A Surface Modifier for the Production of Selectively Activated Amino Surface Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
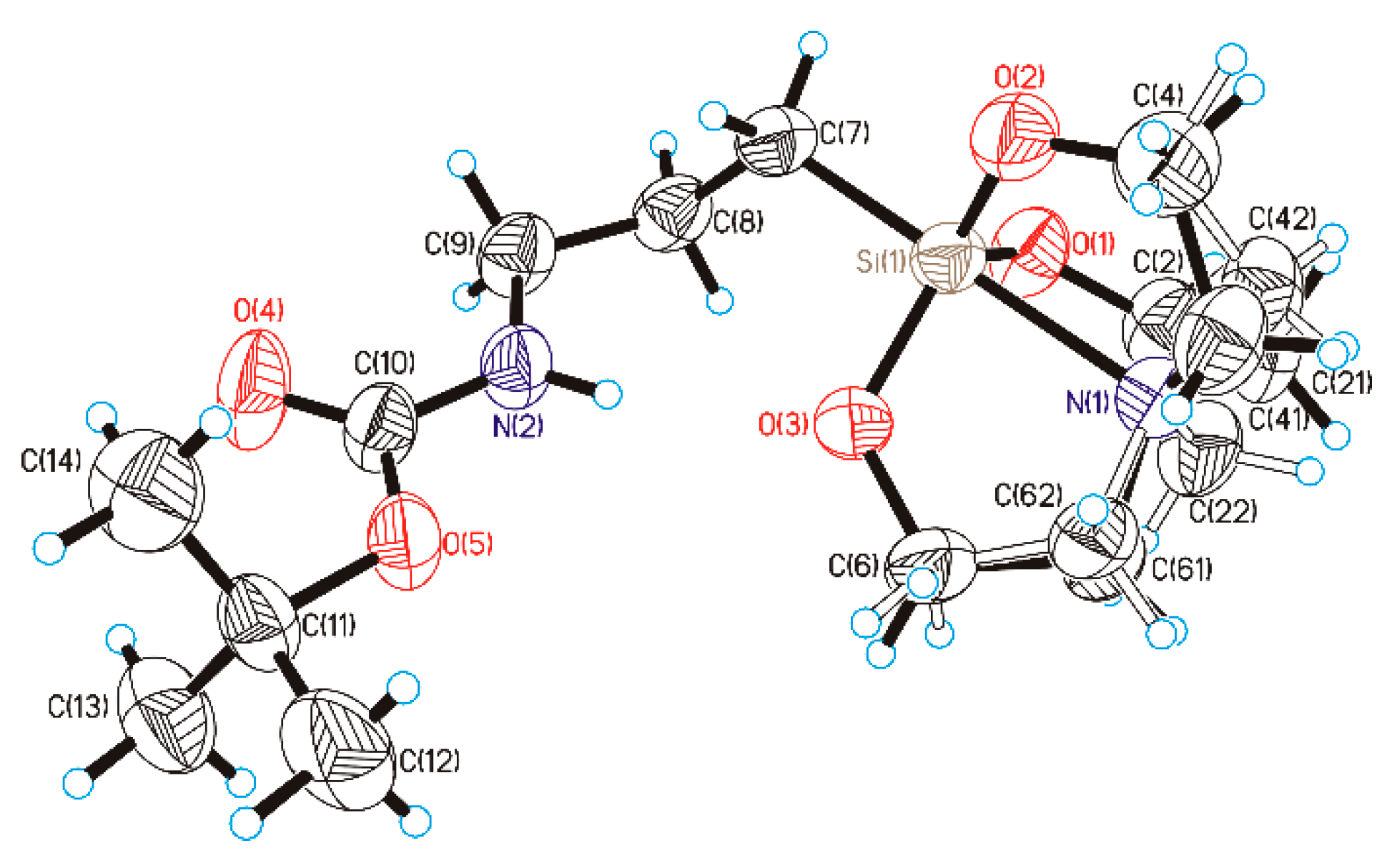
2.2. NMR and Crystallography Measurements
2.3. Monolayer Preparation and Modification
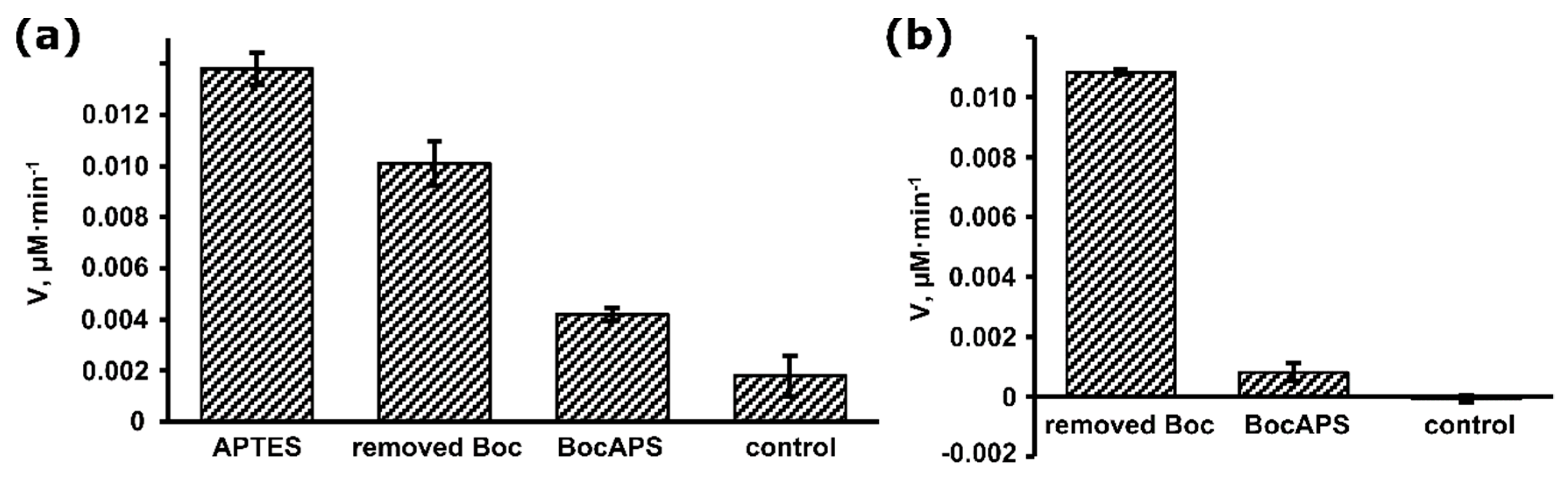
2.4. Measurement of Enzyme Activity
3. Results and Discussion
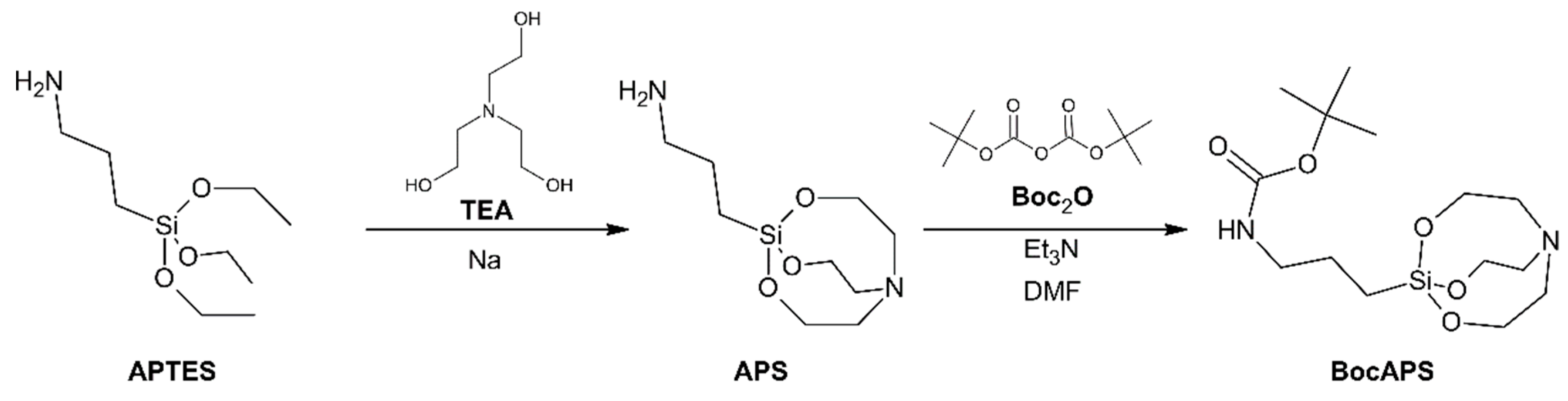
3.1. Synthesis and Characterization of BocAPS
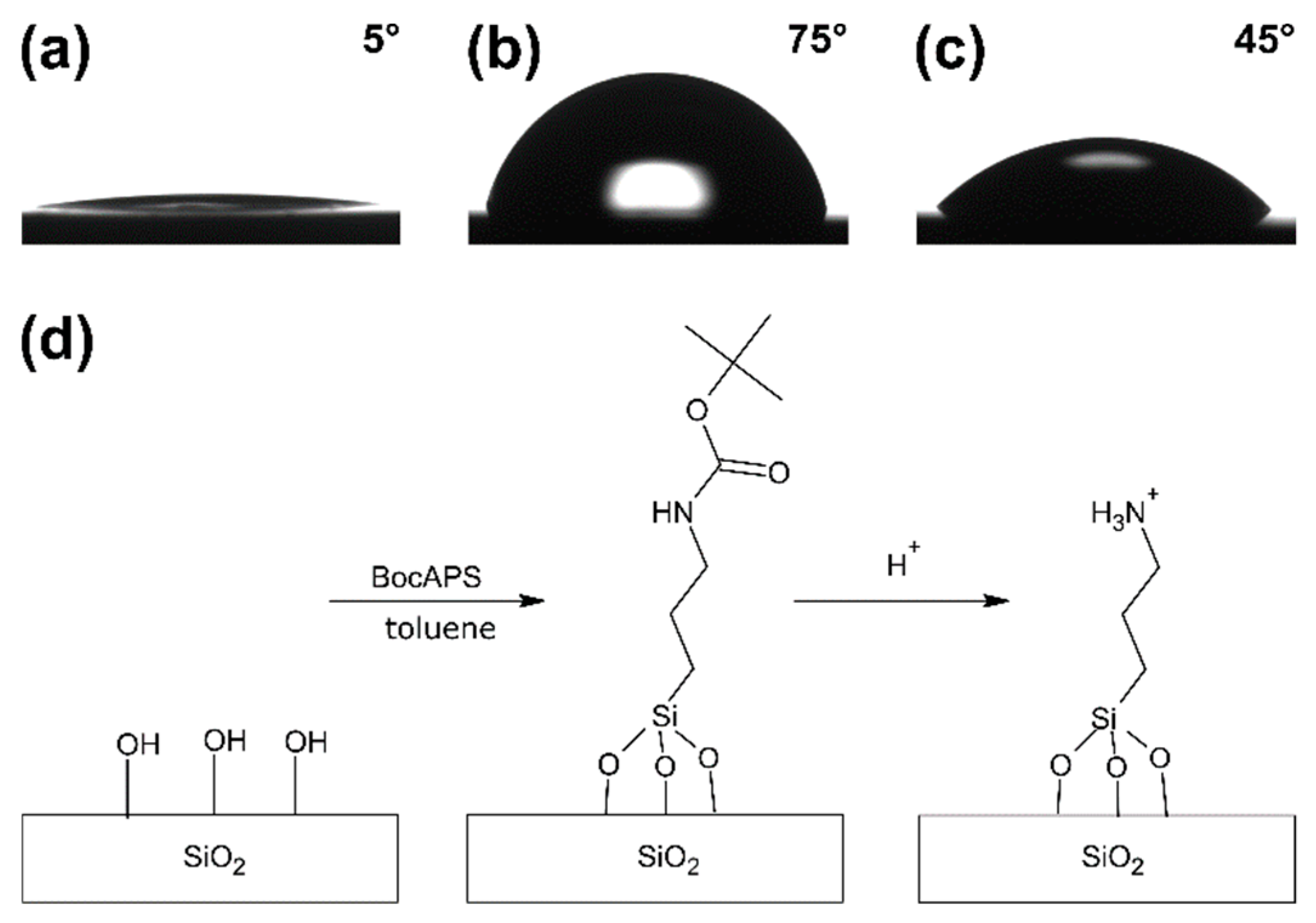
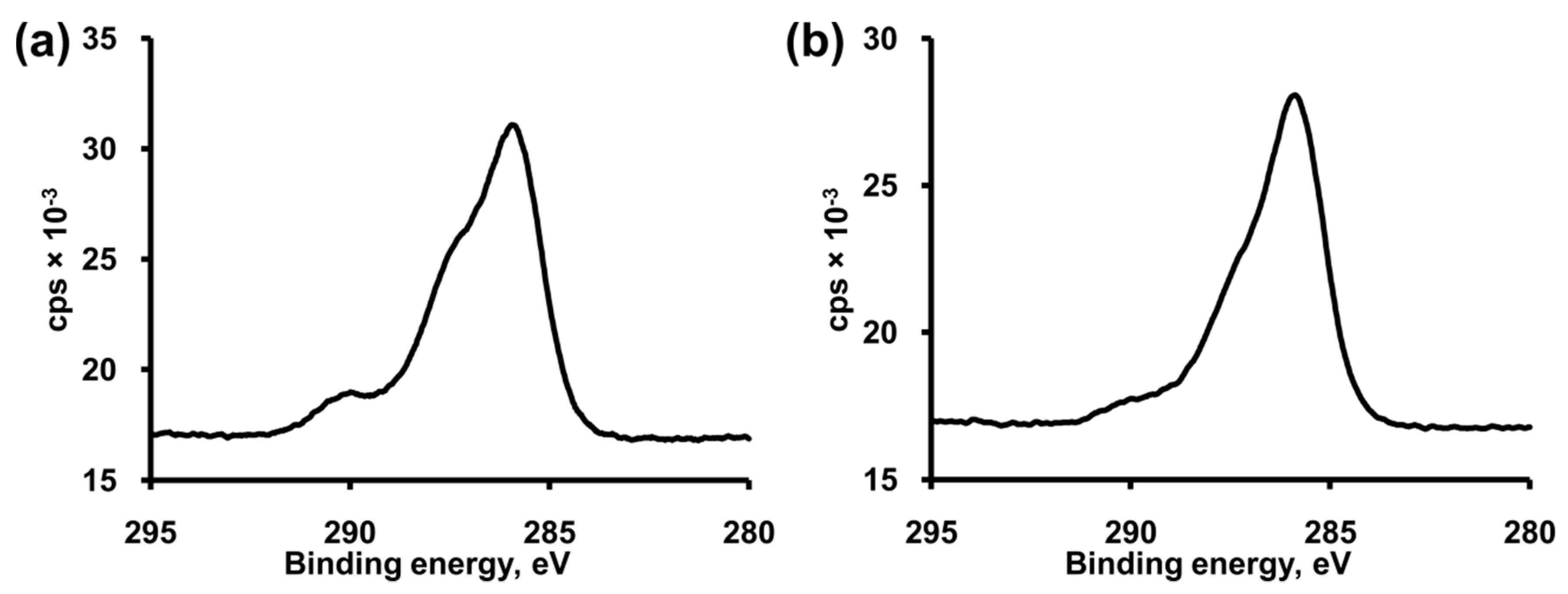
3.2. Formation and Modification of a BocAPS SAM
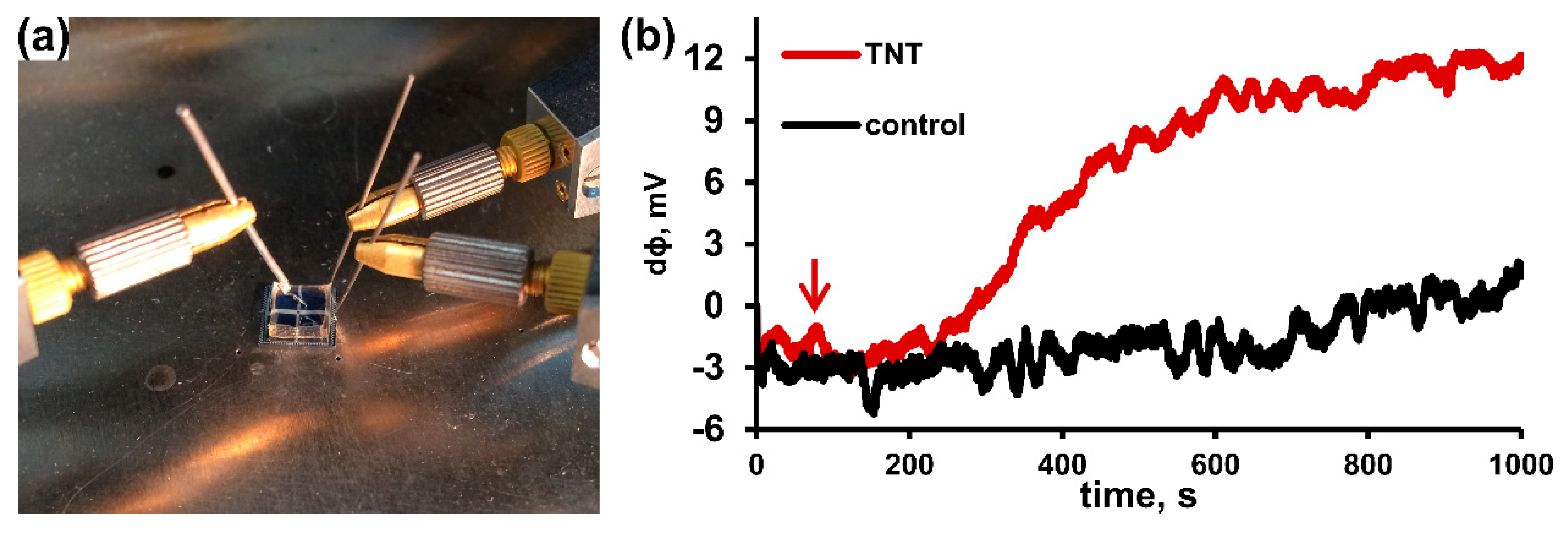
3.3. Biosensor Fabrication Based on BocAPS SAM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Kopelman, R. Mechanism of organosilane self-assembled monolayer formation on silica studied by second-harmonic generation. J. Phys. Chem. 1996, 100, 11014–11018. [Google Scholar] [CrossRef]
- Aswal, D.K.; Lenfant, S.; Guerin, D.; Yakhmi, J.V.; Vuillaume, D. Self assembled monolayers on silicon for molecular electronics. Anal. Chim. Acta. 2006, 568, 84–108. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Lam, E.; Hrapovic, S.; Male, K.B.; Luong, J.H.T. Immobilization of antibodies and enzymes on 3-aminopropyltriethoxysilane-functionalized bioanalytical platforms for biosensors and diagnostics. Chem. Rev. 2014, 114, 11083–11130. [Google Scholar] [CrossRef] [PubMed]
- Howarter, J.A.; Youngblood, J.P. Optimization of silica silanization by 3-aminopropyltriethoxysilane. Langmuir 2006, 22, 11142–11147. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-W.; Hsieh, C.-W.; Kan, H.-C.; Hsieh, M.-L.; Hsieh, S.; Chau, L.-K.; Cheng, T.-E.; Lin, W.-T. Improved performance of aminopropylsilatrane over aminopropyltriethoxysilane as a linker for nanoparticle-based plasmon resonance sensors. Sens. Actuators, B. 2012, 163, 207–215. [Google Scholar] [CrossRef]
- Lyubchenko, Y.L.; Shlyakhtenko, L.S. AFM for analysis of structure and dynamics of DNA and protein–DNA complexes. Methods 2009, 47, 206–213. [Google Scholar] [CrossRef]
- Shlyakhtenko, L.S.; Gall, A.A.; Filonov, A.; Cerovac, Z.; Lushnikov, A.; Lyubchenko, Y.L. Silatrane-based surface chemistry for immobilization of DNA, protein-DNA complexes and other biological materials. Ultramicroscopy 2003, 97, 279–287. [Google Scholar] [CrossRef]
- Greene, T.W.; Wuts, P.G.M. Protective Groups in Organic Synthesis, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Tian, J.; Ma, K.; Saaem, I. Advancing high-throughput gene synthesis technology. Mol. Bio. Syst. 2009, 5, 714–722. [Google Scholar] [CrossRef]
- Clausmeyer, J.; Schuhmann, W.; Plumeré, N. Electrochemical patterning as a tool for fabricating biomolecule microarrays. TrAC, Trends Anal. Chem. 2014, 58, 23–30. [Google Scholar] [CrossRef]
- Johnson, E.K.; Adams, D.J.; Cameron, P.J. Directed self-assembly of dipeptides to form ultrathin hydrogel membranes. J. Am. Chem. Soc. 2010, 132, 5130–5136. [Google Scholar] [CrossRef]
- Egeland, R.D.; Southern, E.M. Electrochemically directed synthesis of oligonucleotides for DNA microarray fabrication. Nucleic Acids Res. 2005, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Maurer, K.; McShea, A.; Strathmann, M.; Dill, K. The removal of the t-BOC group by electrochemically generated acid and use of an addressable electrode array for peptide synthesis. J. Comb. Chem. 2005, 7, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Puchnin, K.; Andrianova, M.; Kuznetsov, A.; Kovalev, V. Field-effect transition sensor for KI detection based on self-assembled calixtube monolayers. Biosens. Bioelectron. 2017, 98, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Gubanova, O.; Andrianova, M.; Saveliev, M.; Komarova, N.; Kuznetsov, E.; Kuznetsov, A. Fabrication and package of ISFET biosensor for micro volume analysis with the use of direct ink writing approach. Mater. Sci. Semicond. Process. 2017, 60, 71–78. [Google Scholar] [CrossRef]
- Andrianova, M.S.; Gubanova, O.V.; Komarova, N.V.; Kuznetsov, E.V.; Kuznetsov, A.E. Development of a biosensor based on phosphotriesterase and n-channel ISFET for detection of pesticides. Sens. Actuators, B. 2015, 221, 1017–1026. [Google Scholar] [CrossRef]
- Hencsei, P.; Kovács, I.; Fülöp, V. The crystal structure of 1-(γ-mercaptopropyl) silatrane. J. Organomet. Chem. 1989, 377, 19–23. [Google Scholar] [CrossRef]
- Komarova, N.V.; Andrianova, M.S.; Saveliev, M.I.; Kuznetsov, A.E. Optimization of silicon dioxide surface functionalization protocol for designing the receptor layer of a biosensor for detecting explosives. Mosc. Univ. Chem. Bulletin. 2016, 71, 25–31. [Google Scholar] [CrossRef]
- Examples of Boc deprotection. Available online: http://commonorganicchemistry.com/Rxn_Pages/Boc_Protection/Boc_Protection_TFA.htm (accessed on 10 September 2019).
- Chow, B.Y.; Emig, C.J.; Jacobson, J.M. Photoelectrochemical synthesis of DNA microarrays. PNAS. 2009, 106, 15219–15224. [Google Scholar] [CrossRef]
- Lund, H.; Hammerich, O. Org. Electrochem., 4th ed.; Marcel Dekker, Inc.: New York, NY, USA, 2001; p. 459. [Google Scholar]
- Marques, M.E.; Mansur, A.A.P.; Mansur, H.S. Chemical functionalization of surfaces for building three-dimensional engineered biosensors. Appl. Surf. Sci. 2013, 275, 347–360. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puchnin, K.; Grudtsov, V.; Andrianova, M.; Bezzubov, S.; Kuznetsov, A. A Surface Modifier for the Production of Selectively Activated Amino Surface Groups. Coatings 2019, 9, 726. https://doi.org/10.3390/coatings9110726
Puchnin K, Grudtsov V, Andrianova M, Bezzubov S, Kuznetsov A. A Surface Modifier for the Production of Selectively Activated Amino Surface Groups. Coatings. 2019; 9(11):726. https://doi.org/10.3390/coatings9110726
Chicago/Turabian StylePuchnin, Kirill, Vitaliy Grudtsov, Maria Andrianova, Stanislav Bezzubov, and Alexander Kuznetsov. 2019. "A Surface Modifier for the Production of Selectively Activated Amino Surface Groups" Coatings 9, no. 11: 726. https://doi.org/10.3390/coatings9110726
APA StylePuchnin, K., Grudtsov, V., Andrianova, M., Bezzubov, S., & Kuznetsov, A. (2019). A Surface Modifier for the Production of Selectively Activated Amino Surface Groups. Coatings, 9(11), 726. https://doi.org/10.3390/coatings9110726






