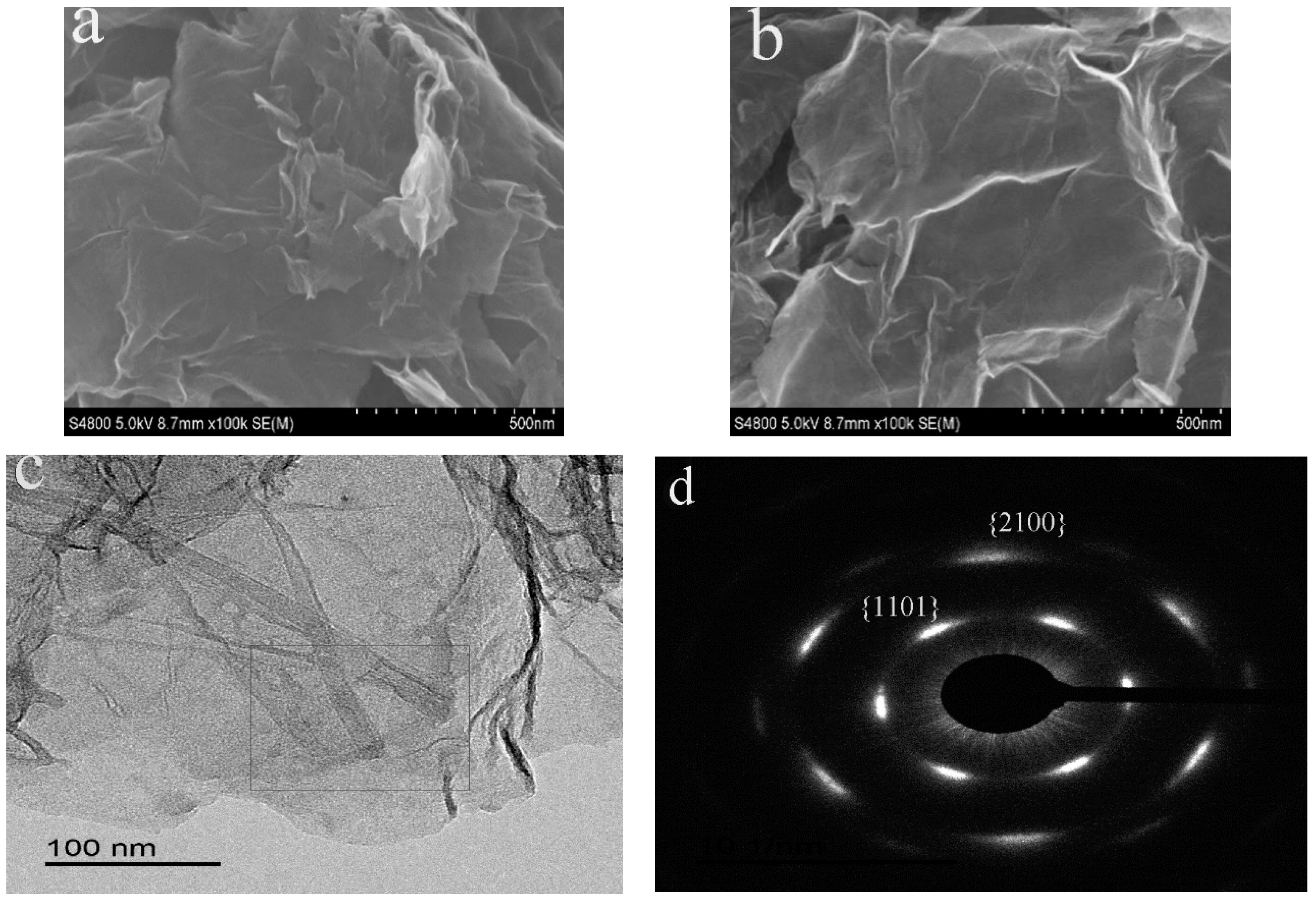
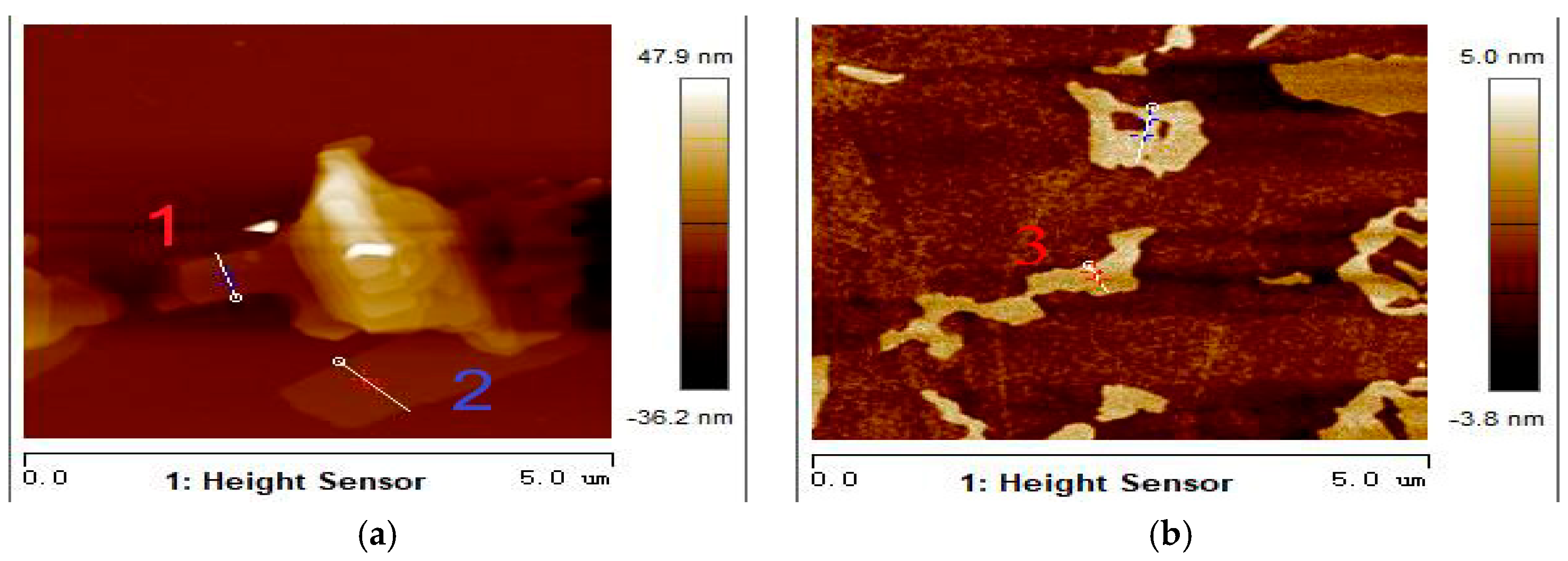
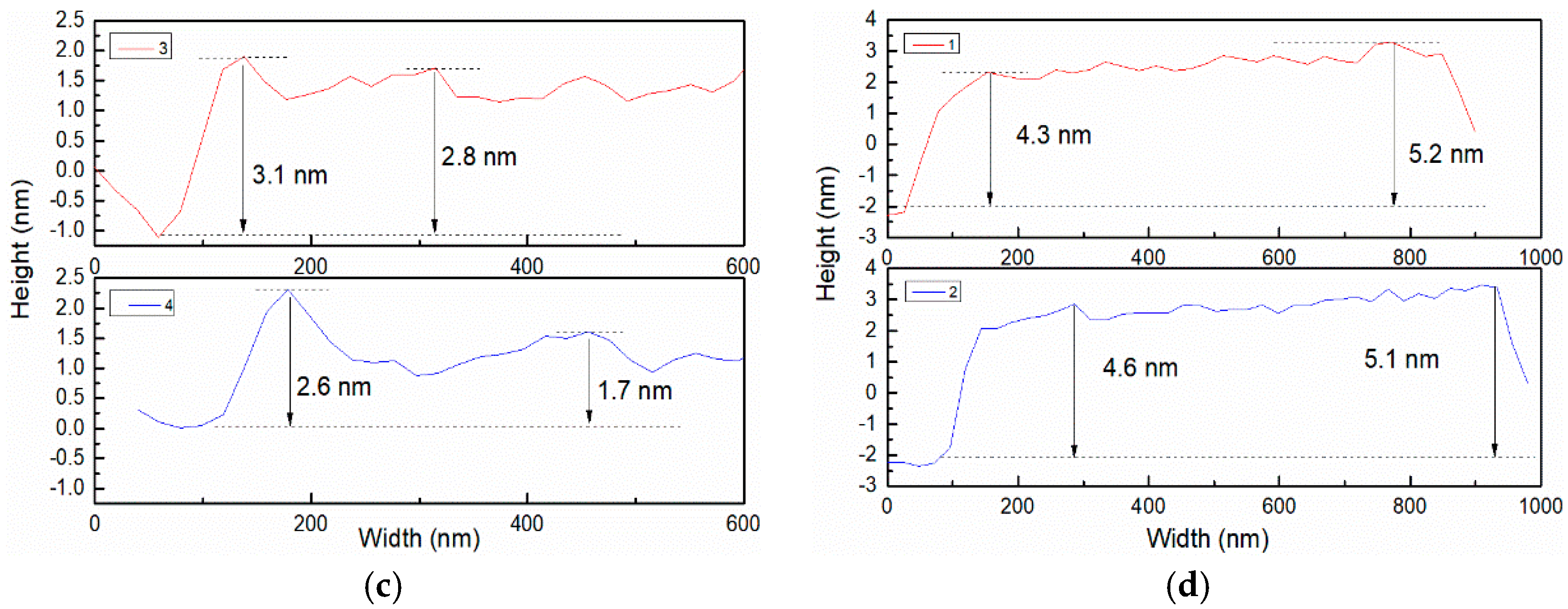
3.2. Morphology Analysis
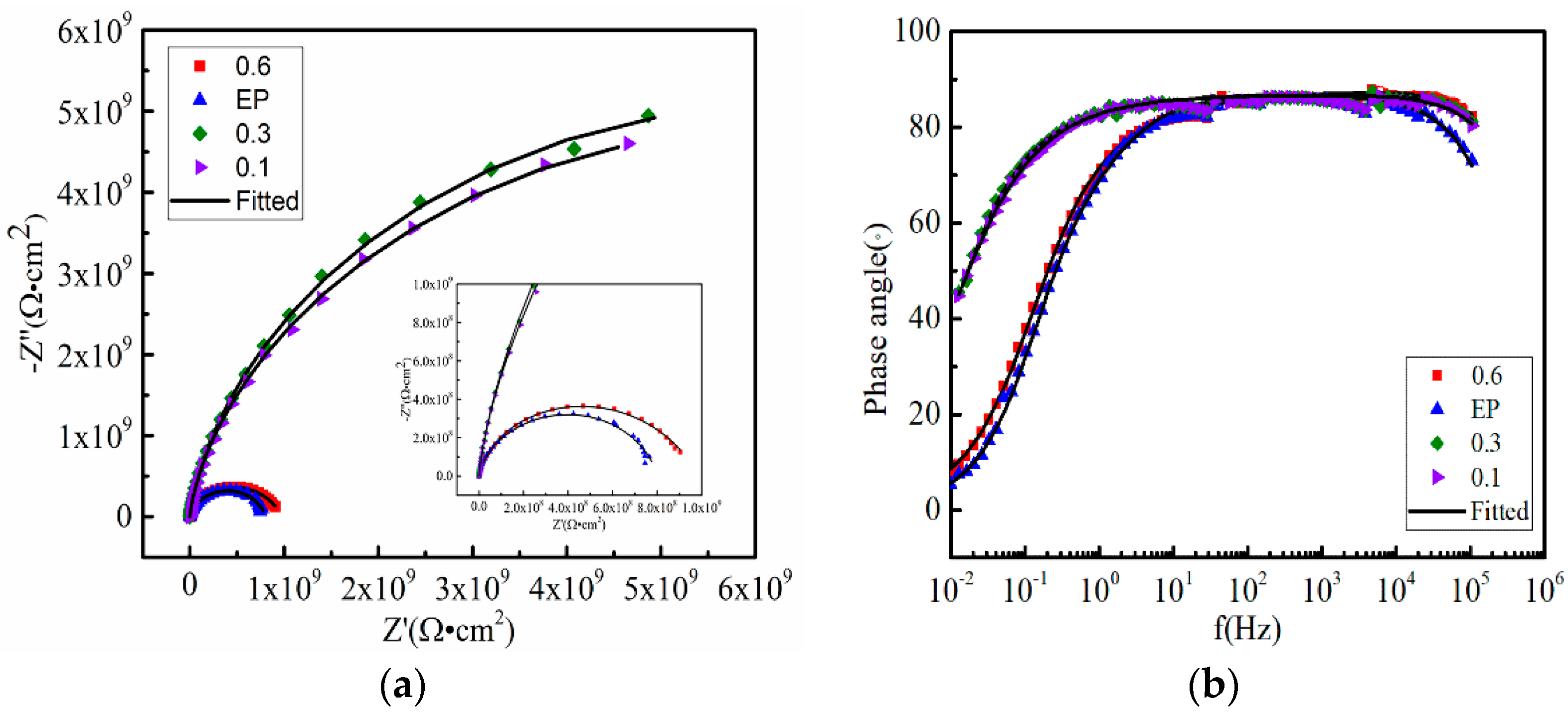
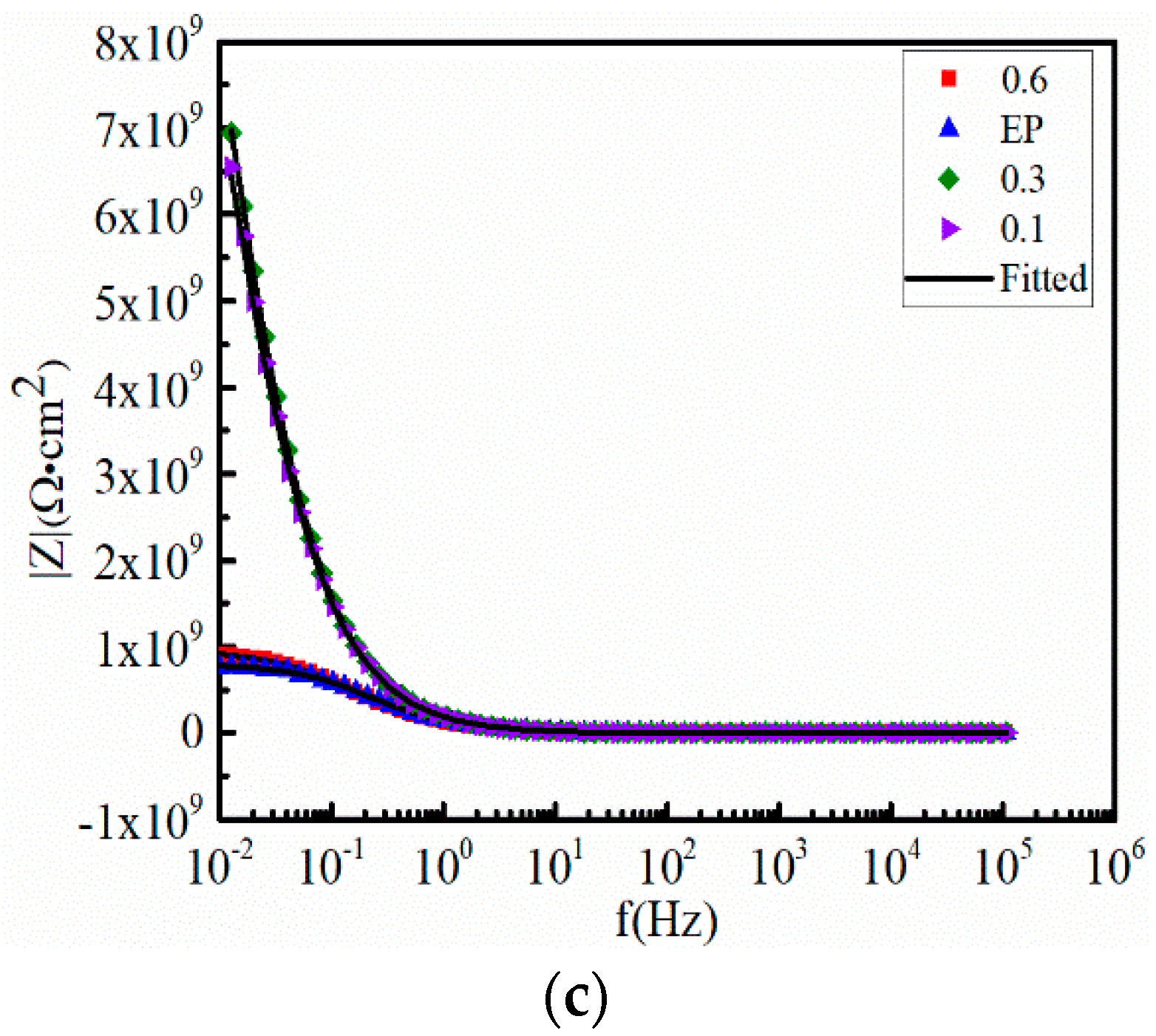
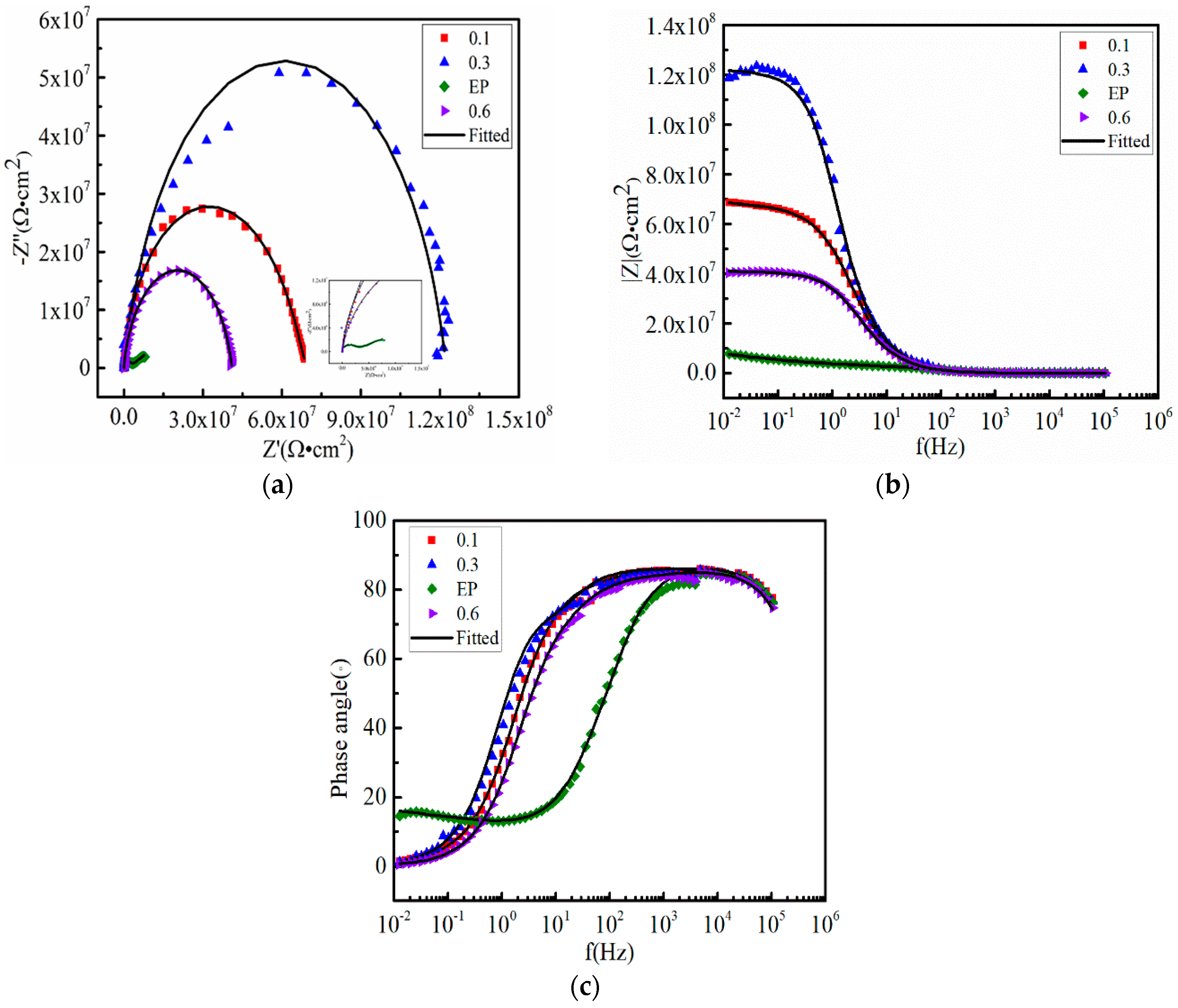
3.3. Anticorrosive Performance
3.4. Polarization-Curve Test
3.5. Spray Test for the Hybrid Coating
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Bohm, S. Graphene against corrosion. Nat. Nanotechnol. 2014, 9, 741–742. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.Y.; Lee, H.-J.; Go, H.-A.; Lee, Y.B.; Bae, T.S.; Kim, J.K.; Choi, W.S. Graphene-based multifunctional iron oxide nanosheets with tunable properties. Chem. Eur. J. 2011, 17, 1214–1219. [Google Scholar] [CrossRef]
- Parhizkar, N.; Shahrabi, T.; Ramezanzadeh, B. A new approach for enhancement of the corrosion protection properties and interfacial adhesion bonds between the epoxy coating and steel substrate through surface treatment by covalently modified amino functionalized graphene oxide film. Corros. Sci. 2017, 123, 55–75. [Google Scholar] [CrossRef]
- Mogera, U.; Kurra, N.; Radhakrishnan, D.; Narayana, C.; Kulkarni, G.U. Low cost, rapid synthesis of graphene on Ni: An efficient barrier for corrosion and thermal oxidation. Carbon 2014, 78, 384–391. [Google Scholar] [CrossRef]
- Prasai, K.; Chen, G.; Drabold, D.A. Amorphous to amorphous insulator-metal transition in GeSe3:Ag glasses. Phys. Rev. Mater. 2017, 1, 015603. [Google Scholar] [CrossRef]
- Ersan, G.; Apul, O.G.; Perreault, F.; Karanfil, T. Adsorption of organic contaminants by graphene nanosheets: A review. Water Res. 2017, 126, 385–398. [Google Scholar] [CrossRef]
- Chen, C.; He, Y.; Xiao, G.; Wu, Y.; He, Z.; Zhong, F. Zirconia doped in carbon fiber by electrospinning method and improve the mechanical properties and corrosion resistance of epoxy. Prog. Org. Coat. 2018, 125, 420–431. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S. Graphene nanosheet: Synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem. Soc. Rev. 2011, 40, 2644–2672. [Google Scholar] [CrossRef]
- Chen, B.; Ni, B.-J.; Liu, W.-T.; Ye, Q.-Y.; Liu, S.-Y.; Zhang, H.-X.; Yoon, K.-B. Mechanical properties of epoxy nanocomposites filled with melamine functionalized molybdenum disulfide. RSC Adv. 2018, 8, 20450–20455. [Google Scholar] [CrossRef]
- Eksik, O.; Gao, J.; Shojaee, S.A.; Thomas, A.; Chow, P.; Bartolucci, S.F.; Lucca, D.A.; Koratkar, N. Epoxy nanocomposites with two-dimensional transition metal dichalcogenide additives. ACS Nano 2014, 8, 5282–5289. [Google Scholar] [CrossRef]
- Ghanbari, A.; Attar, M.M. A study on the anticorrosion performance of epoxy nanocomposite coatings containing epoxy-silane treated nano-silica on mild steel substrate. J. Ind. Eng. Chem. 2015, 23, 145–153. [Google Scholar] [CrossRef]
- An, A.K.; Guo, J.; Lee, E.-J.; Jeong, S.; Zhao, Y.; Wang, Z.; Leiknes, T. PDMS/PVDF hybrid electrospun membrane with superhydrophobic property and drop impact dynamics for dyeing wastewater treatment using membrane distillation. J. Membr. Sci. 2017, 525, 57–67. [Google Scholar] [CrossRef]
- Gong, G.; Gao, K.; Wu, J.; Sun, N.; Zhou, C.; Zhao, Y.; Jiang, L. A highly durable silica/polyimide superhydrophobic nanocomposite film with excellent thermal stability and abrasion-resistant performance. J. Mater. Chem. A 2015, 3, 713–718. [Google Scholar] [CrossRef]
- Mao, J.; Ge, M.; Huang, J.; Lai, Y.; Lin, C.; Zhang, K.; Meng, K.; Tang, Y. Constructing multifunctional MOF@rGO hydro-/aerogels by the self-assembly process for customized water remediation. J. Mater. Chem. A 2017, 5, 11873–11881. [Google Scholar] [CrossRef]
- Nine, M.J.; Cole, M.A.; Tran, D.N.H.; Losic, D. Graphene: A multipurpose material for protective coatings. J. Mater. Chem. A 2015, 3, 12580–12602. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, C.; Song, L.; Ma, Y.; Ali, Z.; Gu, J.; Zhang, B.; Zhang, H.; Zhang, Q. A stable 3D sol-gel network with dangling fluoroalkyl chains and rapid self-healing ability as a long-lived superhydrophobic fabric coating. Chem. Eng. J. 2018, 334, 598–610. [Google Scholar] [CrossRef]
- Lin, Y.; Han, J.; Cai, M.; Liu, W.; Luo, X.; Zhang, H.; Zhong, M. Durable and robust transparent superhydrophobic glass surfaces fabricated by a femtosecond laser with exceptional water repellency and thermostability. J. Mater. Chem. A 2018, 6, 9049–9056. [Google Scholar] [CrossRef]
- Seo, J.-W.T.; Green, A.A.; Antaris, A.L.; Hersam, M.C. High-concentration aqueous dispersions of graphene using nonionic, biocompatible block copolymers. J. Phys. Chem. Lett. 2011, 2, 1004–1008. [Google Scholar] [CrossRef]
- Shang, W.; Wu, F.; Wen, Y.; He, C.; Zhan, X.; Li, Y. Corrosion resistance and mechanism of graphene oxide composite coatings on magnesium alloy. Ind. Eng. Chem. Res. 2018, 58, 1200–1211. [Google Scholar] [CrossRef]
- Shen, L.; Li, Y.; Zhao, W.; Miao, L.; Xie, W.; Lu, H.; Wang, K. Corrosion protection of graphene-modified zinc-rich epoxy coatings in dilute nacl solution. ACS Appl. Nano Mater. 2018, 2, 180–190. [Google Scholar] [CrossRef]
- Wu, Y.Q.; He, Y.; Chen, C.L.; Li, H.J.; Xia, Y.Q.; Zhou, T.G. MoS2-CNFs composites to enhance the anticorrosive and mechanical performance of epoxy coating. Prog. Org. Coat. 2019, 129, 178–186. [Google Scholar] [CrossRef]
- Xia, Y.; He, Y.; Chen, C.; Wu, Y.; Chen, J. MoS2 nanosheets modified SiO2 to enhance the anticorrosive and mechanical performance of epoxy coating. Prog. Org. Coat. 2019, 132, 316–327. [Google Scholar] [CrossRef]
- Wu, F.; Xie, A.; Sun, M.; Wang, Y.; Wang, M. Reduced graphene oxide (RGO) modified spongelike polypyrrole (PPy) aerogel for excellent electromagnetic absorption. J. Mater. Chem. A 2015, 3, 14358–14369. [Google Scholar] [CrossRef]
- Baux, J.; Causse, N.; Esvan, J.; Delaunay, S.; Tireau, J.; Roy, M.; You, D.; Pebere, N. Impedance analysis of film-forming amines for the corrosion protection of a carbon steel. Electrochim. Acta 2018, 283, 699–707. [Google Scholar] [CrossRef]
- Chen, C.; He, Y.; Xiao, G.; Xia, Y.; Li, H.; He, Z. Two-dimensional hybrid materials: MoS2-RGO nanocomposites enhanced the barrier properties of epoxy coating. Appl. Surf. Sci. 2018, 444, 511–521. [Google Scholar] [CrossRef]
- Tian, S.; Sun, J.; Yang, S.; He, P.; Wang, G.; Di, Z.; Ding, G.; Xie, X.; Jiang, M. Controllable edge oxidation and bubbling exfoliation enable the fabrication of high quality water dispersible graphene. Sci. Rep. 2016, 6, 34127. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, Q.; Hsu, H.S.; Raina, S.; Xu, Y.; Kang, J.B.; Chen, J.; Deng, S.; Xu, N.; Kang, W.P. Morphology effect of vertical graphene on the high performance of supercapacitor electrode. ACS Appl. Mater. Interfaces 2016, 8, 7363–7369. [Google Scholar] [CrossRef]
- Song, H.; Hao, L.; Tian, Y.; Wan, X.; Zhang, L.; Lv, Y. Stable and water-dispersible graphene nanosheets: Sustainable preparation, functionalization, and high-performance adsorbents for Pb2+. ChemPlusChem 2012, 77, 379–386. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, C.; Li, X.; Liu, Z. In situ green synthesis of Ag nanoparticles on tea polyphenols-modified graphene and their catalytic reduction activity of 4-nitrophenol. Colloids Surf. Physicochem. Eng. Asp. 2015, 485, 102–110. [Google Scholar] [CrossRef]
- Akhavan, O.; Kalaee, M.; Alavi, Z.S.; Ghiasi, S.M.A.; Esfandiar, A. Increasing the antioxidant activity of green tea polyphenols in the presence of iron for the reduction of graphene oxide. Carbon 2012, 50, 3015–3025. [Google Scholar] [CrossRef]
- Pant, B.; Saud, P.S.; Park, M.; Park, S.J.; Kim, H.Y. General one-pot strategy to prepare Ag-TiO2 decorated reduced graphene oxide nanocomposites for chemical and biological disinfectant. J. Alloys Compd. 2016, 671, 51–59. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.J.; Kim, H.Y. One-pot synthesis of CdS sensitized TiO2 decorated reduced graphene oxide nanosheets for the hydrolysis of ammonia-borane and the effective removal of organic pollutant from water. Ceram. Int. 2016, 42, 15247–15252. [Google Scholar] [CrossRef]
- Pant, B.; Pokharel, P.; Tiwari, A.P.; Saud, P.S.; Park, M.; Ghouri, Z.K.; Choi, S.; Park, S.J.; Kim, H.Y. Characterization and antibacterial properties of aminophenol grafted and Ag NPs decorated graphene nanocomposites. Ceram. Int. 2015, 41, 5656–5662. [Google Scholar] [CrossRef]
- Ding, R.; Li, W.; Wang, X.; Gui, T.; Li, B.; Han, P.; Tian, H.; Liu, A.; Wang, X.; Liu, X.; et al. A brief review of corrosion protective films and coatings based on graphene and graphene oxide. J. Alloys Compd. 2018, 764, 1039–1055. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.J.; Li, Y.M.; Wang, Y.; Li, J.H. P25-graphene composite as a high performance photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Zhao, H.; Xue, Q.; Wang, L. Polydopamine coated graphene oxide for anticorrosive reinforcement of water-borne epoxy coating. Chem. Eng. J. 2018, 335, 255–266. [Google Scholar] [CrossRef]
- Faure, M.; Billon, F.; Haghiri-Gosnet, A.M.; Tribollet, B.; Deslouis, C.; Pailleret, A.; Gamby, J. Influence of the atomic nitrogen content in amorphous carbon nitride thin films on the modulation of their polarizable interfaces properties. Electrochim. Acta 2018, 280, 238–247. [Google Scholar] [CrossRef]
- Jayaraj, J.; Raj, S.A.; Srinivasan, A.; Ananthakumar, S.; Pillai, U.T.S.; Dhaipule, N.G.K.; Mudali, U.K. Composite magnesium phosphate coatings for improved corrosion resistance of magnesium AZ31 alloy. Corros. Sci. 2016, 113, 104–115. [Google Scholar] [CrossRef]

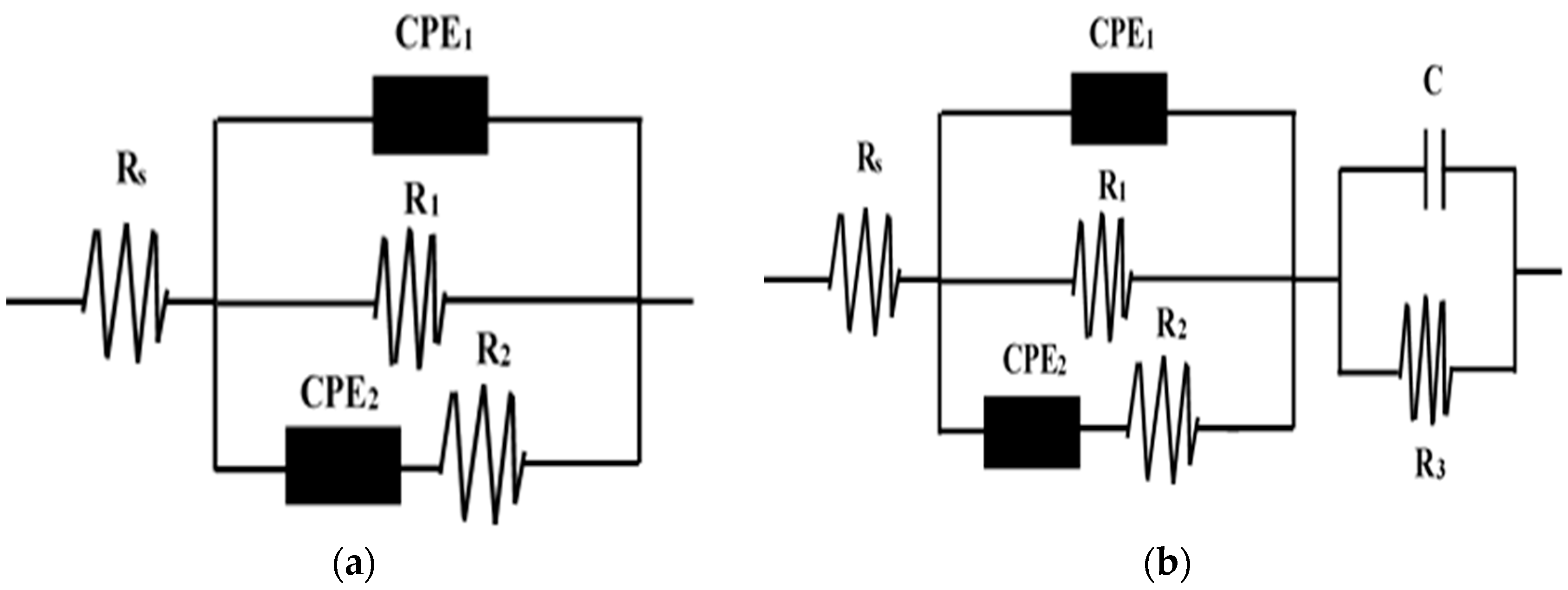
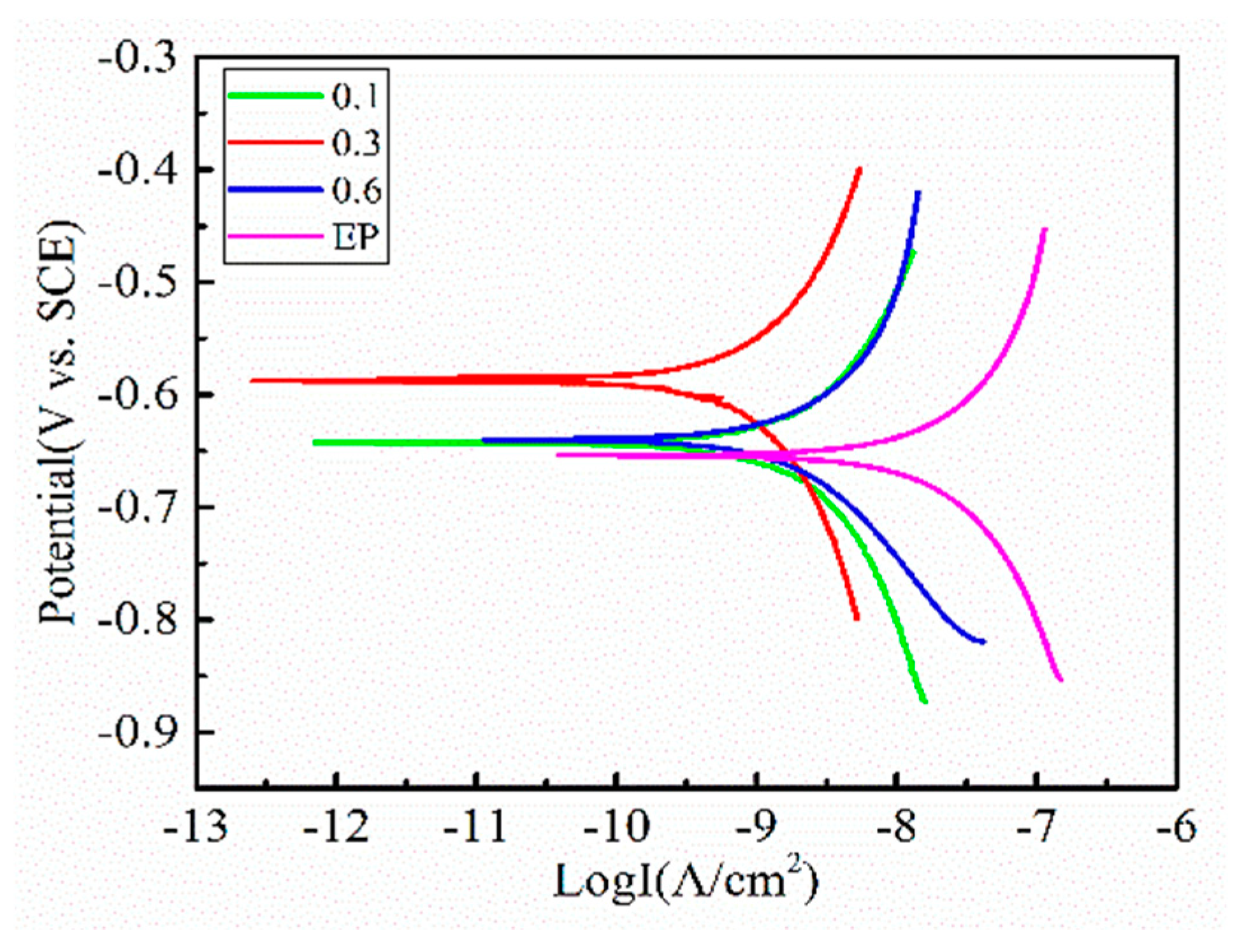

| System | Ecorr (V) | βa (mV) | βc (mV) | Icorr (A/cm2) | Corrosion Rate (mm/a) |
|---|---|---|---|---|---|
| Epoxy | −0.65305 | 154.18 | 156.02 | 1.5896 × 10−8 | 1.8648 × 10−4 |
| TP/GE (0.1 wt.%) | −0.64122 | 110.24 | 131.21 | 1.2515 × 10−9 | 1.4682 × 10−5 |
| TP/GE (0.3 wt.%) | −0.58949 | 148.15 | 146.83 | 6.1457 × 10−10 | 7.2096 × 10−6 |
| TP/GE (0.6 wt.%) | −0.64003 | 362.35 | 230.53 | 4.6053 × 10−9 | 5.4026 × 10−5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Xiao, G.; Zhang, W.; Zhang, N.; Chen, C.; Fan, Y.; Li, H.; Liu, X.; He, Y. One-Step Method for Preparing Dispersive Tea Polyphenol/Graphene Nanosheets Enhanced with Anticorrosion Performance. Coatings 2019, 9, 731. https://doi.org/10.3390/coatings9110731
Guo Y, Xiao G, Zhang W, Zhang N, Chen C, Fan Y, Li H, Liu X, He Y. One-Step Method for Preparing Dispersive Tea Polyphenol/Graphene Nanosheets Enhanced with Anticorrosion Performance. Coatings. 2019; 9(11):731. https://doi.org/10.3390/coatings9110731
Chicago/Turabian StyleGuo, Youwei, Guoqing Xiao, Wei Zhang, Nange Zhang, Chunling Chen, Yi Fan, Hongjie Li, Xuewei Liu, and Yi He. 2019. "One-Step Method for Preparing Dispersive Tea Polyphenol/Graphene Nanosheets Enhanced with Anticorrosion Performance" Coatings 9, no. 11: 731. https://doi.org/10.3390/coatings9110731
APA StyleGuo, Y., Xiao, G., Zhang, W., Zhang, N., Chen, C., Fan, Y., Li, H., Liu, X., & He, Y. (2019). One-Step Method for Preparing Dispersive Tea Polyphenol/Graphene Nanosheets Enhanced with Anticorrosion Performance. Coatings, 9(11), 731. https://doi.org/10.3390/coatings9110731




