Mangrove Inspired Anti-Corrosion Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Observation of the Mangrove Leaves
2.2. Fabrication of the Mangrove-Inspired Coatings
2.3. Electrochemical Measurements
2.4. Wettability and Ion-Resistant Property
3. Results and Discussion
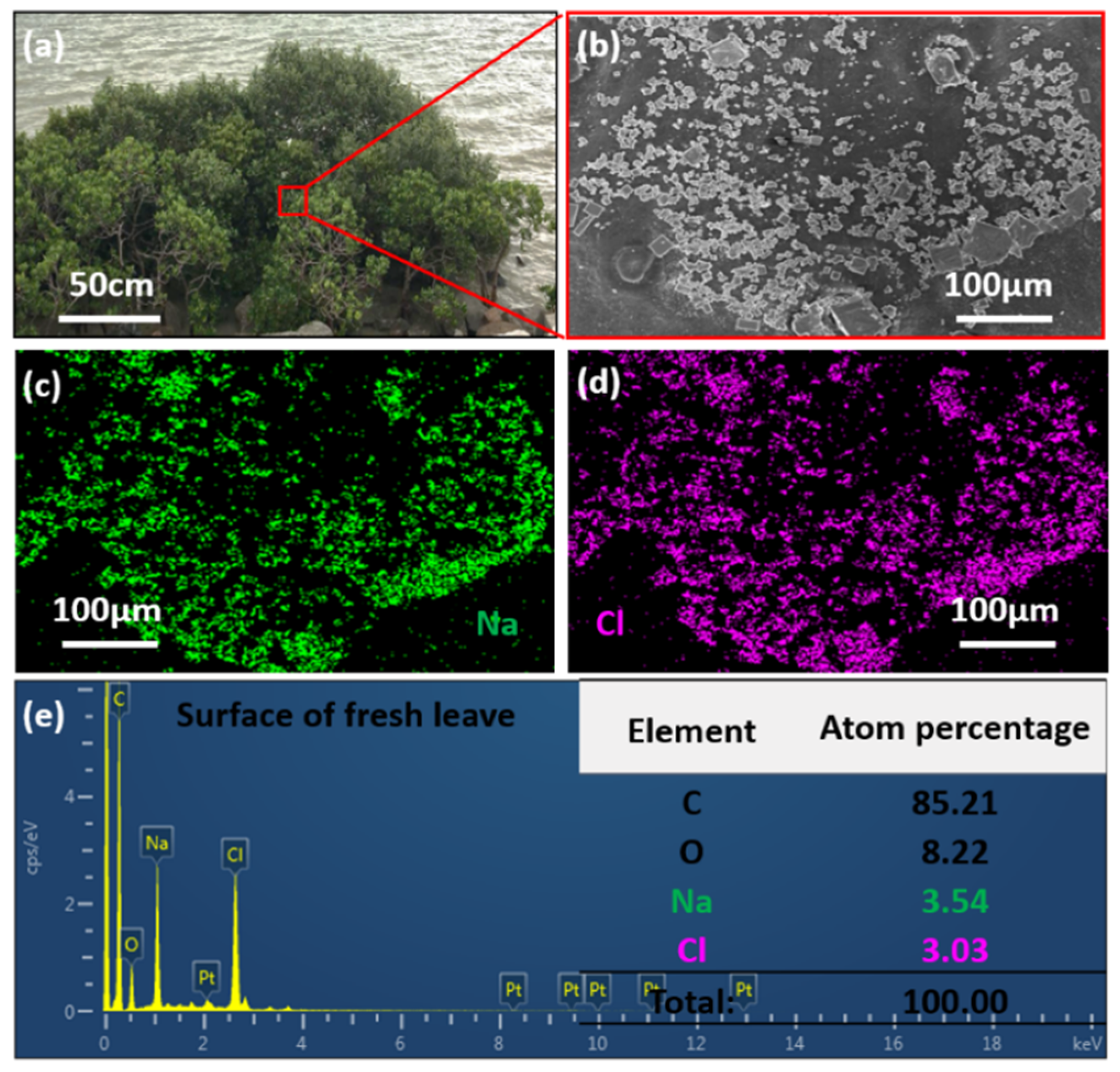
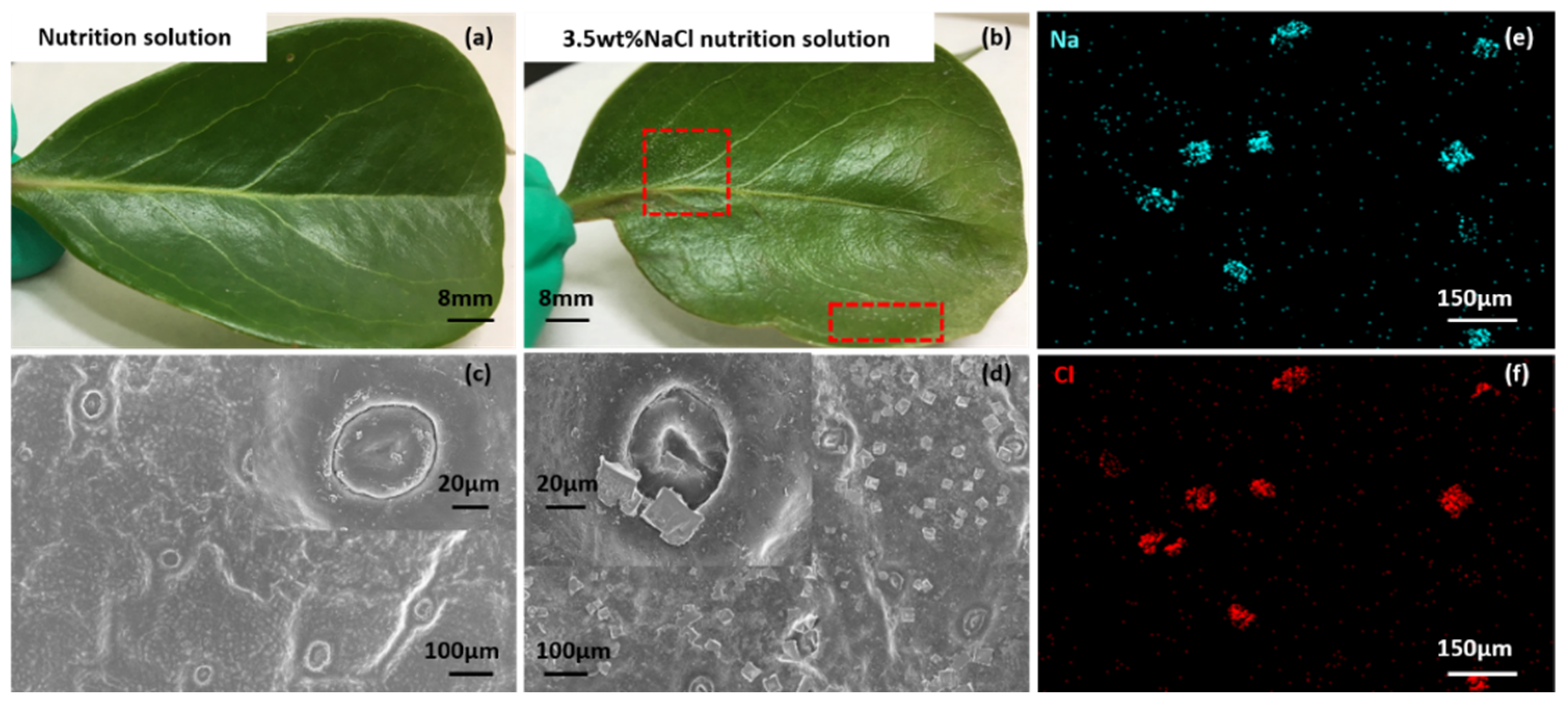
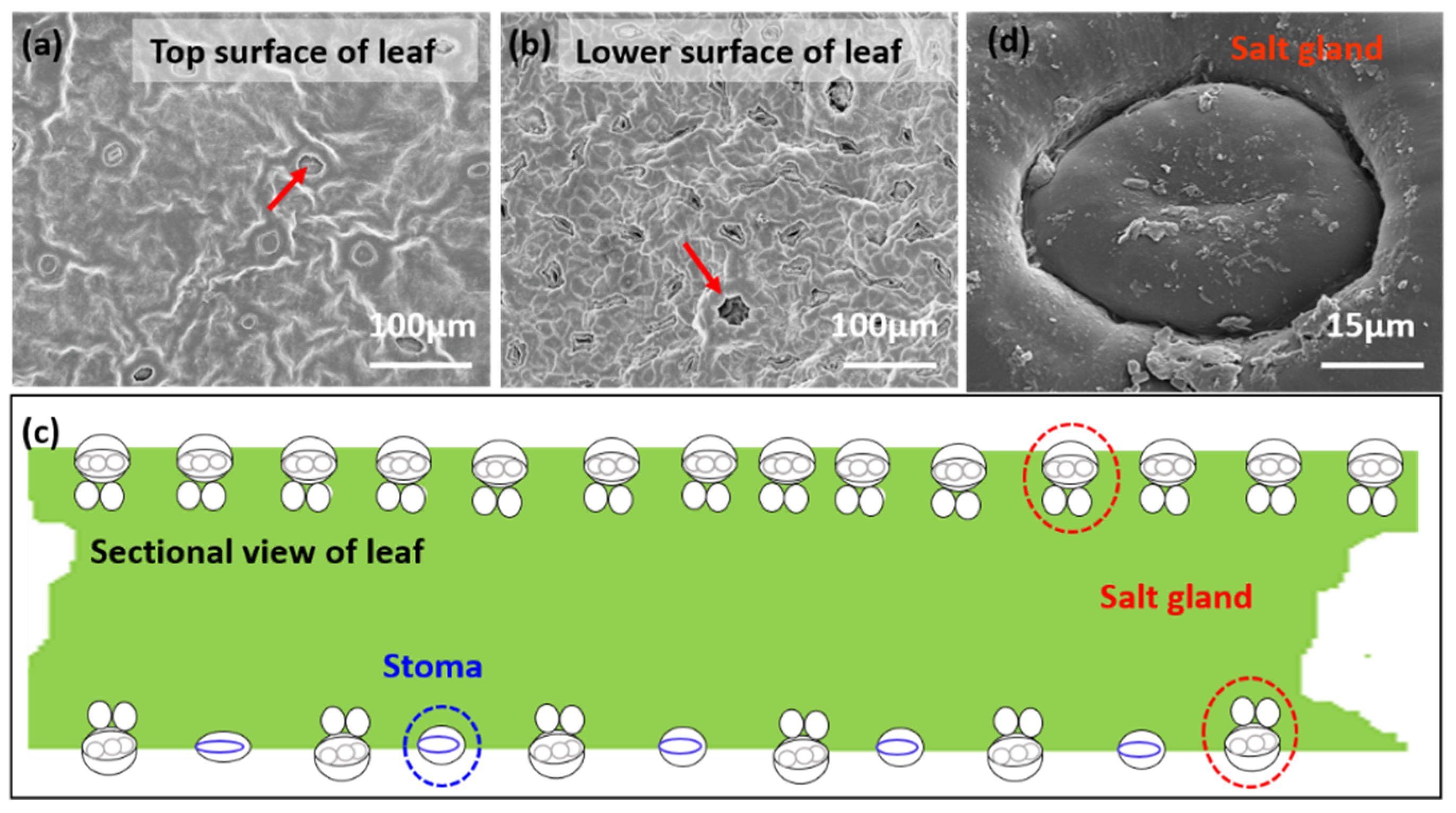
3.1. Salt Particles Deposited on Mangrove Leaves and Salt Gland
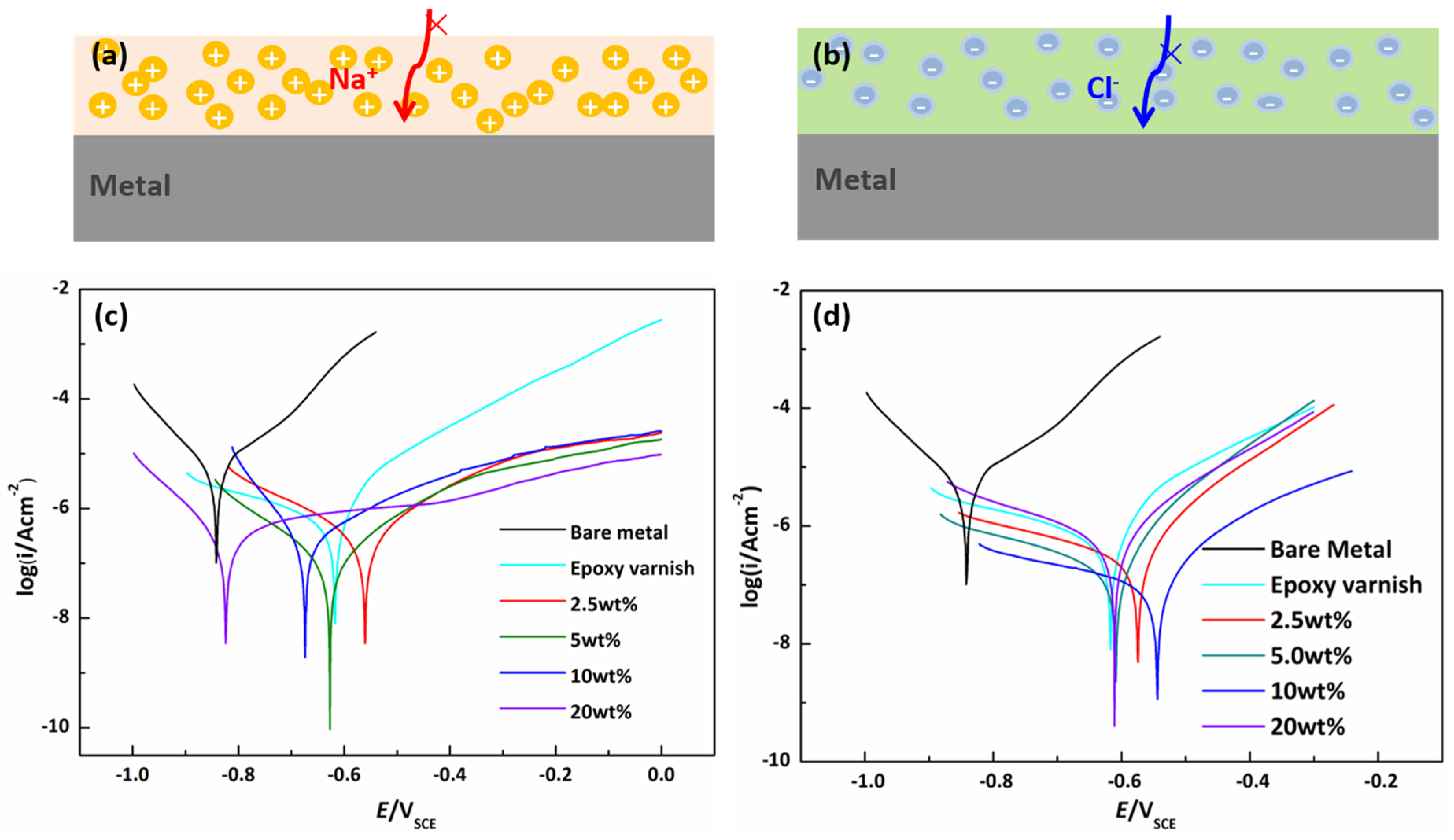
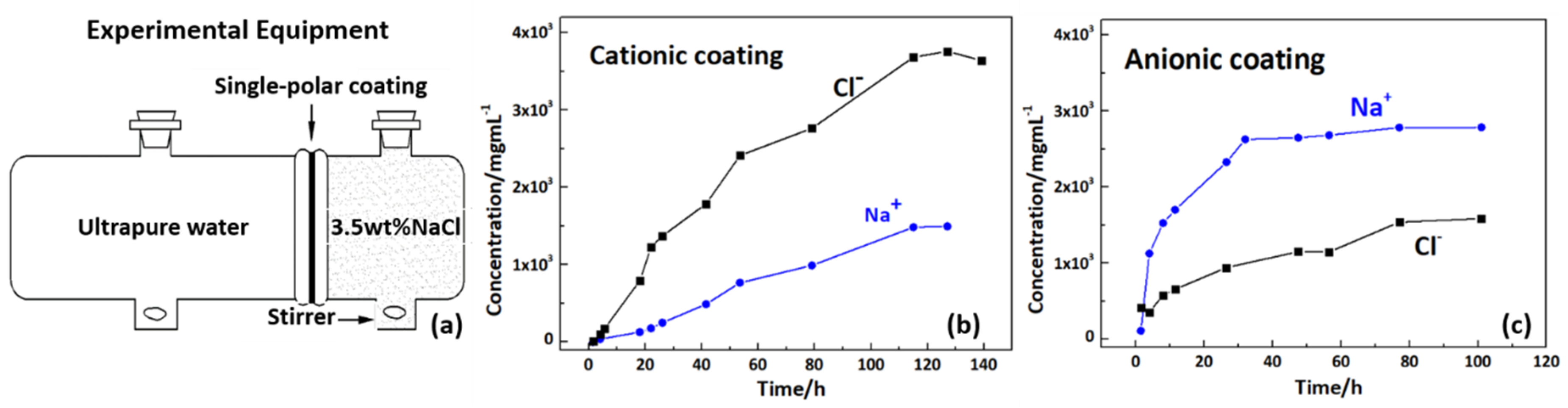
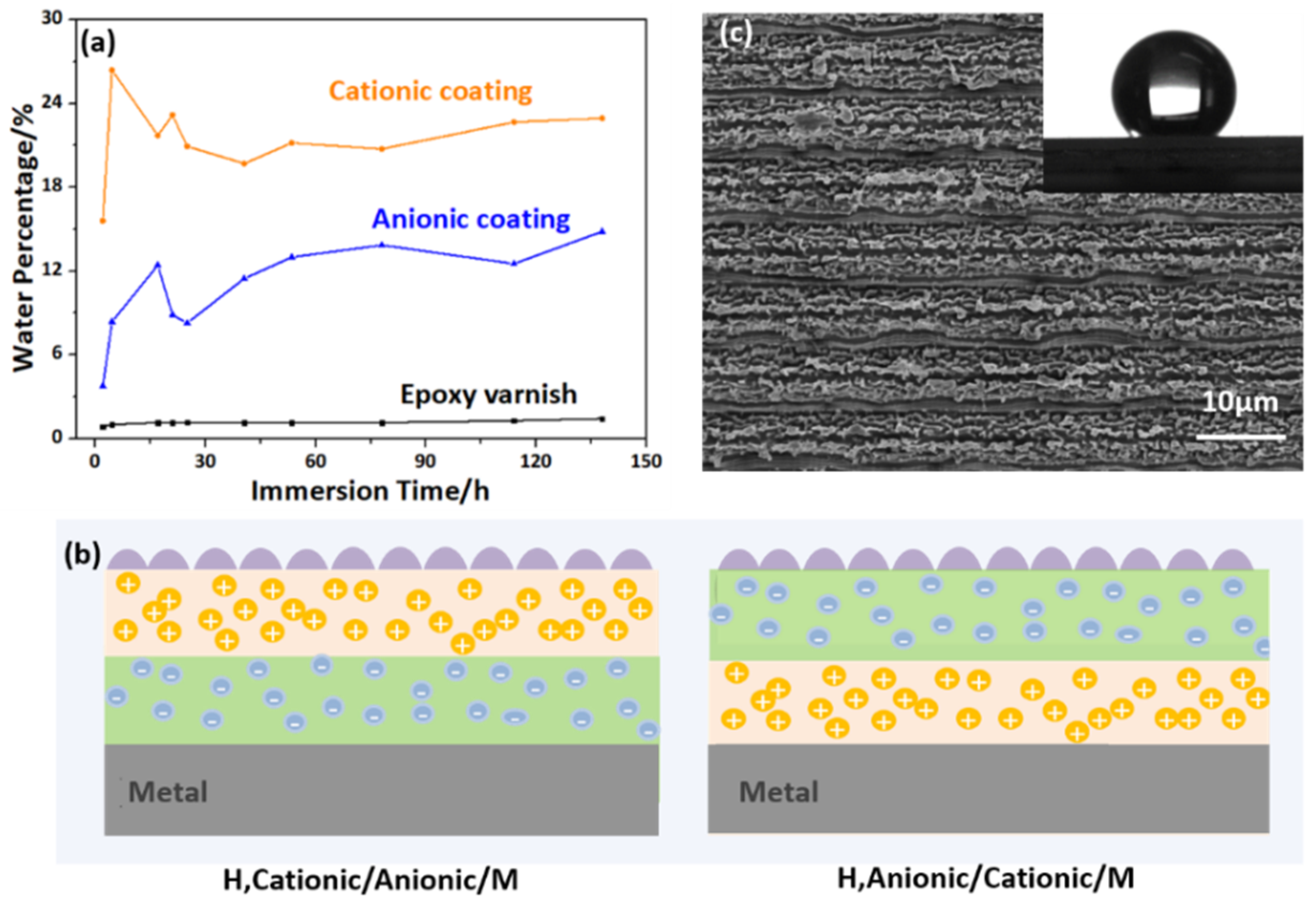
3.2. Design and Properties of Mangrove-Inspired, Single-Polar and Bipolar Coatings
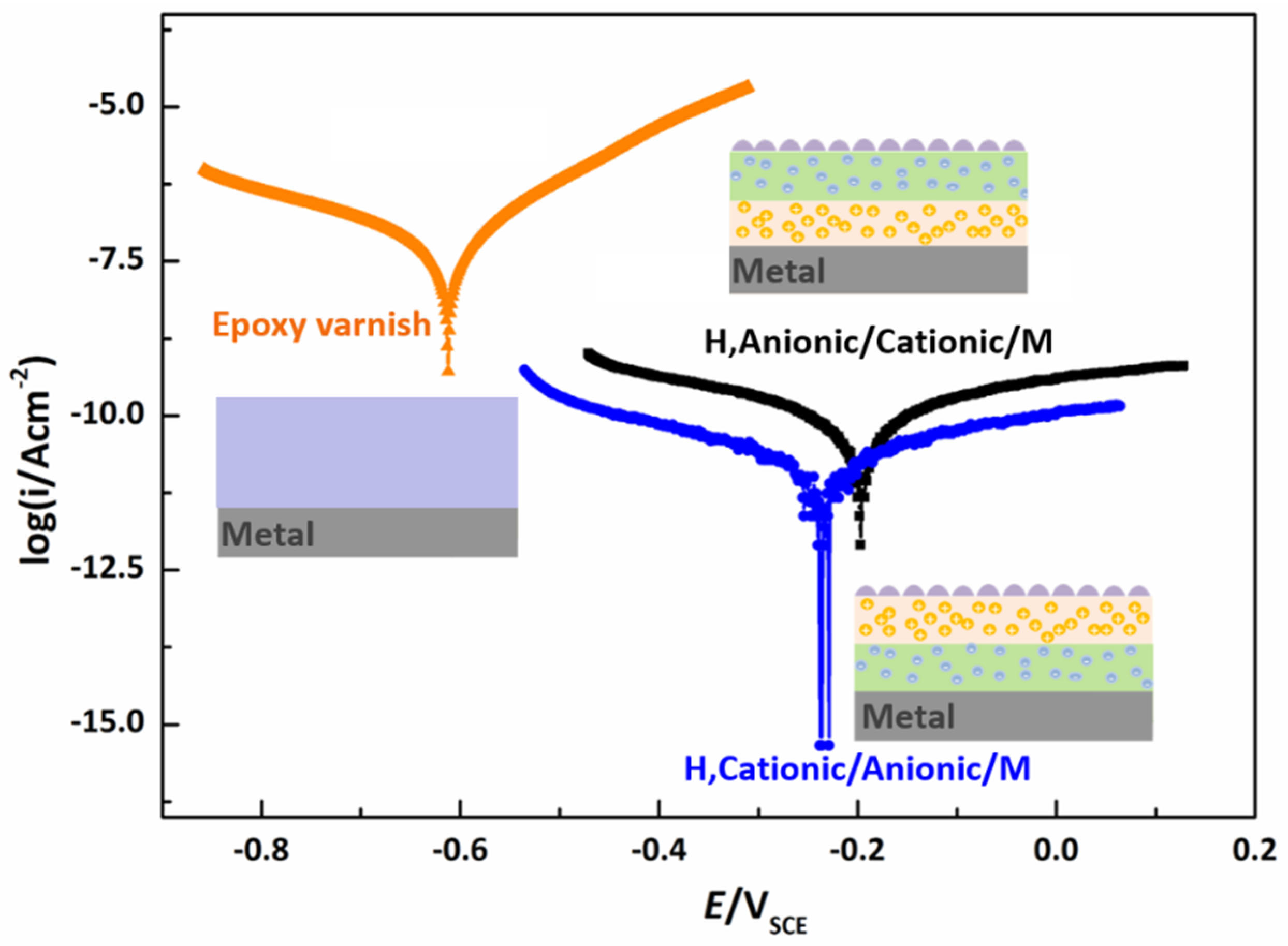
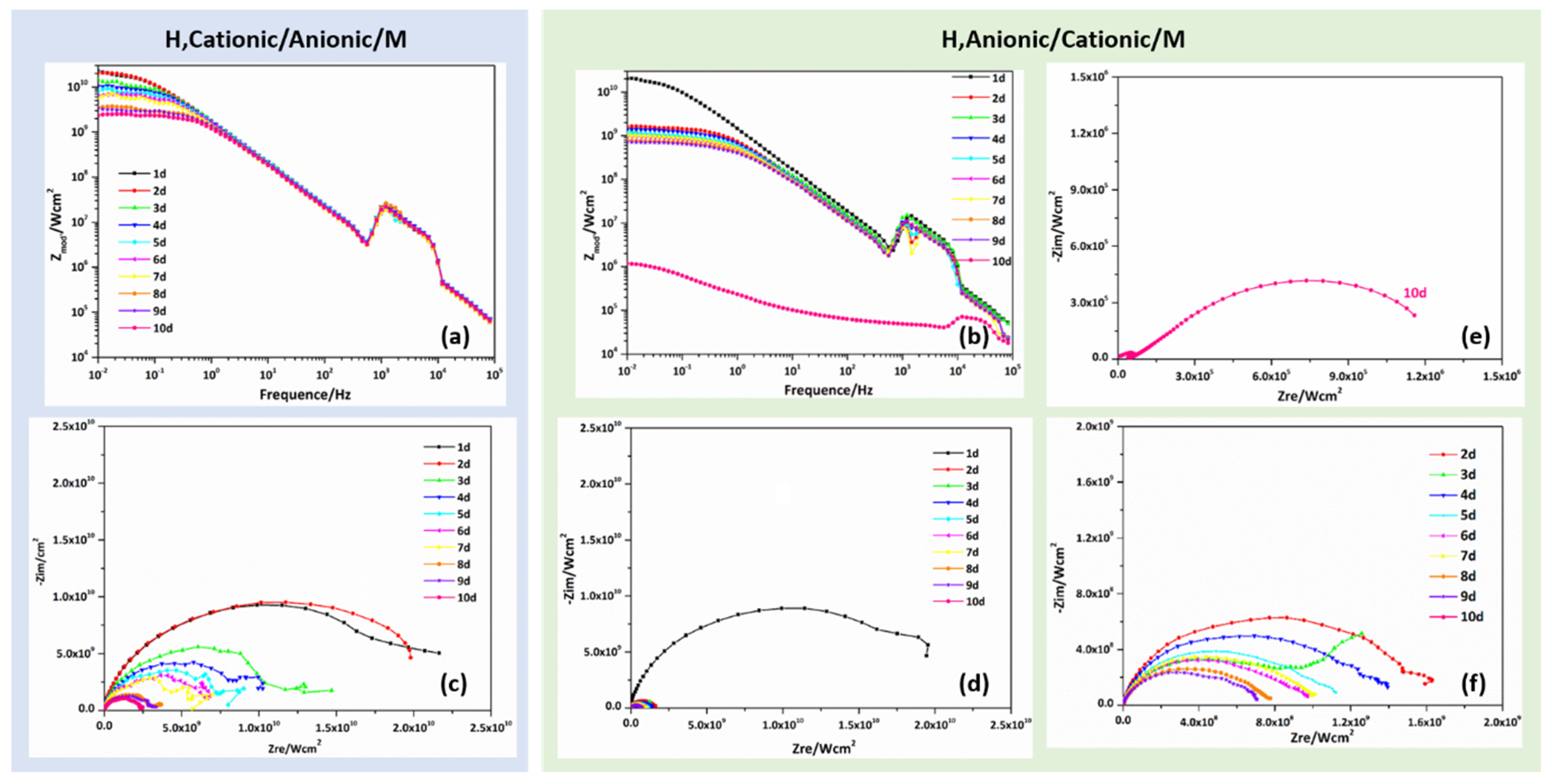
3.3. Anti-Corrosion Performance of the Bipolar, Hydrophobic Coating
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baeckmann, W.V.; Schwenk, W.; Prinz, W. Handbook of Cathodic Corrosion Protection. Biochem. Biophys. Res. Commun. 1997, 307, 810–813. [Google Scholar]
- Baeckmann, W.V.; Schwenk, W.; Prinz, W. The Invention of Protective Devices. In Handbook of Cathodic Corrosion Protection, 3rd ed.; Baeckmann, W.V., Schwenk, W., Eds.; Elsevier: Houston, TX, USA, 1997; pp. 224–229. [Google Scholar]
- International Measures of Prevention, Application, and Economics of Corrosion Technologies Study. Available online: http://impact.nace.org/economic-impact.aspx (accessed on 1 March 2016).
- Cui, M.M.; Wang, B.; Wang, Z.K. Nature-Inspired strategy for anti-corrosion. Adv. Eng. Mater. 2019, 21. [Google Scholar] [CrossRef]
- Wang, B.B.; Wang, Z.Y.; Cao, G.W.; Liu, Y.J.; Ke, W. Local corrosion behavior of 2024 aluminum alloy in salt lake atmosphere of western China. Acta Metall. Sin. 2014, 50, 49–56. [Google Scholar]
- Petrov, N.N.; Falina, I.V.; Koval’, T.V.; Gorokhov, R.V.; Shel’deshov, N.V.; Bukov, N.N. Electrical-percolation effects in epoxy resin/ion-exchange resin/polyaniline anti-corrosion composite materials. Prot. Met. Phys. Chem. 2017, 53, 725–732. [Google Scholar] [CrossRef]
- Dong, Y.H.; Liu, Y.N.; Zhou, Q. Comparative studies on the corrosion protection effect of different ions selective polyaniline coatings. Adv. Mater. Res. 2011, 287, 2464–2469. [Google Scholar] [CrossRef]
- Dong, Y.H.; Zhang, Q.Y.; Su, X.M.; Zhou, Q. Preparation and investigation of the protective properties of bipolar coatings. Prog. Org. Coat. 2013, 76, 662–669. [Google Scholar] [CrossRef]
- Wang, J.G.; Torardi, C.C.; Duch, M.W. Polyaniline-related ion-barrier anti-corrosion coatings. Synth. Met. 2007, 157, 846–850. [Google Scholar] [CrossRef]
- Couture, G.; Alaaeddine, A.; Boschet, F.; Ameduri, B. Polymeric materials as anion-exchange membranes for alkaline fuel cells. Prog. Polym. Sci. 2011, 36, 1521–1557. [Google Scholar] [CrossRef]
- Lyon, S.B.; Bingham, R.; Mills, D.J. Advances in corrosion protection by organic coatings: What we know and what we would like to know. Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Dam-Johansen, K.; Weinell, C.E.; Kiil, S. Cathodic delamination of seawater-immersed anticorrosive coatings: Mapping of parameters affecting the rate. Prog. Org. Coat. 2010, 68, 283–292. [Google Scholar] [CrossRef]
- Montoya, R.; García-Galván, F.R.; Jiménez-Morales, A.; Galván, J.C. A cathodic delamination study of coatings with and without mechanical defects. Corros. Sci. 2014, 84, 432–436. [Google Scholar] [CrossRef]
- Kalendová, A. Effects of particle sizes and shapes of zinc metal on the properties of anticorrosive coatings. Prog. Org. Coat. 2003, 46, 324–332. [Google Scholar]
- Wang, Z.C.; Zhang, Y.Z.; Gu, Z.J. A study on anti-corrosion performance of ion-selective phenolic coatings for carbon steel by EIS. Mater. Prot. 1998, 31, 1–4. [Google Scholar]
- Wang, Z.C.; Gu, Z.J. Corrosion behaviour of copper covered with ion-selective phenolic coatings by EIS. ElectroChemistry 1997, 3, 271–276. [Google Scholar]
- Wang, Z.C.; Gu, Z.J. Electrochemical and corrosion behaviour of A3 steel covered with ion-selective organic coating. J. Xiamen Univ. 1997, 36, 387–393. (In Chinese) [Google Scholar]
- Pereira da Silva, J.E.; Córdoba de Torresi, S.I.; Torresi, R.M. Polyaniline acrylic coatings for corrosion inhibition: The role played by counter-ions. Corros. Sci. 2005, 47, 811–822. [Google Scholar] [CrossRef]
- Wang, H.L.; Liao, S.Y. Salt glands and epidermal non-glandular hair structures in mangrove and coastal plants in DaYa bay. J. Oceanogr. Taiwan Strait 2000, 19, 372–378. [Google Scholar]
- Fu, X.M.; Su, L.R.; Wang, X.Y. A study on the framework of blance sheet of marine biological resources. Pac. J. 2017, 25, 104. [Google Scholar]
- Hao, C.L.; Li, J.; Liu, Y.; Zhou, X.F.; Liu, Y.H.; Liu, R.; Che, L.F.; Zhou, W.Z.; Sun, D.; Li, L.; et al. Superhydrophobic-Like tunable droplet bouncing on slippery liquid interfaces. Nat. Commun. 2015, 6, 7986. [Google Scholar] [CrossRef]
- Liu, C.R.; Sun, J.; Xiang, C.H.; Che, L.F.; Wang, Z.K.; Zhou, X.F. Long-Range spontaneous droplet self-propulsion on wettability gradient surfaces. Sci. Rep. 2017, 7, 7552. [Google Scholar] [CrossRef]
- Liang, W.L.; Ma, X.L.; Wan, P.; Liu, L.Y. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.K.; Lin, Q.S.; Lim, T.M.; Kumar, P.; Loh, C.S. Dynamic secretion changes in the salt glands of the mangrove tree species avicennia officinalis in response to a changing saline environment. Plant Cell Environ. 2013, 36, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Tyerman, S.D. The devil in the detail of secretions. Plant Cell Environ. 2013, 36, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Dassanayake, M.; Larkin, J.C. Making plants break a sweat: The structure, function, and evolution of plant salt glands. Front. Plant Sci. 2017, 8, 406. [Google Scholar] [CrossRef]
| Parameters | Bare Metal | Epoxy Varnish | 2.50 wt % | 5.00 wt % | 10.00 wt % | 20.00 wt % |
|---|---|---|---|---|---|---|
| Ecorr/V | −0.84156 | −0.6171 | −0.56056 | −0.6270 | −0.674 | −0.82369 |
| Icorr/Acm−2 | 3.1212 × 10−6 | 6.1134 × 10−7 | 4.2421 × 10−7 | 1.2796 × 10−7 | 3.0586 × 10−7 | 4.6885 × 10−7 |
| Rp/Ωcm2 | 2.6962 × 105 | 8.9052 × 105 | 1.3214 × 106 | 4.9000 × 106 | 2.2036 × 106 | 1.7568 × 106 |
| Parameters | Bare Metal | Epoxy Varnish | 2.50 wt % | 5.00 wt % | 10.00 wt % | 20.00 wt % |
|---|---|---|---|---|---|---|
| Ecorr/V | −0.84156 | −0.6171 | −0.5724 | −0.5740 | −0.5450 | −0.59219 |
| Icorr/Acm−2 | 3.1213 × 10−6 | 6.1134 × 10−7 | 3.0475 × 10−7 | 2.9602 × 10−7 | 1.0091 × 10−7 | 8.1367 × 10−7 |
| Rp/Ωcm2 | 2.6962 × 105 | 8.9052 × 105 | 1.88 × 106 | 1.89 × 106 | 1.9357 × 106 | 1.9203 × 106 |
| Parameters | Epoxy Varnish | H, Anionic/Cationic/M | H, Cationic/Anionic/M |
|---|---|---|---|
| Ecorr/V | −0.6117 | −0.1941 | −0.2308 |
| Icorr/Acm−2 | 6.1134 × 10−7 | 8.3562 × 10−11 | 1.6966 × 10−11 |
| Rp/Ωcm2 | 8.9052 × 105 | 2.3228 × 109 | 1.3604 × 1010 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, M.; Wang, P.-Y.; Wang, Z.; Wang, B. Mangrove Inspired Anti-Corrosion Coatings. Coatings 2019, 9, 725. https://doi.org/10.3390/coatings9110725
Cui M, Wang P-Y, Wang Z, Wang B. Mangrove Inspired Anti-Corrosion Coatings. Coatings. 2019; 9(11):725. https://doi.org/10.3390/coatings9110725
Chicago/Turabian StyleCui, Miaomiao, Peng-Yuan Wang, Zuankai Wang, and Bin Wang. 2019. "Mangrove Inspired Anti-Corrosion Coatings" Coatings 9, no. 11: 725. https://doi.org/10.3390/coatings9110725
APA StyleCui, M., Wang, P.-Y., Wang, Z., & Wang, B. (2019). Mangrove Inspired Anti-Corrosion Coatings. Coatings, 9(11), 725. https://doi.org/10.3390/coatings9110725






