Synthesis, Characterization, and Corrosion Inhibition Potential of Novel Thiosemicarbazone on Mild Steel in Sulfuric Acid Environment
Abstract
1. Introduction
2. Experimental
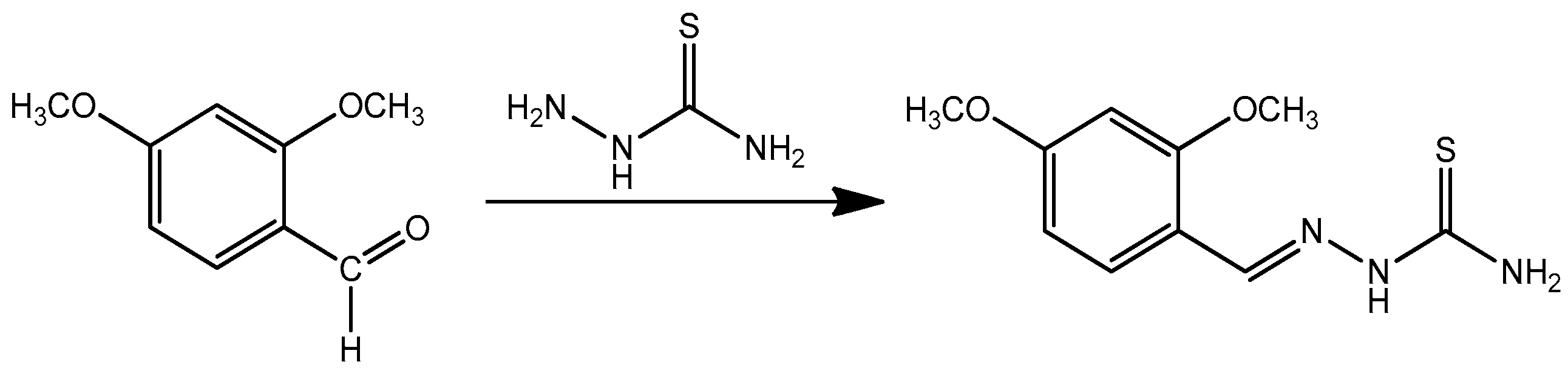
2.1. Chemistry
2.2. Corrosion Tests
2.2.1. Electrochemical Impedance Spectroscopy Measurements
2.2.2. Weight Loss Measurements
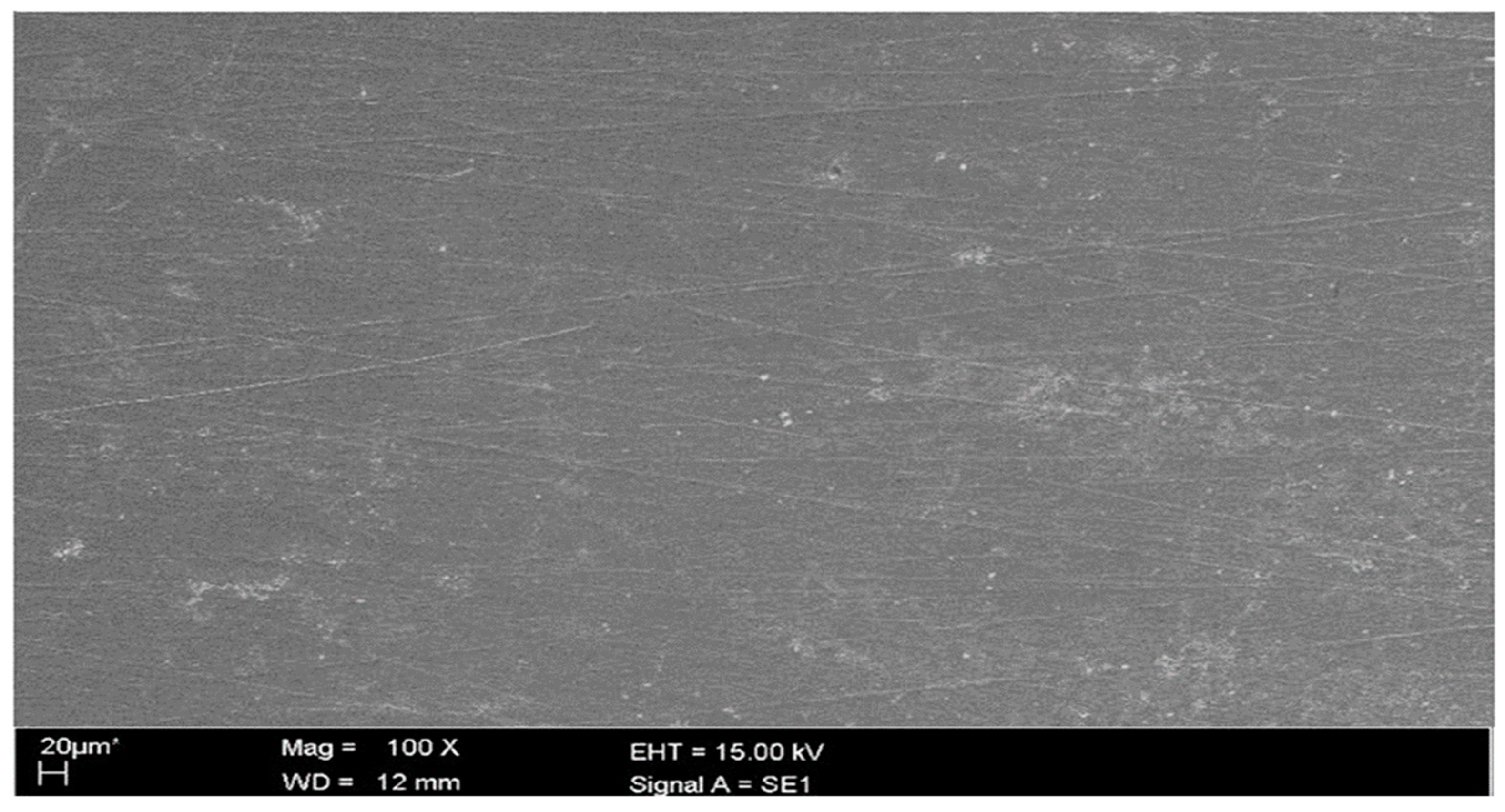
2.3. Scanning Electron Microscope Examination
3. Results and Discussion
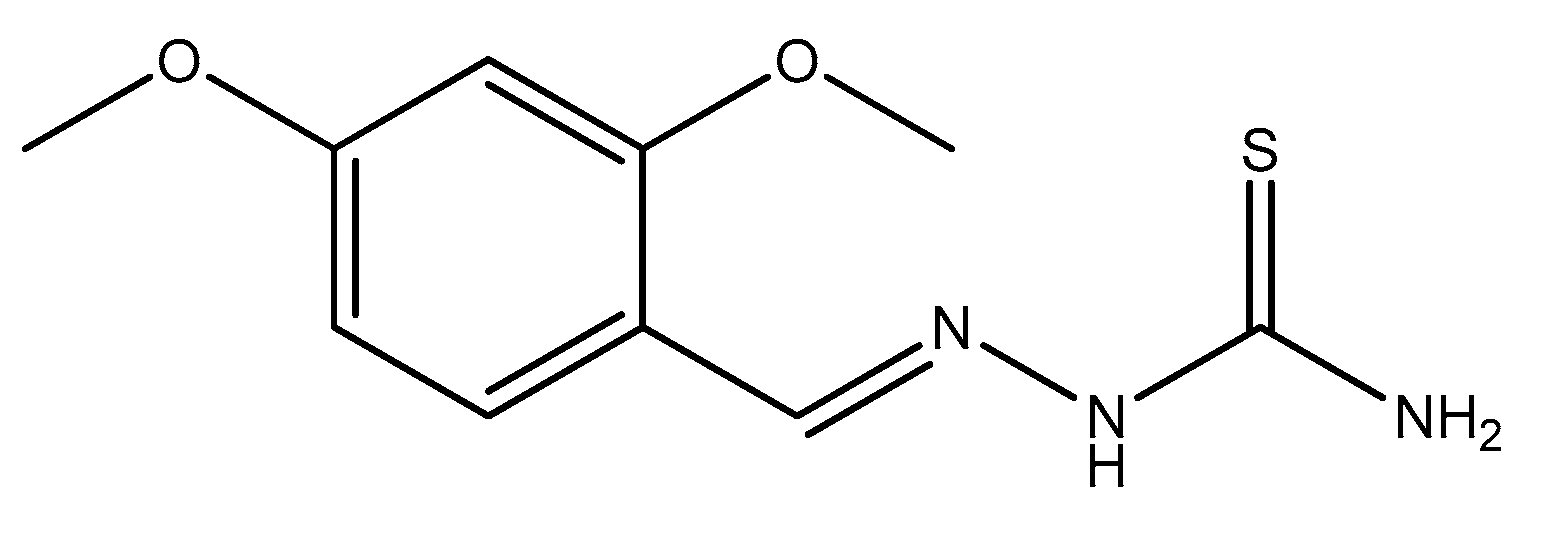
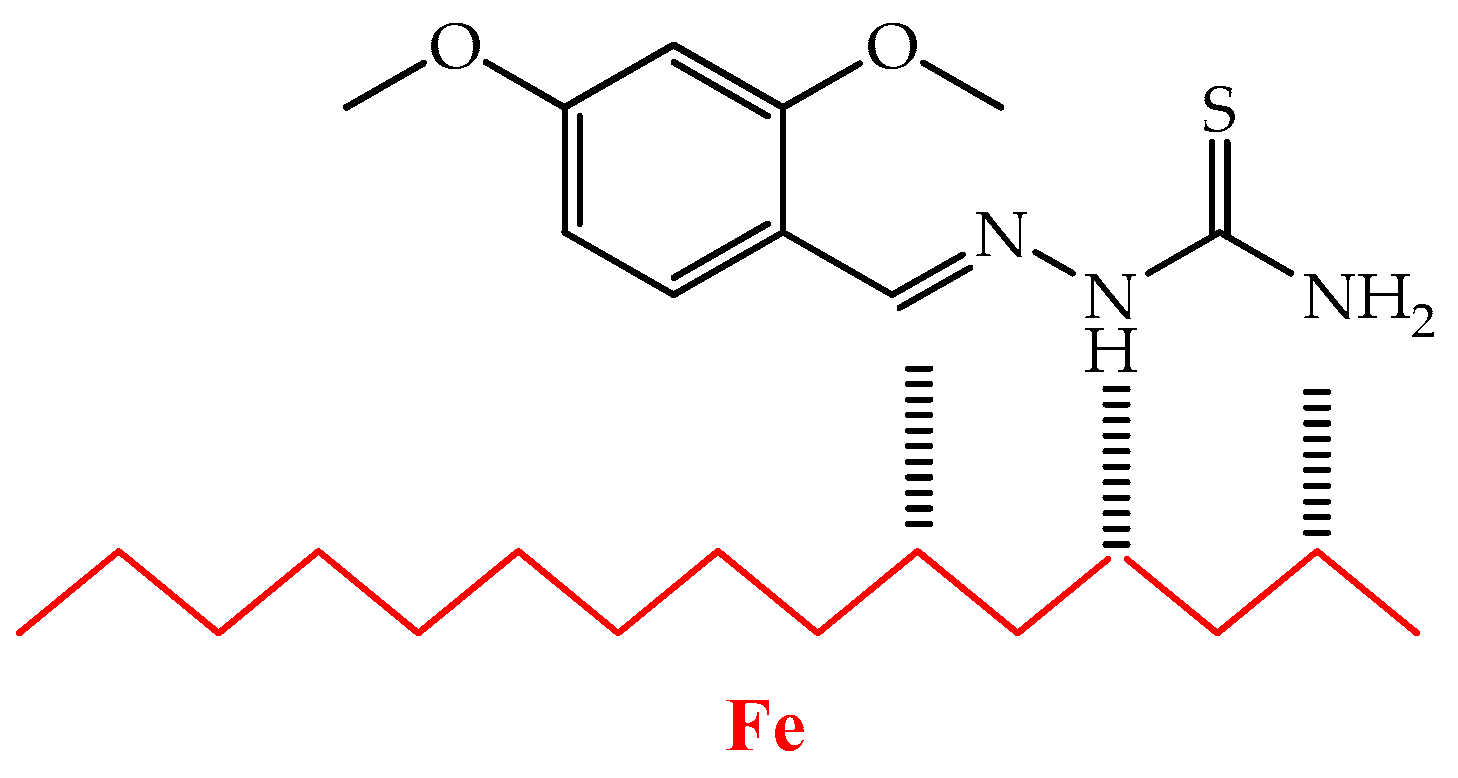
3.1. Chemistry
3.2. Electrochemical Impedance Spectroscopy (EIS)
3.2.1. Open Circuit Potential (OCP)
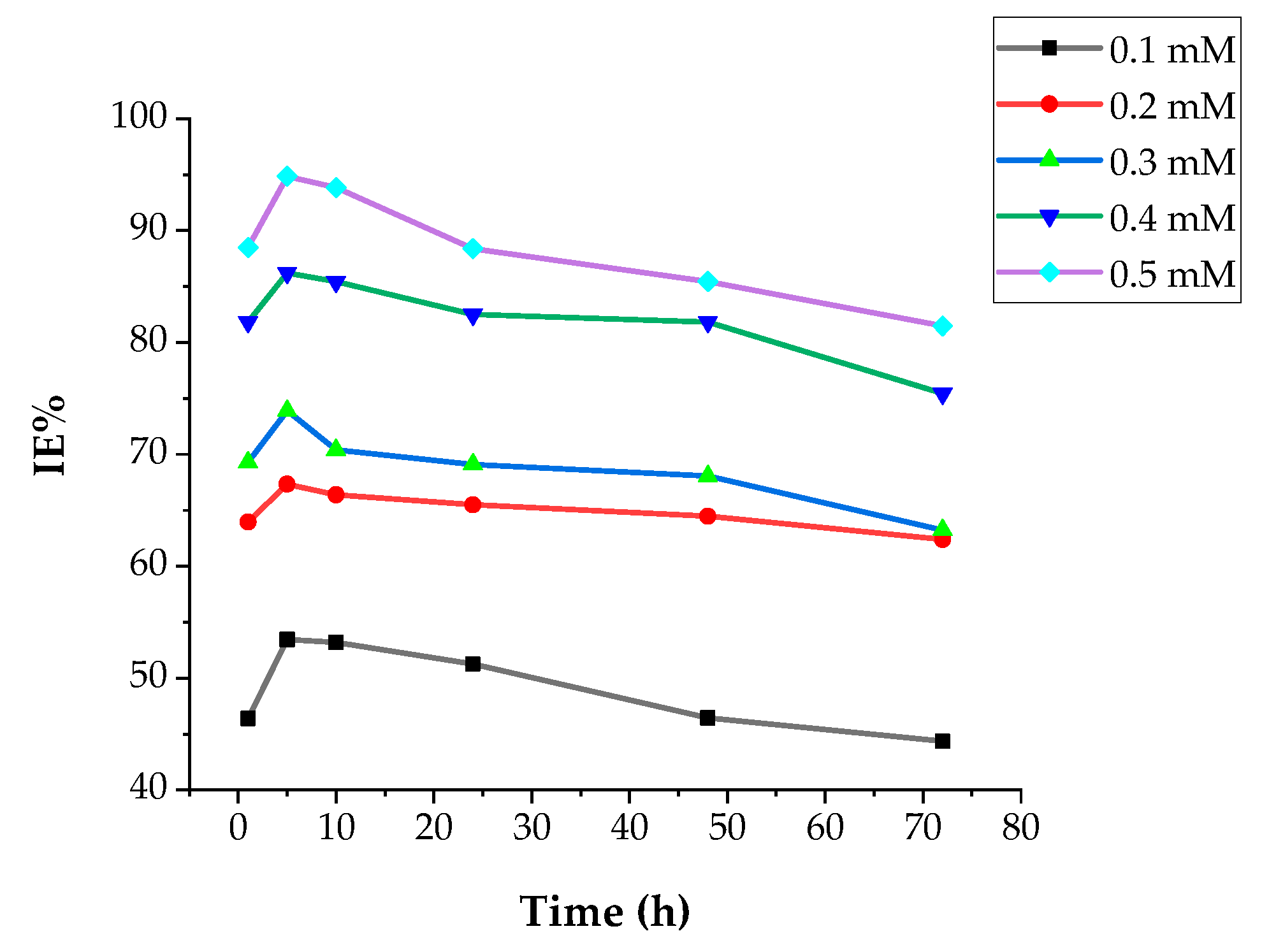
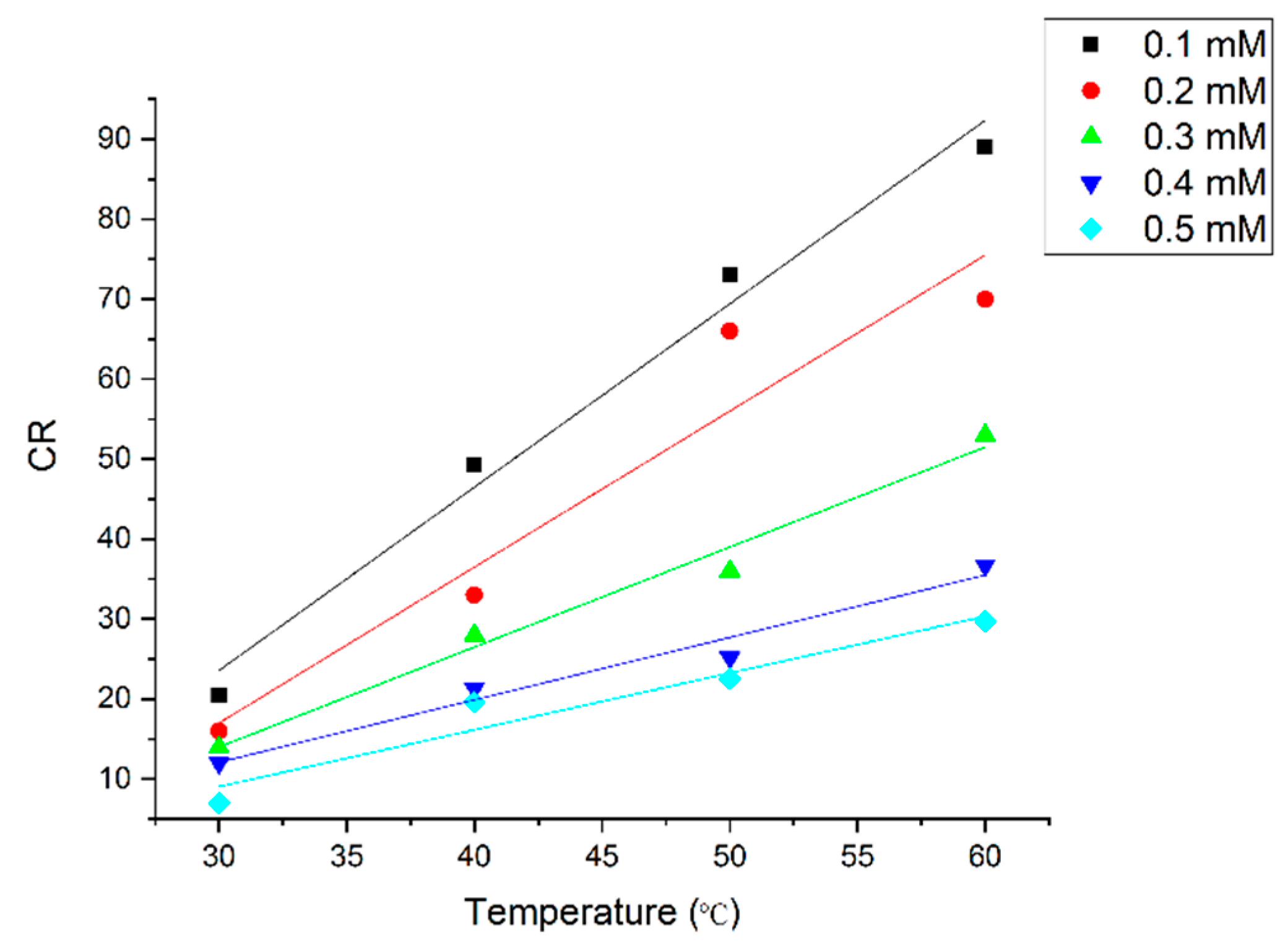
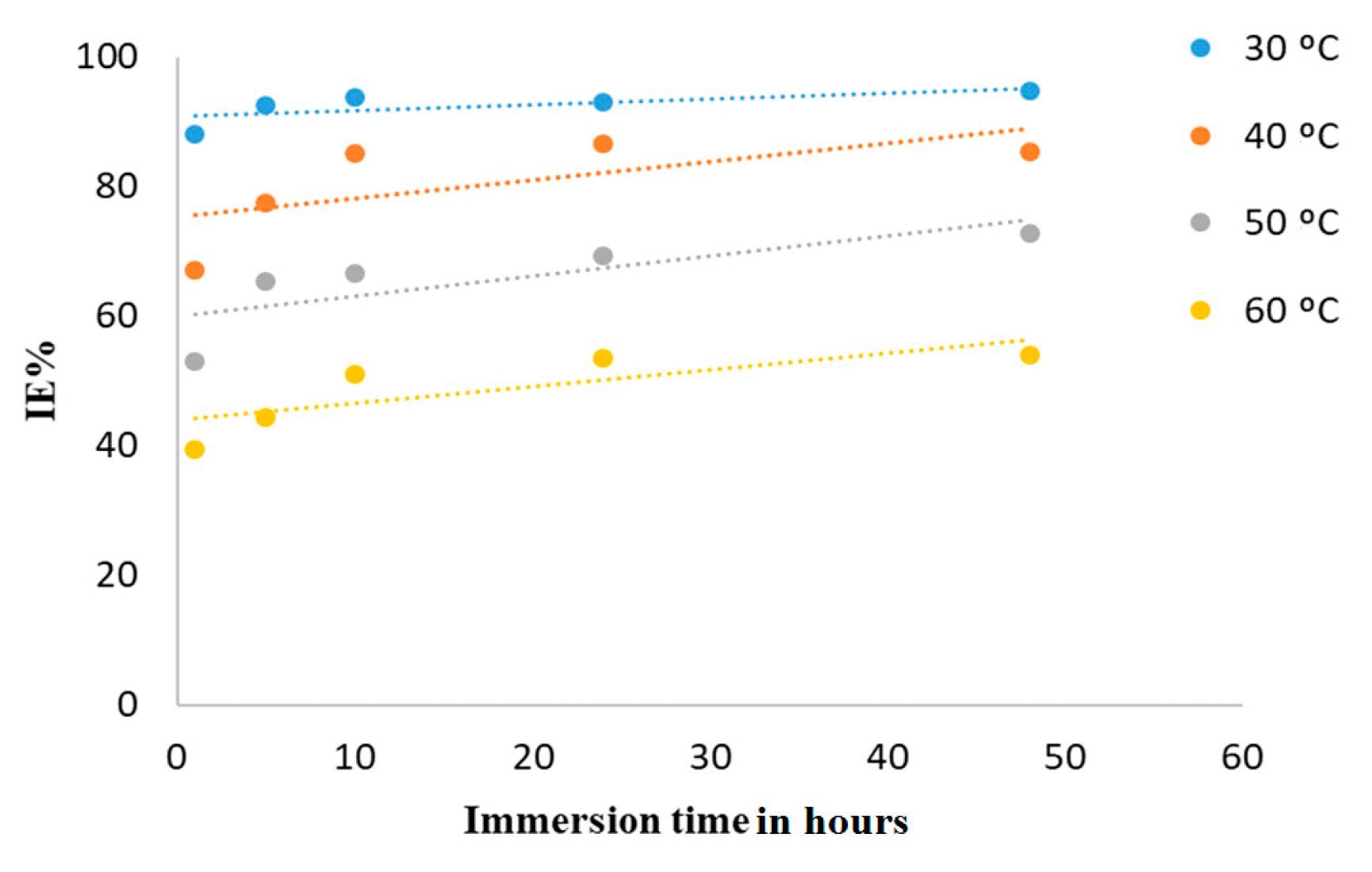
3.2.2. Weight Loss Studies
Concentration Effect
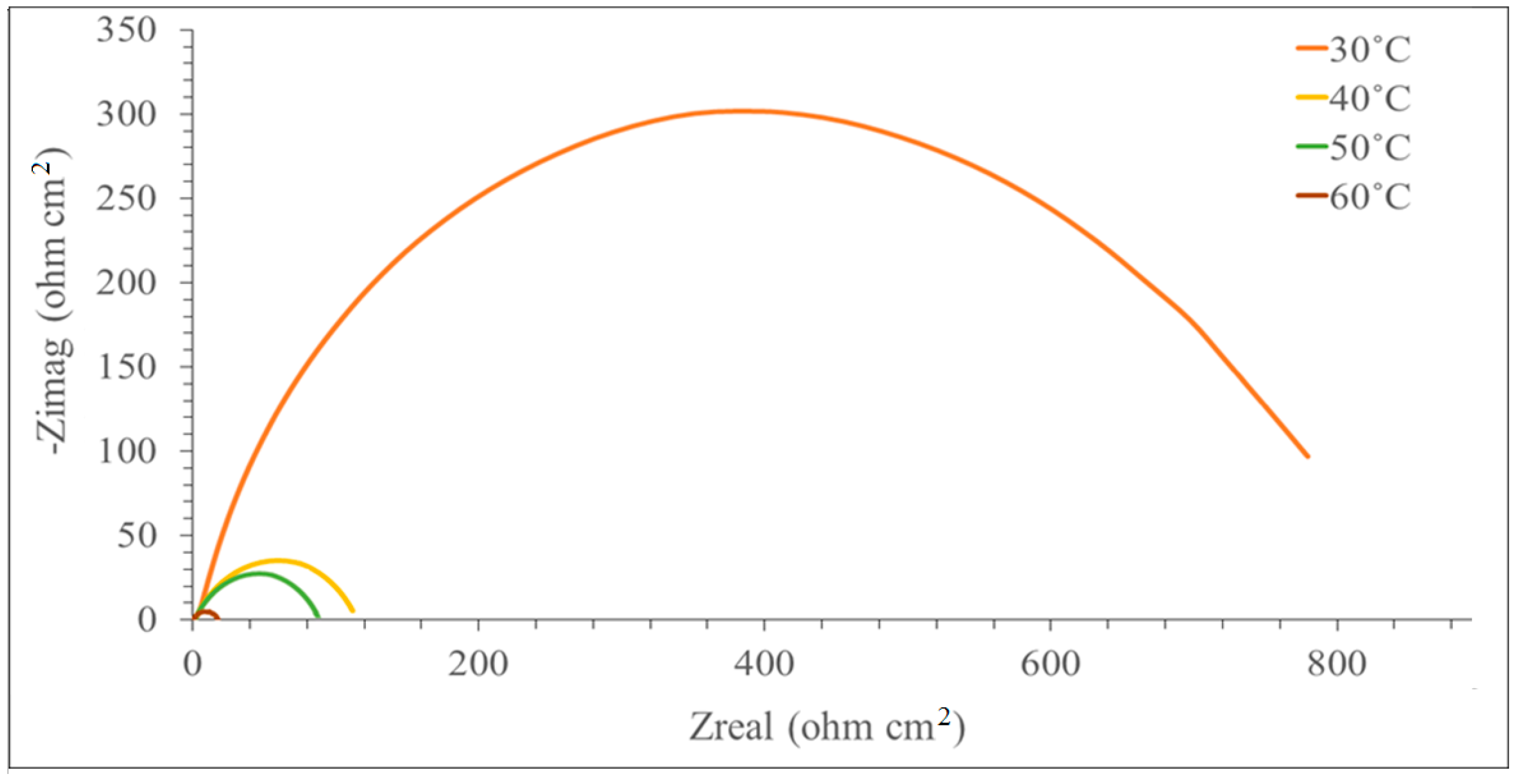
Temperature Impact
3.2.3. Inhibitive Mechanism
3.2.4. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, M.H.O.; Al-Amiery, A.; Al-Majedy, Y.K.; Kadhum, A.A.H.; Mohamad, A.B.; Gaaz, T.S. Synthesis and characterization of a novel organic corrosion inhibitor for mild steel in 1 M hydrochloric acid. Results Phys. 2018, 8, 728–733. [Google Scholar] [CrossRef]
- Al-Amiery, A.; Kassim, F.A.B.; Kadhum, A.A.H.; Mohamad, A.B. Synthesis and characterization of a novel eco-friendly corrosion inhibition for mild steel in 1 M hydrochloric acid. Sci. Rep. 2016, 6, 19890. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiery, A.; Kadhum, A.; Mohamad, A.; Junaedi, S. A novel hydrazinecarbothioamide as a potential corrosion inhibitor for mild steel in HCl. Materials 2013, 6, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Rubaye, A.; Abdulwahid, A.; Al-Baghdadi, S.B.; Al-Amiery, A.; Kadhum, A.; Mohamad, A. Cheery sticks plant extract as a green corrosion inhibitor complemented with LC-EIS/MS spectroscopy. Int. J. Electrochem. Sci. 2015, 10, 8200–8209. [Google Scholar]
- Trabanelli, G. Inhibitors An Old Remedy for a New Challenge. Corrosion 1991, 47, 410–419. [Google Scholar] [CrossRef]
- Data Research Analyst. Worldofchemicals.com. Available online: https://www.worldofchemicals.com/430/chemistry-articles/industrial-applications-of-sulfuric-acid.html (accessed on 15 August 2019).
- Bentiss, F.; Lagrenee, M.; Traisnel, M.; Hornez, J.C. The corrosion inhibition of mild steel in acidic media by a new triazole derivative. Corros. Sci. 1999, 41, 789. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, S.; Qiang, Y.; Feng, L.; Liao, C.; Xu, Y.; Chen, S. Investigation of the inhibition effect of Montelukast Sodium on the copper corrosion in 0.5 mol/L H2SO4. J. Mol. Liq. 2017, 248, 902–910. [Google Scholar] [CrossRef]
- Habeeb, H.J.; Luaibi, H.M.; Abdullah, T.A.; Dakhil, R.M.; Kadhum, A.A.H.; Al-Amiery, A. Case study on thermal impact of novel corrosion inhibitor on mild steel. Case Stud. Therm. Eng. 2018, 12, 64–68. [Google Scholar] [CrossRef]
- Al-Baghdadi, S.B.; Hashim, F.G.; Salam, A.Q.; Abed, T.K.; Gaaz, T.S.; Al-Amiery, A. Synthesis and corrosion inhibition application of NATN on mild steel surface in acidic media complemented with DFT studies. Results Phys. 2018, 8, 1178–1184. [Google Scholar] [CrossRef]
- Kadhum, A.; Mohamad, A.; Hammed, L.; Al-Amiery, A.; San, N.; Musa, A. Inhibition of mild steel corrosion in hydrochloric acid solution by new coumarin. Materials 2014, 7, 4335–4348. [Google Scholar] [CrossRef]
- Mohamad, A.B.; Kadhum, A.A.H.; Al-Amiery, A.A.; Ying, L.C.; Musa, A.Y. Synergistic of a coumarin derivative with potassium iodide on the corrosion inhibition of aluminum alloy in 1.0 NH2SO4. Met. Mater. Int. 2014, 20, 459–467. [Google Scholar] [CrossRef]
- Al-Amiery, A.; Kadhum, A.; Alobaidy, A.H.; Mohamad, A.; Hoon, P. Novel corrosion inhibitor for mild steel in HCl. Materials 2014, 7, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiery, A.; Kadhum, A.; Mohamad, A.; Musa, A.; Li, C. Electrochemical study on newly synthesized chlorocurcumin as an inhibitor for mild steel corrosion in hydrochloric acid. Materials 2013, 6, 5466–5477. [Google Scholar] [CrossRef] [PubMed]
- Kadhum, A.; Al-Amiery, A.; Shikara, M.; Mohamad, A. Synthesis, structure elucidation and DFT studies of new thiadiazoles. Int. J. Physi. Sci. 2011, 6, 6692–6697. [Google Scholar]
- Kelland, M.A. Production chemicals for the oil and gas industry. Chromatographia 2010, 72, 199. [Google Scholar]
- Eddy, N.O.; Ebenso, E.E.; Ibok, U.J. Adsorption, synergistic inhibitive effect and quantum chemical studies of ampicillin (AMP) and halides for the corrosion of mild steel in H2SO4. J. Appl. Electrochem. 2010, 40, 445–456. [Google Scholar] [CrossRef]
- Yaro, A.S.; Khadom, A.A.; Wael, R.K. Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid. Alex. Eng. J. 2013, 52, 129–135. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Fu, H. Triazolyl blue tetrazolium bromide as a novel corrosion inhibitor for steel in HCl and H2SO4 solutions. Corros. Sci. 2011, 53, 302–309. [Google Scholar] [CrossRef]
- Jacob, K.S.; Parameswaran, G. Corrosion inhibition of mild steel in hydrochloric acid solution by Schiff base furoin thiosemicarbazone. Corros. Sci. 2010, 52, 224–228. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Ebbedi, N.O. Adsorption properties and inhibition of mild steel corrosion in sulfuric acid solution by ketoconazole: Experimental and theoretical investigation. Corros. Sci. 2010, 52, 198–204. [Google Scholar] [CrossRef]
- Kumar, S.L.A.; Gopiraman, M.; Kumar, M.S.; Sreekanth, A. 2-Acetylpyridine-N(4)-Morpholine Thiosemicarbazone (HAcpMTSc) as a Corrosion Inhibitor on Mild Steel in HCl. Ind. Eng. Chem. Res. 2011, 50, 7824–7832. [Google Scholar] [CrossRef]
- Singh, D.D.N.; Singh, M.M.; Chaudhary, R.S.; Agrawal, C.V. Inhibitive effects of isatin, thiosemicarbazide and isatin-3-(3-thiosemicarbazone) on the corrosion of aluminium alloys in nitric acid. J. Appl. Electrochem. 1980, 10, 587–592. [Google Scholar] [CrossRef]
- Aytac, A.; Ozmen, U.; Kabasakaloglu, M. Investigation of some Schiff bases as acidic corrosion of alloy AA3102. Mater. Chem. Phys. 2005, 89, 176–181. [Google Scholar] [CrossRef]
- Li, S.L.; Ma, H.Y.; Lei, S.B.; Yu, R.; Chen, S.H.; Liu, D.X. Inhibition of copper corrosion with Schiff base derived from 3-methoxysalicylaldehyde and O-phenyldiamine in chloride media. Corrosion 1998, 54, 947. [Google Scholar] [CrossRef]
- Li, S.; Chen, S.; Lei, S.; Ma, H.; Yu, R.; Liu, D. Investigation on some Schiff bases as HCl corrosion inhibitors for copper. Corros. Sci. 1999, 41, 1273–1287. [Google Scholar] [CrossRef]
- Fouda, A.S.; Madkour, L.H.; El-Shafei, A.A.; Abd Elmaksoud, S.A. Corrosion inhibitor for Zinc in 2 M HCl solution. Bull. Korean Chem. Soc. 1995, 16, 454–458. [Google Scholar]
- Lashgari, M.; Arshadi, M.R.; Miandari, S. The enhancing power of iodide on corrosion prevention of mild steel in the presence of a synthetic soluble Schiff base: Electrochemical and surface analyses. Electrochim. Acta 2010, 55, 6058–6063. [Google Scholar] [CrossRef]
- Küstü, C.; Emregül, K.C.; Atakol, O. Schiff bases of increasing complexity as mild steel corrosion inhibitors in 2 M HCl. Corros. Sci. 2007, 49, 2800–2814. [Google Scholar]
- Asan, A.; Kabasakaloglu, M.; Isıklan, M.; Kılıc, Z. Corrosion inhibition of brass in presence of terdentate ligands in chloride solution. Corros. Sci. 2005, 47, 1534–1544. [Google Scholar] [CrossRef]
- El Azhar, M.; Mernari, B.; Traisnel, M.; Bentiss, F.; Lagrenée, M. Corrosion inhibition of mild steel by the new class of inhibitors [2,5-bis(n-pyridyl)-1,3,4-thiadiazoles] in acidic media. Corros. Sci. 2001, 43, 2229–2238. [Google Scholar] [CrossRef]
- Alobaidy, A.; Kadhum, A.; Al-Baghdadi, S.; Al-Amiery, A.; Kadhum, A.; Yousif, E. Eco-friendly corrosion inhibitor: Experimental studies on the corrosion inhibition performance of creatinine for mild steel in HCl complemented with quantum chemical calculations. Int. J. Electrochem. Sci. 2015, 10, 3961–3972. [Google Scholar]
- Amin, M.A.; Ibrahim, M.M. Corrosion and corrosion control of mild steel in concentrated H2SO4 solutions by a newly synthesized glycine derivative. Corros Sci. 2011, 53, 873–885. [Google Scholar] [CrossRef]
- Salman, T.A.; Zinad, D.S.; Jaber, S.H.; Al-Ghezi, M.; Mahal, A.; Takriff, M.S.; Al-Amiery, A.A. Effect of 1,3,4-Thiadiazole Scaffold on the Corrosion Inhibition of Mild Steel in Acidic Medium: An Experimental and Computational Study. J. Bio Tribo Corros. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Kadhim, A.; Al-Okbi, A.K.; Jamil, D.M.; Qussay, A.; Al-Amiery, A.A.; Gaaz, T.S. Experimental and theoretical studies of benzoxazines corrosion inhibitors. Results Phys. 2017, 7, 4013–4019. [Google Scholar] [CrossRef]
- Jamil, D.M.; Al-Okbi, A.K.; Al-Baghdadi, S.B.; Al-Amiery, A.A.; Kadhim, A.; Gaaz, T.S. Experimental and theoretical studies of Schiff bases as corrosion inhibitors. Chem. Cent. J. 2018, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- ASTM, G. Standard Practice for Preparing, Cleaning and Evaluating Corrosion Test Specimens; American Society for Testing and Materials: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Herrag, L.; Hammouti, B.; Elkadiri, S.; Aouniti, A.; Jama, C.; Vezin, H. Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: Experimental and theoretical investigations. Corros. Sci. 2010, 52, 3042–3051. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.; Robb, M.; Cheeseman, J. Gaussian 09, Revision A 02’; Gaussian, Inc.: Wallingford, CT, USA, 2009; Volume 200, p. 28. [Google Scholar]
- Juttner, K. Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces. Electrochim. Acta 1990, 35, 1501–1508. [Google Scholar] [CrossRef]
- Park, J.R.; Macdonald, D.D. Impedance studies of the growth of porous magnetite films on carbon steel in high temperature aqueous systems. Corros. Sci. 1983, 23, 295–315. [Google Scholar] [CrossRef]
- Walter, G.W. A comparison of single frequency and wide frequency range impedance tests for painted metals. Corros. Sci. 1990, 30, 617–629. [Google Scholar] [CrossRef]
- Walter, G.W. The application of impedance methods to study the effects of water uptake and chloride ion concentration on the degradation of paint films—I. Attached films. Corros. Sci. 1991, 32, 1059–1084. [Google Scholar] [CrossRef]
- Mansfeld, F. Electrochemical impedance spectroscopy (EIS) as a new tool for investigating methods of corrosion protection. Electrochim. Acta 1990, 35, 1533–1544. [Google Scholar] [CrossRef]
- Mendonça, G.L.; Costa, S.N.; Freire, V.N.; Casciano, P.N.; Correia, A.N.; de Lima-Neto, P. Understanding the corrosion inhibition of carbon steel and copper in sulphuric acid medium by amino acids using electrochemical techniques allied to molecular modelling methods. Corros. Sci. 2017, 115, 41–55. [Google Scholar] [CrossRef]
- Ebenso, E. Effect of halide ions on the corrosion inhibition of mild steel in H2SO4 using methyl red: Part 1. Bull. Electrochem. 2003, 19, 209–216. [Google Scholar]
- Khamis, A.; Saleh, M.; Awad, M. The counter ion influence of cationic surfactant and role of chloride ion synergism on corrosion inhibition of mild steel in acidic media. Int. J. Electrochem. Sci. 2012, 7, 10487–10500. [Google Scholar]
- Obot, I.; Obi-Egbedi, N.; Umoren, S. Antifungal drugs as corrosion inhibitors for aluminium in 0.1 M HCl. Corros. Sci. 2009, 51, 1868–1875. [Google Scholar] [CrossRef]
- Ju, H.; Kai, Z.-P.; Li, Y. Aminic nitrogen-bearing polydentate Schiff base compounds as corrosion inhibitors for iron in acidic media: A quantum chemical calculation. Corros. Sci. 2008, 50, 865–871. [Google Scholar] [CrossRef]
- Obayes, H.R.; Al-Amiery, A.A.; Alwan, G.H.; Abdullah, T.A.; Kadhum, A.A.H.; Mohamad, A.B. Sulphonamides as corrosion inhibitor: Experimental and DFT studies. J. Mol. Struct. 2017, 1138, 27–34. [Google Scholar] [CrossRef]
- Al-Azawi, K.F.; Al-Baghdadi, S.B.; Mohamed, A.Z.; Al-Amiery, A.A.; Abed, T.K.; Mohammed, S.A. Synthesis, inhibition effects and quantum chemical studies of a novel coumarin derivative on the corrosion of mild steel in a hydrochloric acid solution. Chem. Cent. J. 2016, 10, 1–11. [Google Scholar] [CrossRef]
- Sanap, S.; Patil, R.; Dubey, R. Corrosion Inhibition of Mild Steel by Using Mixed Ligand Metal Complexes. Int. J. Chem. Sci. 2013, 11, 503–517. [Google Scholar]
- Al-Amiery, A.; Kadhum, A.; Kadihum, A.; Mohamad, A.; How, C.; Junaedi, S. Inhibition of mild steel corrosion in sulfuric acid solution by new Schiff base. Materials 2014, 7, 787–804. [Google Scholar] [CrossRef]
- Junaedi, S.; Kadhum, A.; Al-Amiery, A.; Mohamad, A.; Takriff, M. Synthesis and characterization of novel corrosion inhibitor derived from oleic acid: 2-Amino 5-Oleyl-1, 3, 4-Thiadiazol (AOT). Int. J. Electrochem. Sci. 2012, 7, 3543–3554. [Google Scholar]
- Al-Amiery, A.; Al-Majedy, Y.; Kadhum, A.; Mohamad, A. New coumarin derivative as an eco-friendly inhibitor of corrosion of mild steel in acid medium. Molecules 2015, 20, 366–383. [Google Scholar] [CrossRef] [PubMed]
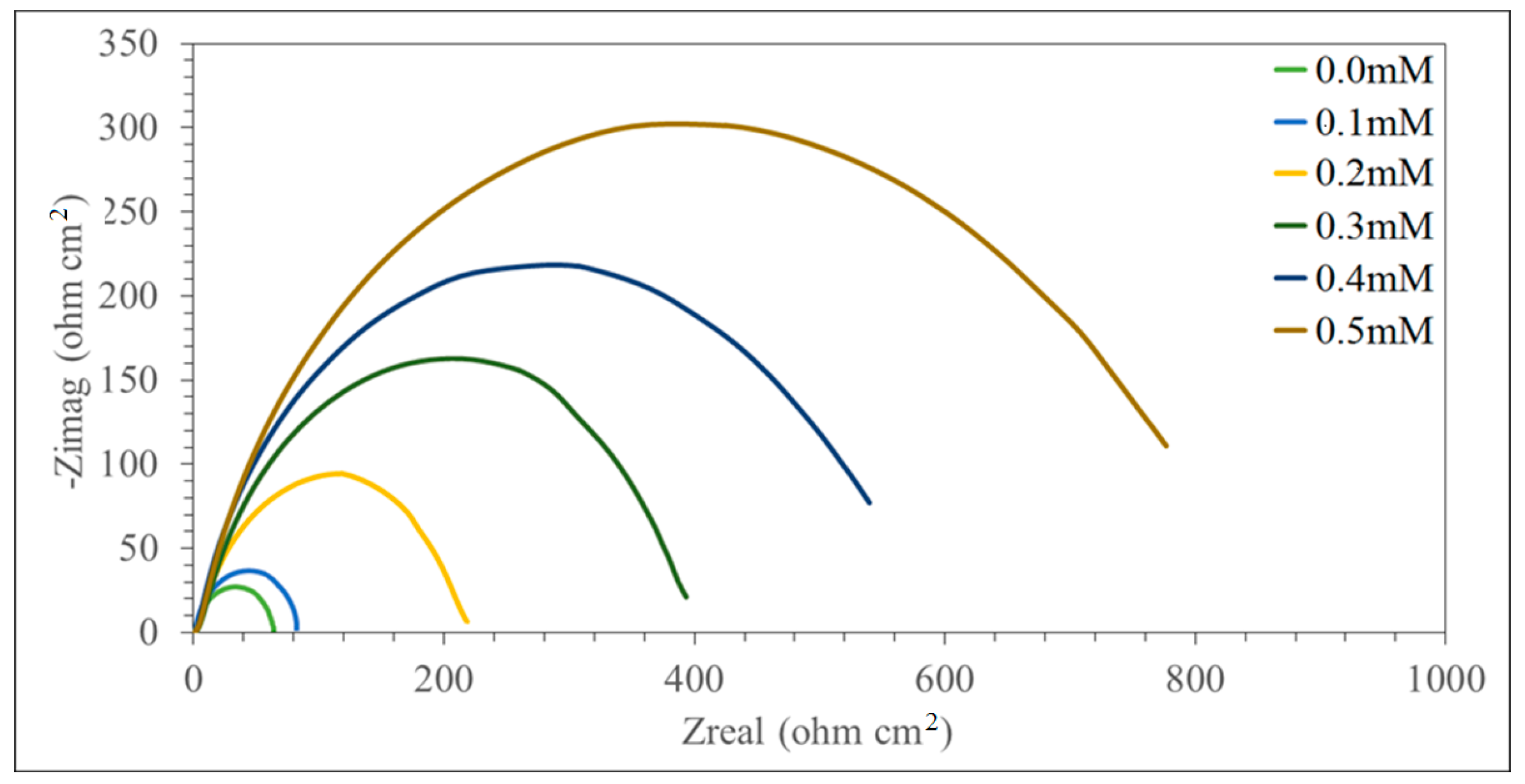
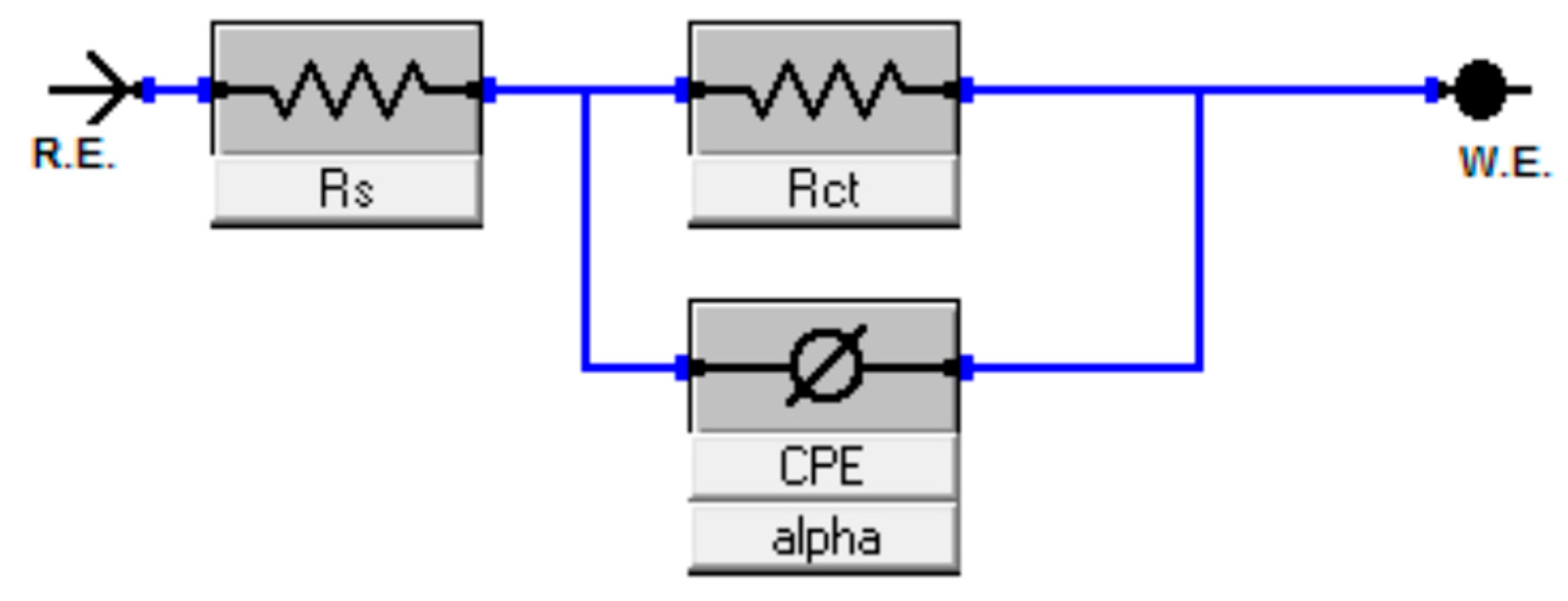
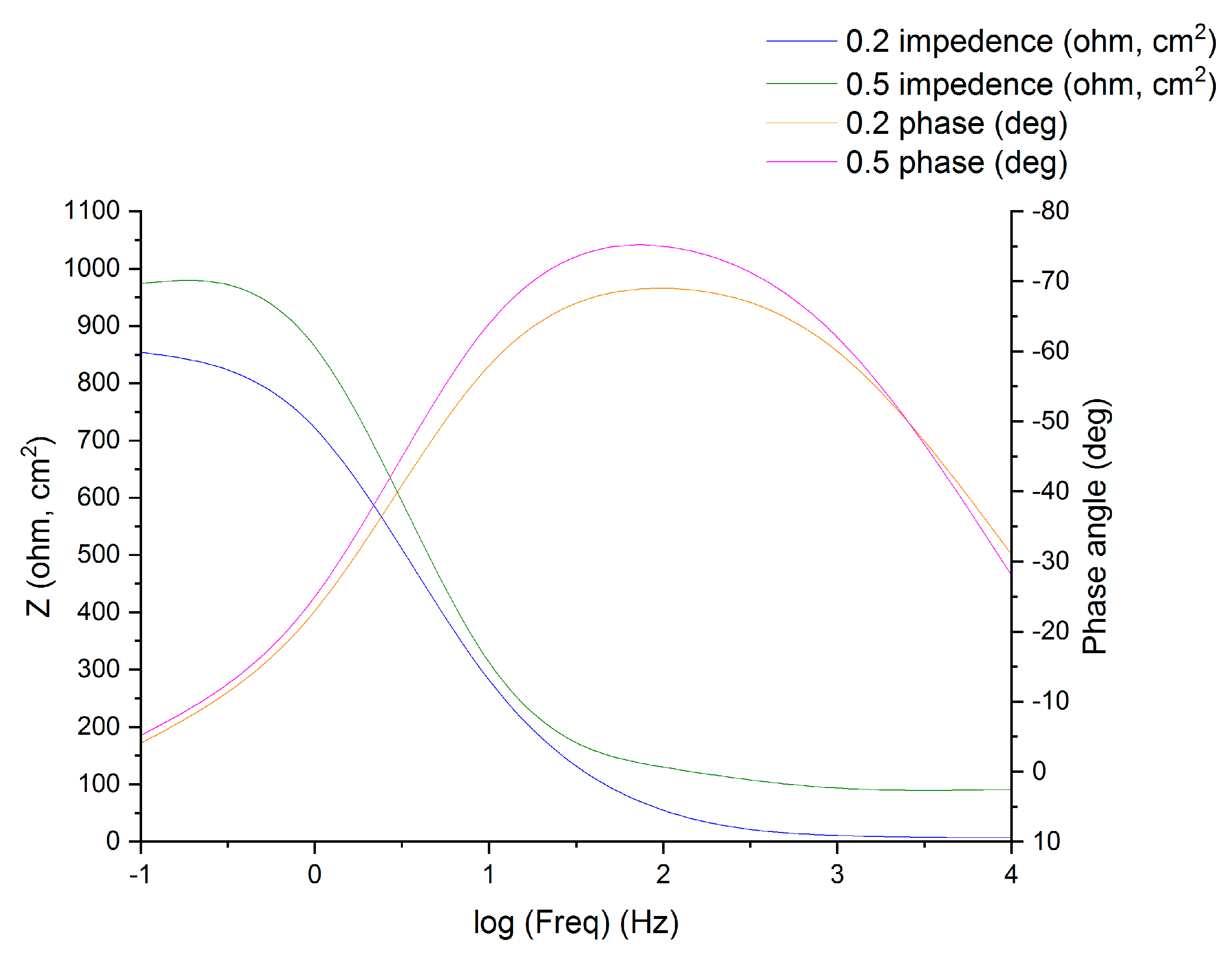
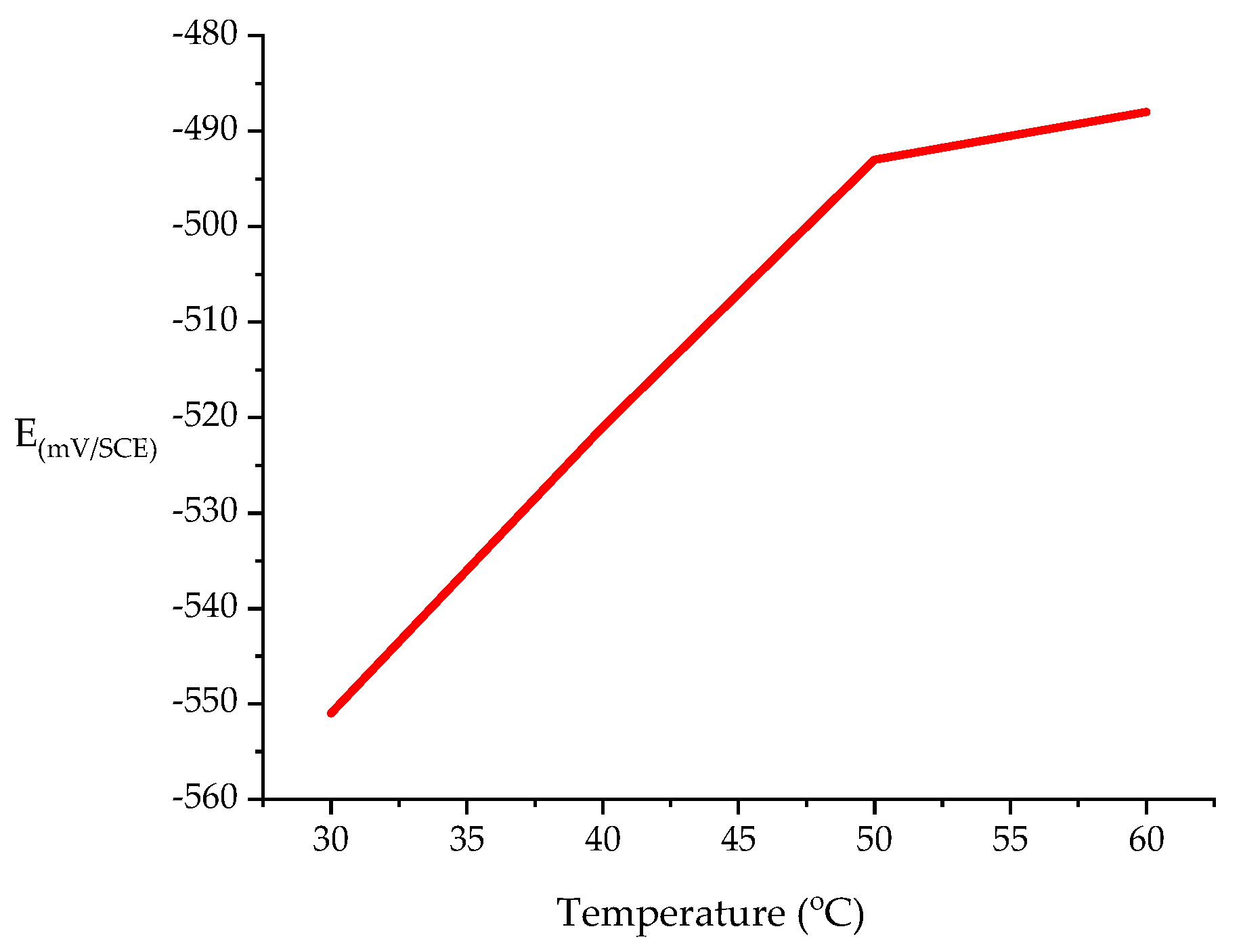
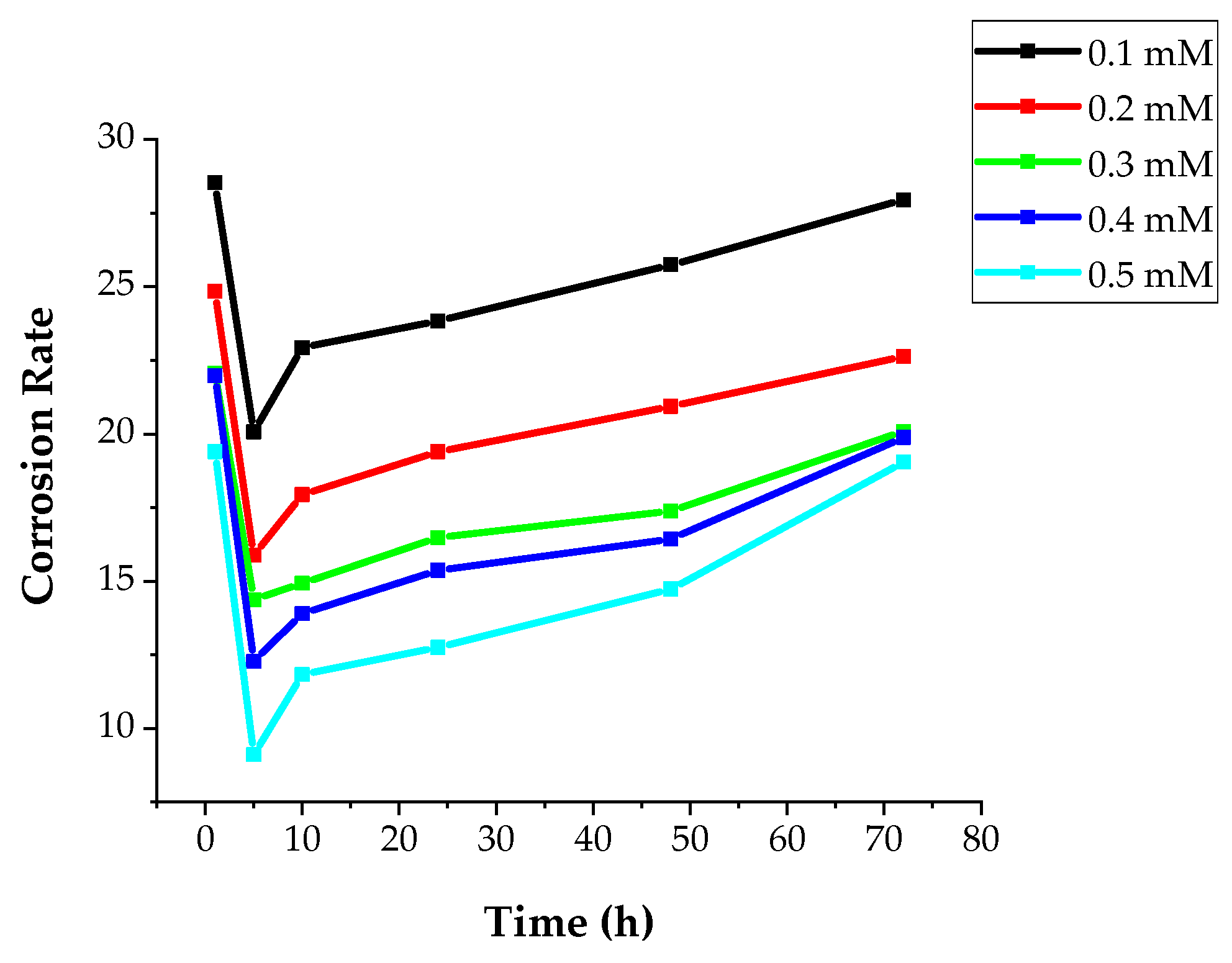

| Concentration (mM) | Rs (ohm cm2) | Rct (ohm cm2) | Cdl (µF cm−2) | IE (%) |
|---|---|---|---|---|
| 0.0 | 1.630 | 46.09 | 512.2 | 0.00 |
| 0.1 | 1.716 | 239.63 | 375.26 | 39.43 |
| 0.2 | 1.572 | 251.52 | 236.64 | 72.62 |
| 0.3 | 1.680 | 433.94 | 182.03 | 82.67 |
| 0.4 | 1.655 | 555.64 | 153.36 | 88.49 |
| 0.5 | 1.610 | 783.61 | 145.24 | 94.30 |
| Temp. °C | Conc. (mM) | Rs (ohm cm2) | Rct (ohm cm2) | Cdl (µF cm−2) | IE (%) |
|---|---|---|---|---|---|
| 30 | 0.0 | 1.630 | 46.09 | 512.2 | 0.00 |
| 30 | 0.5 | 1.610 | 783.61 | 145.24 | 94.30 |
| 40 | 0.0 | 1.236 | 17.64 | 1229 | 0.00 |
| 40 | 0.5 | 1.927 | 79.34 | 313.30 | 85.91 |
| 50 | 0.0 | 1.114 | 8.91 | 1278 | 0.00 |
| 50 | 0.5 | 1.372 | 47.37 | 341.83 | 74.93 |
| 60 | 0.0 | 1063 | 4.27 | 1383 | 0.00 |
| 60 | 0.5 | 1.689 | 11.02 | 658.43 | 58.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. Jawad, Q.; S. Zinad, D.; Dawood Salim, R.; A Al-Amiery, A.; Sumer Gaaz, T.; Takriff, M.S.; H. Kadhum, A.A. Synthesis, Characterization, and Corrosion Inhibition Potential of Novel Thiosemicarbazone on Mild Steel in Sulfuric Acid Environment. Coatings 2019, 9, 729. https://doi.org/10.3390/coatings9110729
A. Jawad Q, S. Zinad D, Dawood Salim R, A Al-Amiery A, Sumer Gaaz T, Takriff MS, H. Kadhum AA. Synthesis, Characterization, and Corrosion Inhibition Potential of Novel Thiosemicarbazone on Mild Steel in Sulfuric Acid Environment. Coatings. 2019; 9(11):729. https://doi.org/10.3390/coatings9110729
Chicago/Turabian StyleA. Jawad, Qusay, Dhafer S. Zinad, Rawaa Dawood Salim, Ahmed A Al-Amiery, Tayser Sumer Gaaz, Mohd S. Takriff, and Abdul Amir H. Kadhum. 2019. "Synthesis, Characterization, and Corrosion Inhibition Potential of Novel Thiosemicarbazone on Mild Steel in Sulfuric Acid Environment" Coatings 9, no. 11: 729. https://doi.org/10.3390/coatings9110729
APA StyleA. Jawad, Q., S. Zinad, D., Dawood Salim, R., A Al-Amiery, A., Sumer Gaaz, T., Takriff, M. S., & H. Kadhum, A. A. (2019). Synthesis, Characterization, and Corrosion Inhibition Potential of Novel Thiosemicarbazone on Mild Steel in Sulfuric Acid Environment. Coatings, 9(11), 729. https://doi.org/10.3390/coatings9110729






