The Effect of Cathodic Arc Guiding Magnetic Field on the Growth of (Ti0.36Al0.64)N Coatings
Abstract
1. Introduction
2. Materials and Methods
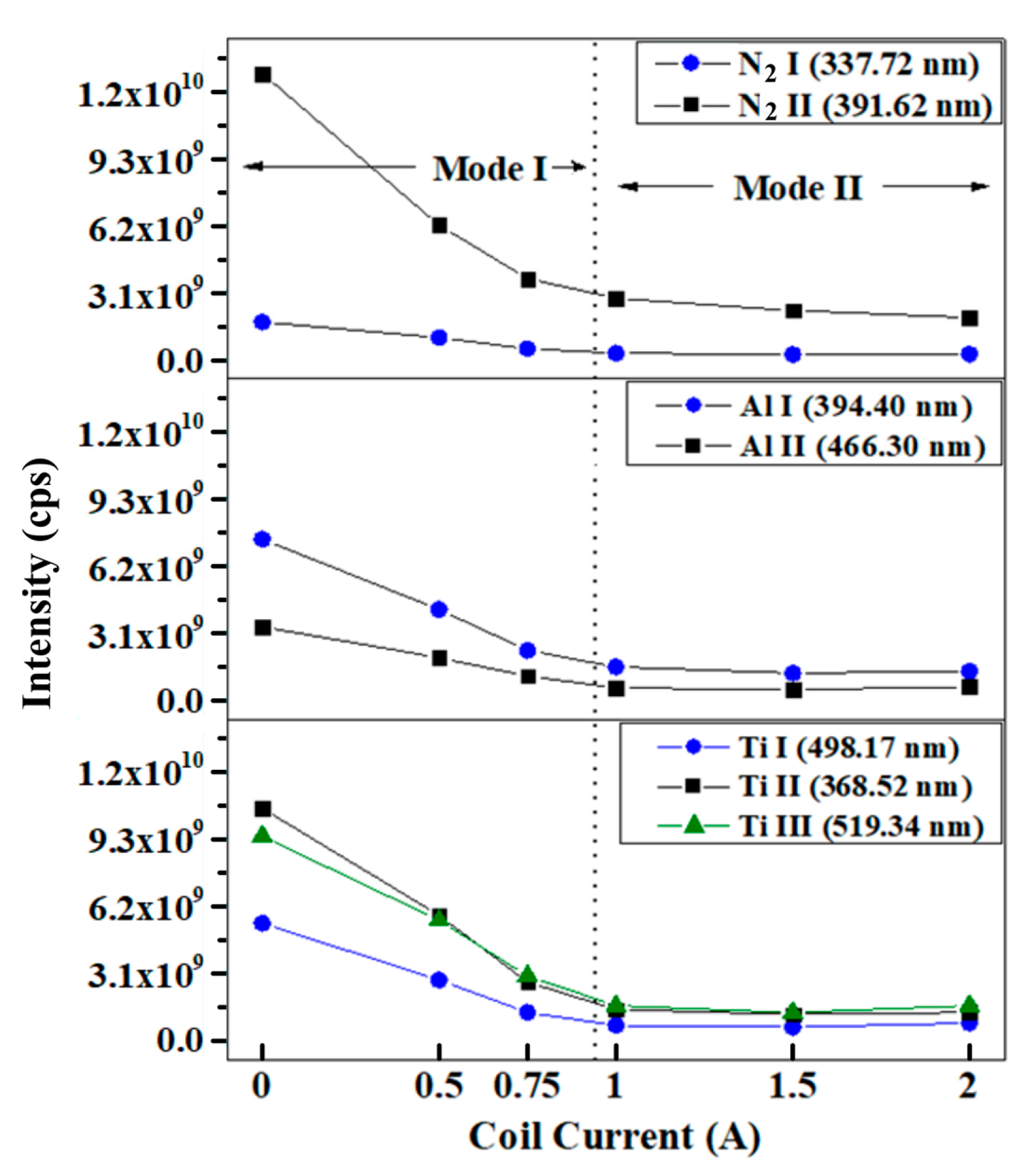
3. Results
4. Discussion
5. Conclusions
- the cathode spots to reside at the periphery of the cathode;
- the increase in cathode spot velocity;
- a decrease in plasma density;
- a decrease in the deposition rate, and
- a decrease of ionization of the plasma species.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Munz, W.D. Titanium aluminum nitride films—A new alternative to tin coatings. J. Vac. Sci. Technol. A 1986, 4, 2717–2725. [Google Scholar] [CrossRef]
- Knutsson, A.; Ullbrand, J.; Rogstrom, L.; Norrby, N.; Johnson, J.S.; Hultman, L.; Almer, J.; Joesaar, M.P.J.; Jansson, B.; Oden, M. Microstructure evolution during the isostructural decomposition of TiAlN-A combined in-situ small angle X-ray scattering and phase field study. J. Appl. Phys. 2013, 113, 213518. [Google Scholar] [CrossRef]
- Bartosik, M.; Rumeau, C.; Hahn, R.; Zhang, Z.L.; Mayrhofer, P.H. Fracture toughness and structural evolution in the TiAlN system upon annealing. Sci. Rep. 2017, 7, 16476. [Google Scholar] [CrossRef] [PubMed]
- Horling, A.; Hultman, L.; Oden, M.; Sjolen, J.; Karlsson, L. Mechanical properties and machining performance of Ti1−xAlxN-coated cutting tools. Surf. Coat. Technol. 2005, 191, 384–392. [Google Scholar] [CrossRef]
- Holleck, H. Metastable coatings—Prediction of composition and structure. Surf. Coat. Technol. 1988, 36, 151–159. [Google Scholar] [CrossRef]
- Greczynski, G.; Mraz, S.; Hans, M.; Lu, J.; Hultman, L.; Schneider, J.M. Control over the phase formation in metastable transition metal nitride thin films by tuning the Al+ subplantation depth. Coatings 2019, 9, 17. [Google Scholar] [CrossRef]
- Shulumba, N.; Hellman, O.; Raza, Z.; Alling, B.; Barrirero, J.; Mucklich, F.; Abrikosov, I.A.; Oden, M. Lattice vibrations change the solid solubility of an alloy at high temperatures. Phys. Rev. Lett. 2016, 117, 205502. [Google Scholar] [CrossRef]
- Horling, A.; Hultman, L.; Oden, M.; Sjolen, J.; Karlsson, L. Thermal stability of arc evaporated high aluminum-conteht Ti1−xAlxN thin films. J. Vac. Sci. Technol. A 2002, 20, 1815–1823. [Google Scholar] [CrossRef]
- Rogstrom, L.; Ullbrand, J.; Almer, J.; Hultman, L.; Jansson, B.; Oden, M. Strain evolution during spinodal decomposition of TiAlN thin films. Thin Solid Film 2012, 520, 5542–5549. [Google Scholar] [CrossRef]
- Calamba, K.M.; Pierson, J.F.; Bruyere, S.; Febvrier, A.L.; Eklund, P.; Barrirero, J.; Mucklich, F.; Boyd, R.; Joesaar, M.P.J.; Oden, M. Dislocation structure and microstrain evolution during spinodal decomposition of reactive magnetron sputtered heteroepixatial c-(Ti-0.37,Al-0.63)N/c-TiN films grown on MgO(001) and (111) substrates. J. Appl. Phys. 2019, 125, 105301. [Google Scholar] [CrossRef]
- Tasnadi, F.; Abrikosov, I.A.; Rogstrom, L.; Almer, J.; Johansson, M.P.; Oden, M. Significant elastic anisotropy in Ti1−xAlxN alloys. Appl. Phys. Lett. 2010, 97, 231902. [Google Scholar] [CrossRef]
- Abrikosov, I.A.; Knutsson, A.; Alling, B.; Tasnadi, F.; Lind, H.; Hultman, L.; Oden, M. Phase stability and elasticity of TiAlN. Materials (Basel) 2011, 4, 1599–1618. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Chen, K.H.; Chen, L.; Zhu, C.J.; Li, P.; Du, Y. Effect of Al and Si additions on microstructure and mechanical properties of TiN coatings. J. Cent. South Univ. Technol. 2011, 18, 310–313. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, Y.C.; Nah, J.W.; Lee, J.J. High temperature oxidation of Ti1−xAlxN coatings made by plasma enhanced chemical vapor deposition. J. Vac. Sci. Technol. A 1999, 17, 133–137. [Google Scholar] [CrossRef]
- Koller, C.M.; Hollerweger, R.; Sabitzer, C.; Rachbauer, R.; Kolozsvari, S.; Paulitsch, J.; Mayrhofer, P.H. Thermal stability and oxidation resistance of arc evaporated TiAlN, TaAlN, TiAlTaN, and TiAlN/TaAlN coatings. Surf. Coat. Technol. 2014, 259, 599–607. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L.; Odén, M. X-ray photoelectron spectroscopy studies of Ti1−xAlxN (0 ≤ x ≤ 0.83) high-temperature oxidation: The crucial role of Al concentration. Surf. Coat. Technol. 2019, 374, 923–934. [Google Scholar] [CrossRef]
- Norrby, N.; Rogstrom, L.; Johansson-Joesaar, M.P.; Schell, N.; Oden, M. In situ X-ray scattering study of the cubic to hexagonal transformation of A1N in Ti1−xAlxN. Acta Mater. 2014, 73, 205–214. [Google Scholar] [CrossRef]
- Joesaar, M.P.J.; Norrby, N.; Ullbrand, J.; M’Saoubi, R.; Oden, M. Anisotropy effects on microstructure and properties in decomposed arc evaporated Ti1−xAlxN coatings during metal cutting. Surf. Coat. Technol. 2013, 235, 181–185. [Google Scholar] [CrossRef]
- Baben, M.T.; Hans, M.; Primetzhofer, D.; Evertz, S.; Ruess, H.; Schneider, J.M. Unprecedented thermal stability of inherently metastable titanium aluminum nitride by point defect engineering. Mater. Res. Lett. 2017, 5, 158–169. [Google Scholar] [CrossRef]
- Norrby, N.; Lind, H.; Parakhonskiy, G.; Johansson, M.P.; Tasnadi, F.; Dubrovinsky, L.S.; Dubrovinskaia, N.; Abrikosov, I.A.; Oden, M. High pressure and high temperature stabilization of cubic AlN in Ti0.60Al0.40N. J. Appl. Phys. 2013, 113, 053515. [Google Scholar] [CrossRef]
- Shulumba, N.; Raza, Z.; Hellman, O.; Janzen, E.; Abrikosov, I.A.; Oden, M. Impact of anharmonic effects on the phase stability, thermal transport, and electronic properties of AlN. Phys. Rev. B 2016, 94, 104305. [Google Scholar] [CrossRef]
- Alling, B.; Oden, M.; Hultman, L.; Abrikosov, I.A. Pressure enhancement of the isostructural cubic decomposition in Ti1−xAlxN. Appl. Phys. Lett. 2009, 95, 181906. [Google Scholar] [CrossRef]
- Knutsson, A.; Johansson, M.P.; Karlsson, L.; Oden, M. Thermally enhanced mechanical properties of arc evaporated Ti(0.34)Al(0.66)N/TiN multilayer coatings. J. Appl. Phys. 2010, 108, 044312. [Google Scholar] [CrossRef]
- Forsen, R.; Johansson, M.; Oden, M.; Ghafoor, N. Decomposition and phase transformation in TiCrAlN thin coatings. J. Vac. Sci. Technol. A 2012, 30, 061506. [Google Scholar] [CrossRef]
- Schramm, I.C.; Joesaar, M.P.J.; Jensen, J.; Mucklich, F.; Oden, M. Impact of nitrogen vacancies on the high temperature behavior of (Ti1−xAlx)Ny alloys. Acta Mater. 2016, 119, 218–228. [Google Scholar] [CrossRef]
- Greczynski, G.; Lu, J.; Johansson, M.; Jensen, J.; Petrov, I.; Greene, J.E.; Hultman, L. Selection of metal ion irradiation for controlling Ti1−xAlxN alloy growth via hybrid HIPIMS/magnetron co-sputtering. Vacuum 2012, 86, 1036–1040. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Geier, M.; Löcker, C.; Chen, L. Influence of deposition conditions on texture development and mechanical properties of TiN coatings. Int. J. Mater. Res. 2009, 100, 1052–1058. [Google Scholar] [CrossRef]
- Andersson, J.M.; Vetter, J.; Müller, J.; Sjölén, J. Structural effects of energy input during growth of Ti1−xAlxN (0.55 ≤ x ≤ 0.66) coatings by cathodic arc evaporation. Surf. Coat. Technol. 2014, 240, 211–220. [Google Scholar] [CrossRef]
- Sarzynski, A.; Skrzeczanowski, W.; Marczak, J. Possibility of the Mechelle 900 spectrometer applications for artwork and technology research. Proc. SPIE 2007, 6598, 65980U. [Google Scholar] [CrossRef]
- Forsberg, P. The spectrum and term system of neutral titanium, Ti-I. Phys. Scr. 1991, 44, 446–476. [Google Scholar] [CrossRef]
- Kaufman, V.; Martin, W.C. Wavelengths and energy-level classifications for the spectra of aluminum (Al-I through Al-Xiii). J. Phys. Chem. Ref. Data 1991, 20, 775–858. [Google Scholar] [CrossRef]
- Sugar, J.; Corliss, C. Atomic-energy levels of the iron-period elements—Potassium through nickel. J. Phys. Chem. Ref. Data 1985, 17, 680. [Google Scholar]
- Edlen, B.; Swensson, J.W. Spectrum of doubly ionized titanium, Ti III. Phys. Scr. 1975, 12, 21–32. [Google Scholar] [CrossRef]
- Lofthus, A.; Krupenie, P.H. Spectrum of molecular nitrogen. J. Phys. Chem. Ref. Data 1977, 6, 113–307. [Google Scholar] [CrossRef]
- Waldo, R.A.; Militello, M.C.; Gaarenstroom, S.W. Quantitative thin-film analysis with an energy-dispersive X-ray detector. Surf. Interface Anal. 1993, 20, 111–114. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Maxwell, A.S.; Owen-Jones, S.; Jennett, N.M. Measurement of Young’s modulus and Poisson’s ratio of thin coatings using impact excitation and depth-sensing indentation. Rev. Sci. Instrum. 2004, 75, 970–975. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic-modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Isberg, B. The spectrum of doubly ionized aluminium, Al III. Ark. Fys. (Stockh.) 1968, 35, 551–562. [Google Scholar]
- Hashmi, S.; Batalha, G.F.; Van Tyne, C.J.; Yilbas, B.S. Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 14; Volume 13, p. 315. [Google Scholar]
- Artru, M.-C.; Kaufman, V. Extension of the analysis of triply ionized aluminum (Al IV). J. Opt. Soc. Am. 1975, 65, 594–599. [Google Scholar] [CrossRef]
- Svensson, L.A. The spectrum of four-times-ionized titanium, Ti V. Phys. Scr. 1976, 13, 235–239. [Google Scholar] [CrossRef]
- Artru, M.-C.; Brillet, W.-Ü.L. Analysis of the spectrum of quadruply ionized aluminum (Al V). J. Opt. Soc. Am. 1974, 64, 1063–1071. [Google Scholar] [CrossRef]
- Swensson, J.W.; Edlén, B. The spectrum of three times ionized titanium, Ti IV. Phys. Scr. 1974, 9, 335–337. [Google Scholar] [CrossRef]
- Oden, M.; Almer, J.; Hakansson, G. The effects of bias voltage and annealing on the microstructure and residual stress of arc-evaporated Cr–N coatings. Surf. Coat. Technol. 1999, 120, 272–276. [Google Scholar] [CrossRef]
- Bilek, M.M.M.; Martin, P.J.; McKenzie, D.R. Influence of gas pressure and cathode composition on ion energy distributions in filtered cathodic vacuum arcs. J. Appl. Phys. 1998, 83, 2965–2970. [Google Scholar] [CrossRef]
- Kimblin, C.W. Erosion and ionization in the cathode spot regions of vacuum arcs. J. Appl. Phys. 1973, 44, 3074–3081. [Google Scholar] [CrossRef]
- Daalder, J.E. Components of cathode erosion in vacuum arcs. J. Phys. D Appl. Phys. 1976, 9, 2379. [Google Scholar] [CrossRef]
- Anders, A. Discharge physics of high power impulse magnetron sputtering. Surf. Coat. Technol. 2011, 205, S1–S9. [Google Scholar] [CrossRef]
- Swift, P.D. Macroparticles in films deposited by steered cathodic arc. J. Phys. D Appl. Phys. 1996, 29, 2025–2031. [Google Scholar] [CrossRef]
- Anders, A. Cathodic Arcs: From Fractal Spots to Energetic Condensation; Springer: New York, NY, USA, 2008; p. 233. [Google Scholar]
- Beilis, I.I. Mechanism of cathode spot splitting in vacuum arcs in an oblique magnetic field. Phys. Plasmas 2015, 22, 103510. [Google Scholar] [CrossRef]
- Kurapov, D.; Krassnitzer, S. Aluminum Titanium Nitride Coating with Adapted Morphology for Enhanced Wear Resistance in Machining Operations and Method Thereof. U.S. Patent 9,447,488 B2, 4 April 2013. [Google Scholar]
- Sanders, D.M.; Anders, A. Review of cathodic arc deposition technology at the start of the new millennium. Surf. Coat. Technol. 2000, 133, 78–90. [Google Scholar] [CrossRef]
- Rosen, J.; Anders, A.; Mraz, S.; Schneider, J.M. Charge-state-resolved ion energy distributions of aluminum vacuum arcs in the absence and presence of a magnetic field. J. Appl. Phys. 2005, 97, 103306. [Google Scholar] [CrossRef]
- Anders, A.; Yushkov, G.Y. Ion flux from vacuum arc cathode spots in the absence and presence of a magnetic field. J. Appl. Phys. 2002, 91, 4824–4832. [Google Scholar] [CrossRef]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2005; Chapter 3; p. 43. [Google Scholar]
- Franz, R.; Polcik, P.; Anders, A. Element- and charge-state-resolved ion energies in the cathodic arc plasma from composite AlCr cathodes in argon, nitrogen and oxygen atmospheres. Surf. Coat. Technol. 2015, 272, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Holec, D.; Rovere, F.; Mayrhofer, P.H.; Barna, P.B. Pressure-dependent stability of cubic and wurtzite phases within the TiN-AlN and CrN-AlN systems. Scr. Mater. 2010, 62, 349–352. [Google Scholar] [CrossRef]
- Bartosik, M.; Daniel, R.; Zhang, Z.; Deluca, M.; Ecker, W.; Stefenelli, M.; Klaus, M.; Genzel, C.; Mitterer, C.; Keckes, J. Lateral gradients of phases, residual stress and hardness in a laser heated Ti0.52Al0.48N coating on hard metal. Surf. Coat. Technol. 2012, 206, 4502–4510. [Google Scholar] [CrossRef] [PubMed]

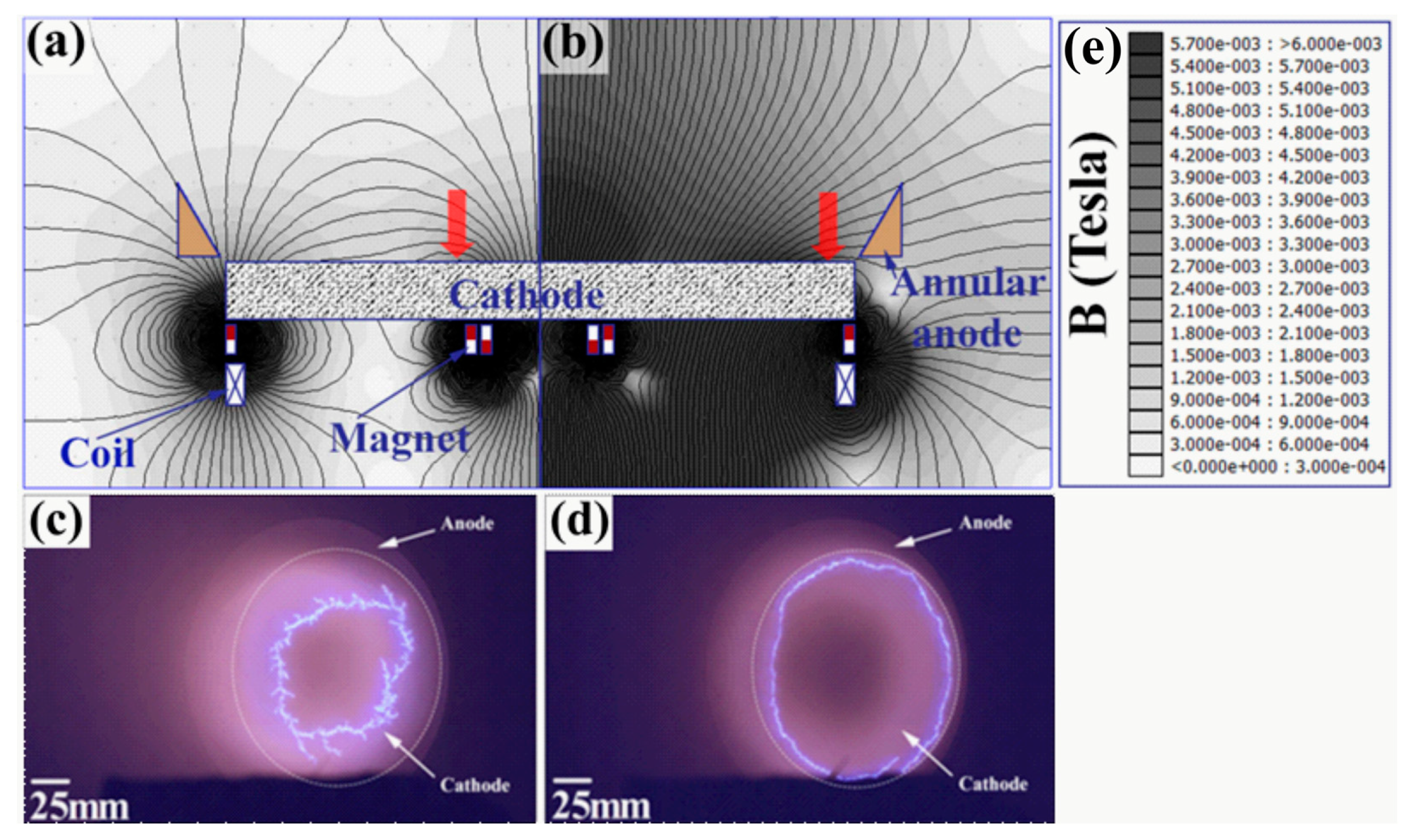
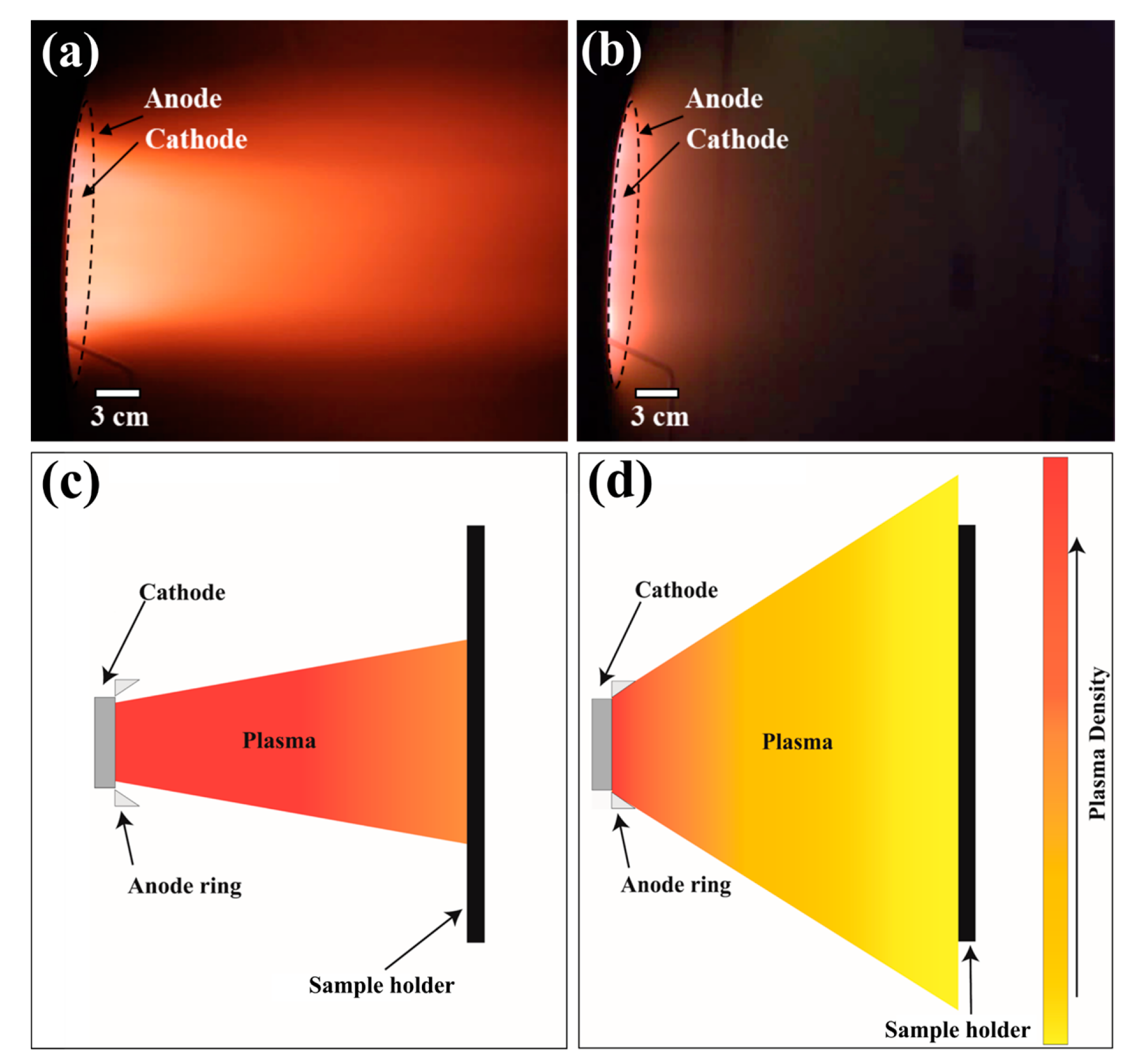
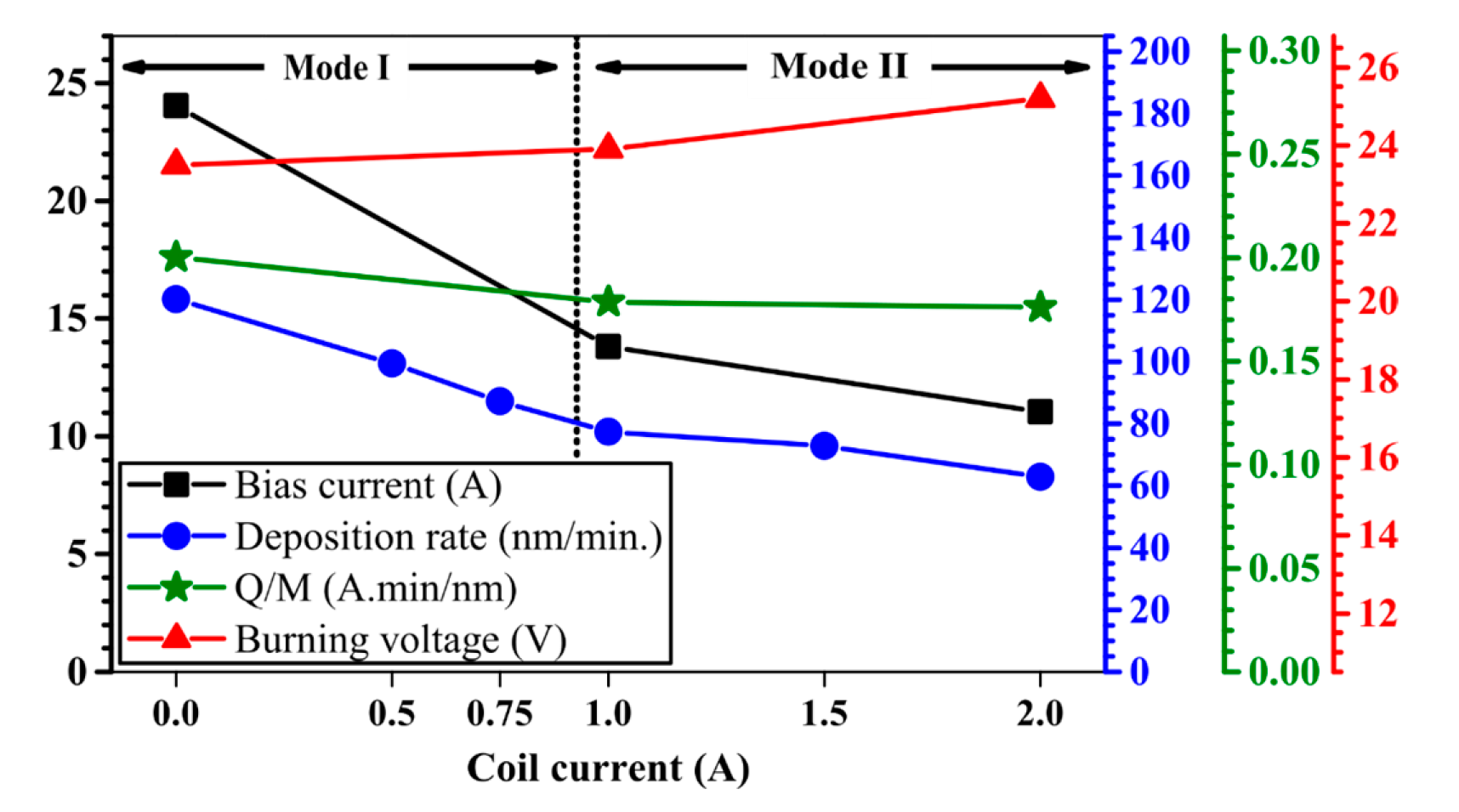
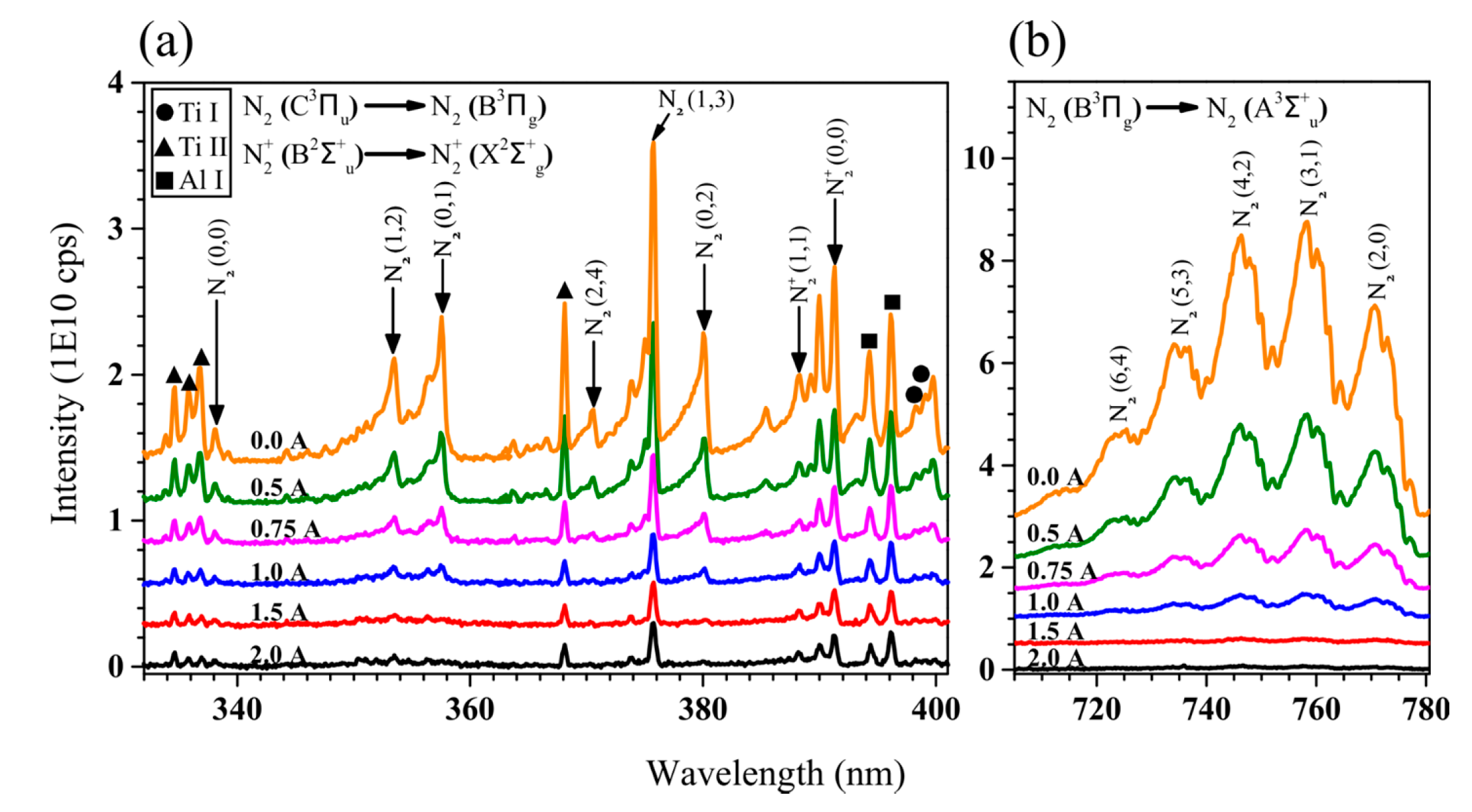
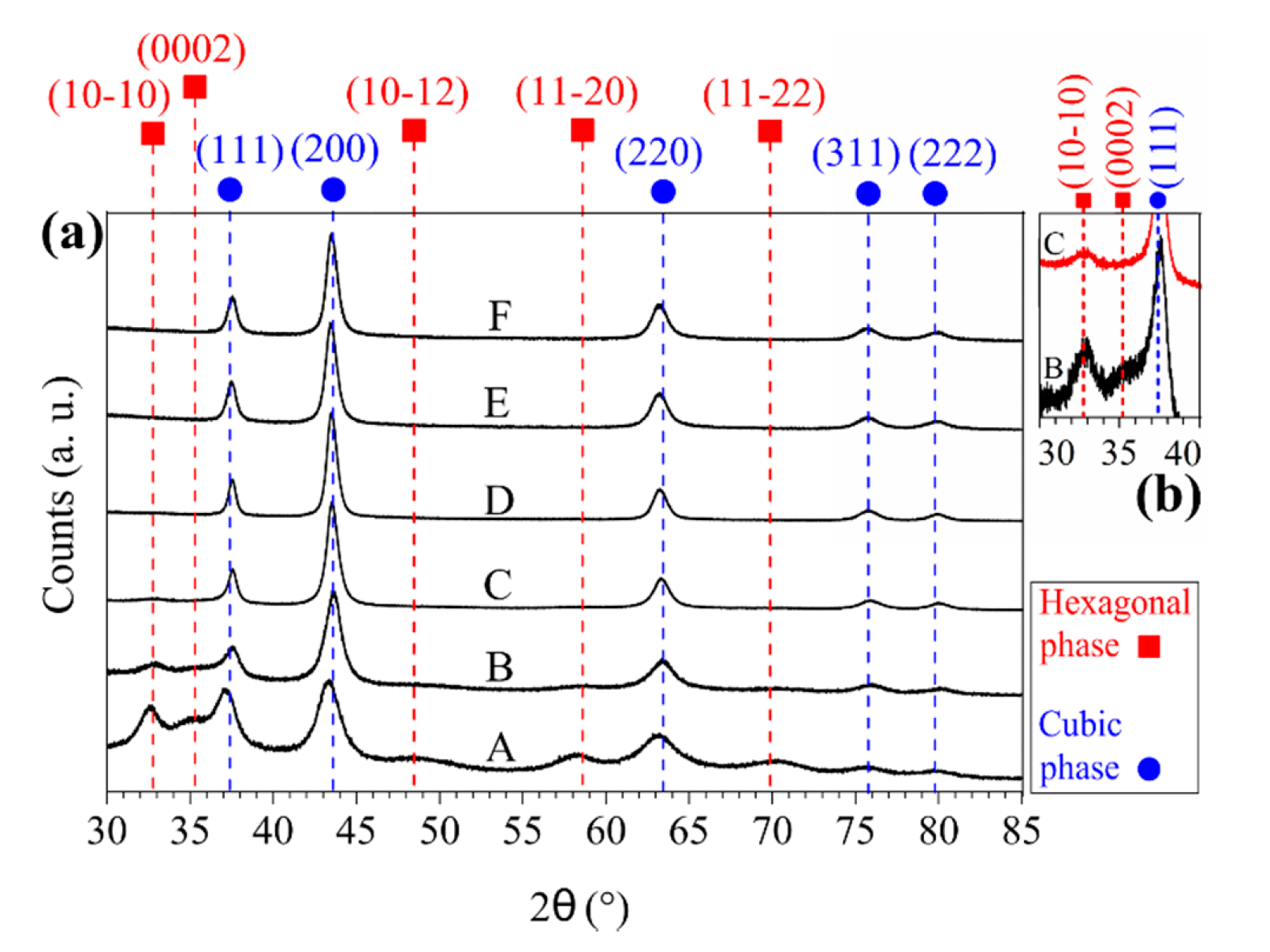
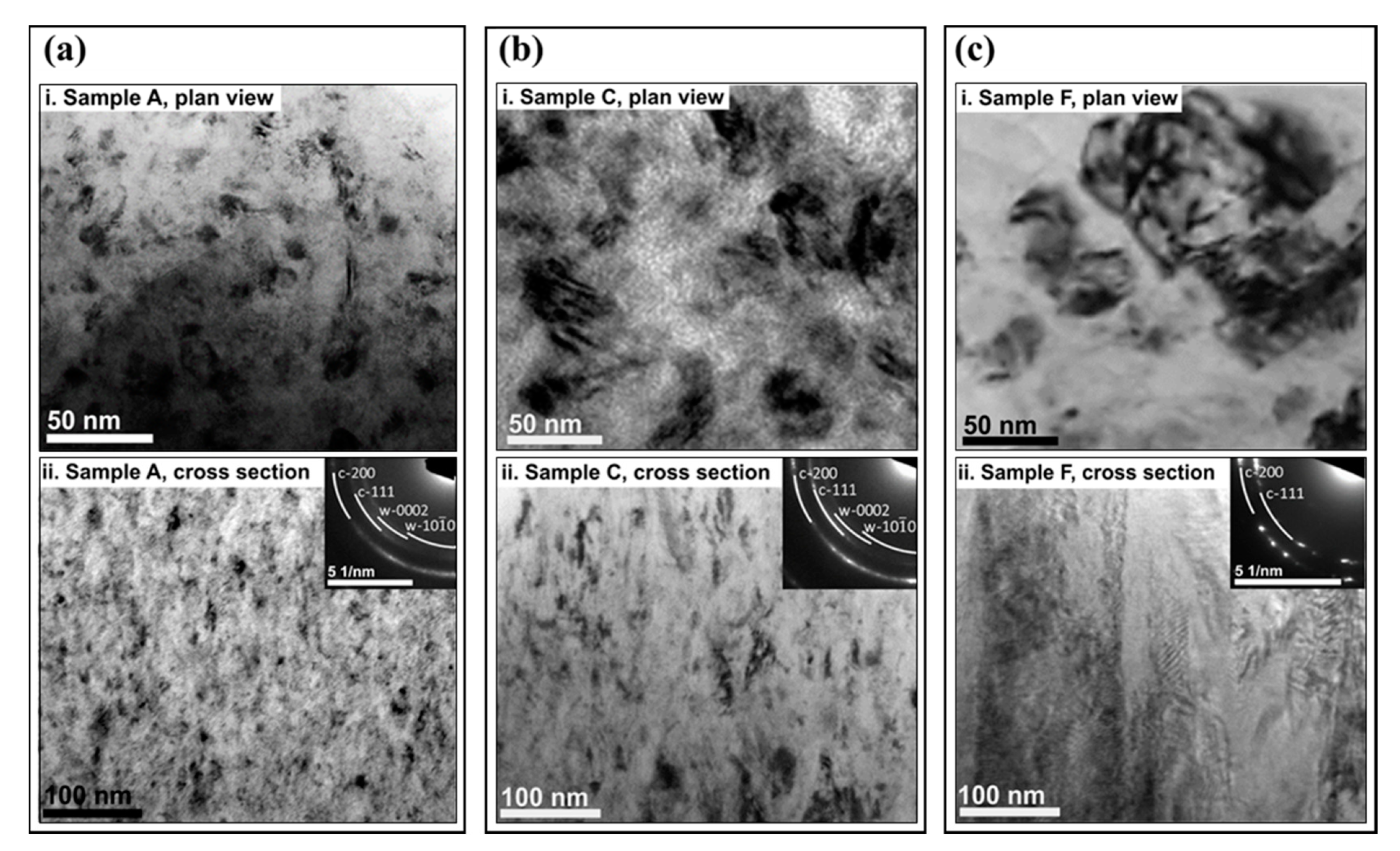
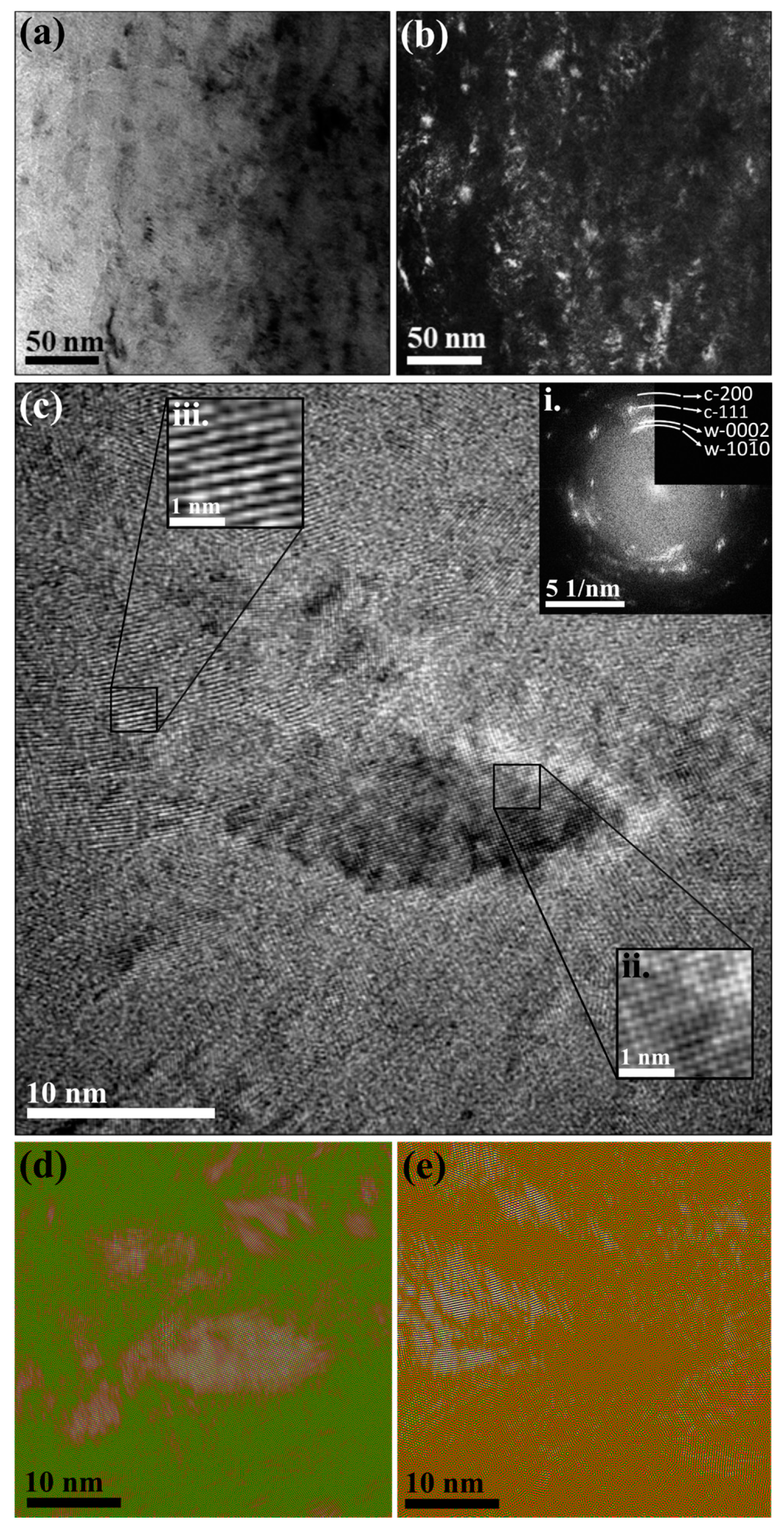
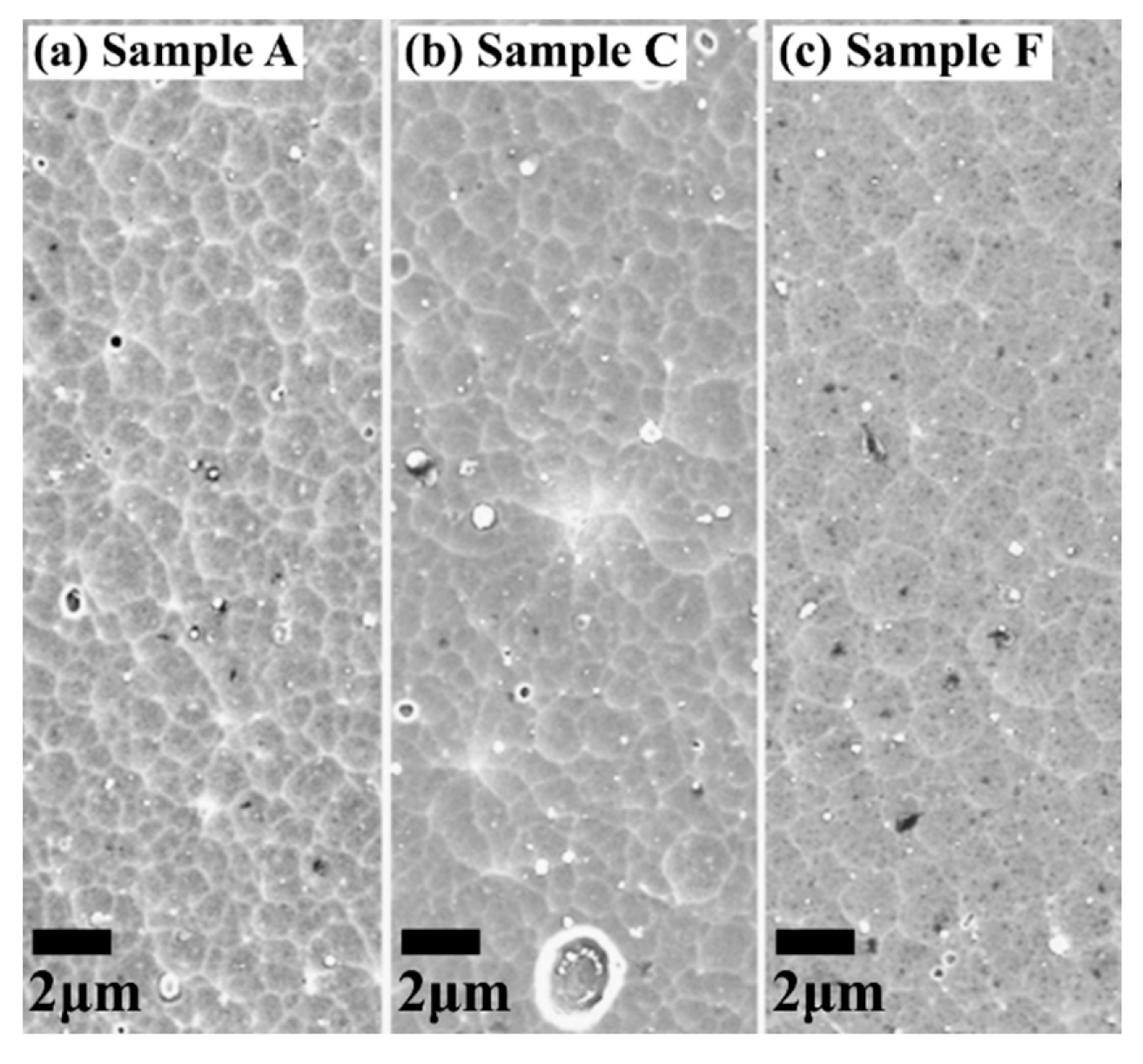
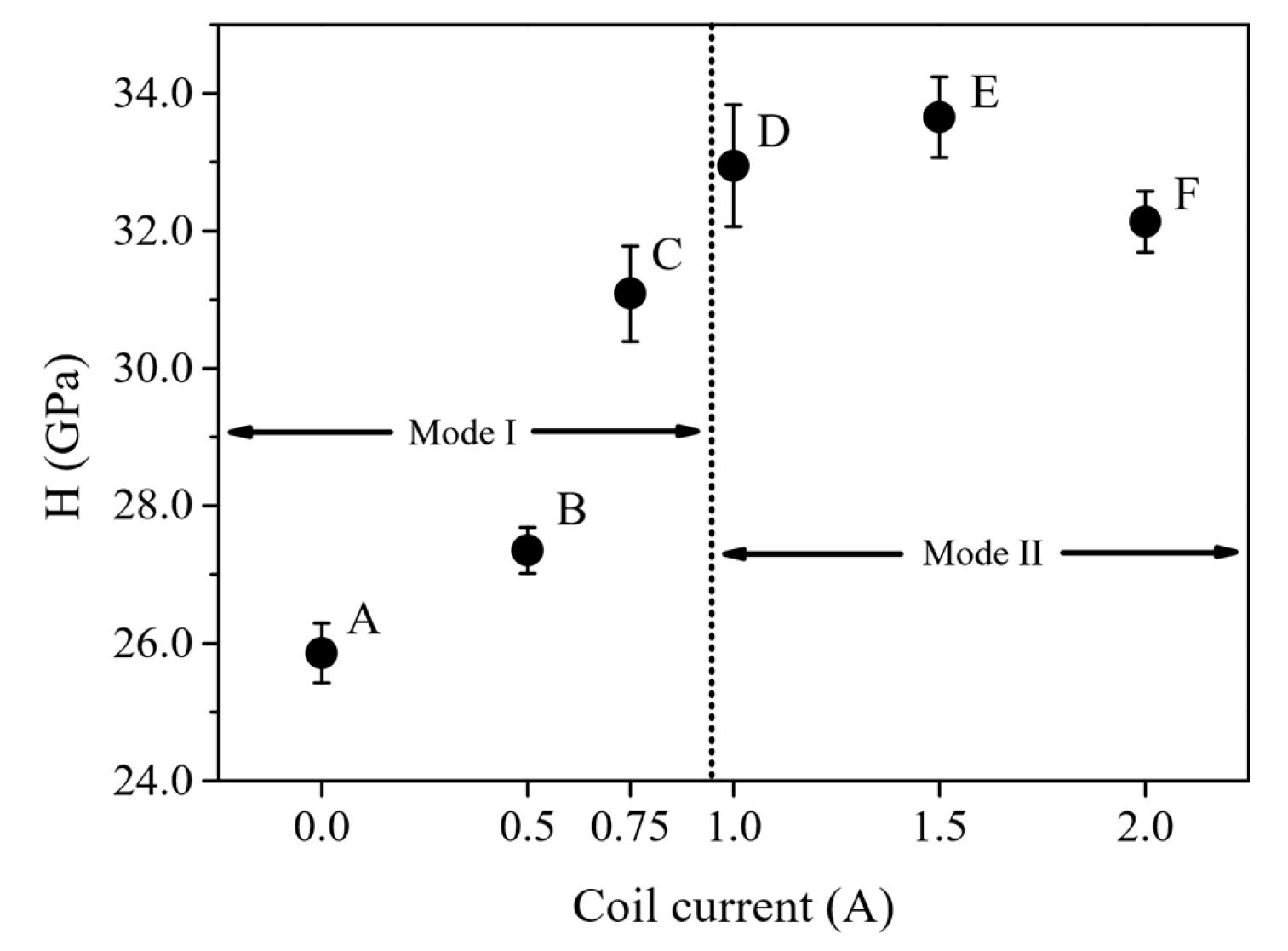
| Sample ID | Coil Current (A) | Coating Thickness (µm) | Residual Stress (GPa) |
|---|---|---|---|
| A | 0.0 | 15 ± 0.4 | −4.6 ± 0.5 |
| B | 0.5 | 12.4 ± 0.3 | −4.6 ± 0.5 |
| C | 0.75 | 10.9 ± 0.4 | −4.5 ± 0.4 |
| D | 1.0 | 9.7 ± 0.4 | −4.4 ± 0.2 |
| E | 1.5 | 9.1 ± 0.4 | −3.4 ± 0.2 |
| F | 2.0 | 7.85 ± 0.3 | −3.6 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaar, A.B.B.; Syed, B.; Hsu, T.-W.; Johansson-Jöesaar, M.; Andersson, J.M.; Henrion, G.; Johnson, L.J.S.; Mücklich, F.; Odén, M. The Effect of Cathodic Arc Guiding Magnetic Field on the Growth of (Ti0.36Al0.64)N Coatings. Coatings 2019, 9, 660. https://doi.org/10.3390/coatings9100660
Chaar ABB, Syed B, Hsu T-W, Johansson-Jöesaar M, Andersson JM, Henrion G, Johnson LJS, Mücklich F, Odén M. The Effect of Cathodic Arc Guiding Magnetic Field on the Growth of (Ti0.36Al0.64)N Coatings. Coatings. 2019; 9(10):660. https://doi.org/10.3390/coatings9100660
Chicago/Turabian StyleChaar, Ana B. B., Bilal Syed, Tun-Wei Hsu, Mats Johansson-Jöesaar, Jon M. Andersson, Gérard Henrion, Lars J. S. Johnson, Frank Mücklich, and Magnus Odén. 2019. "The Effect of Cathodic Arc Guiding Magnetic Field on the Growth of (Ti0.36Al0.64)N Coatings" Coatings 9, no. 10: 660. https://doi.org/10.3390/coatings9100660
APA StyleChaar, A. B. B., Syed, B., Hsu, T.-W., Johansson-Jöesaar, M., Andersson, J. M., Henrion, G., Johnson, L. J. S., Mücklich, F., & Odén, M. (2019). The Effect of Cathodic Arc Guiding Magnetic Field on the Growth of (Ti0.36Al0.64)N Coatings. Coatings, 9(10), 660. https://doi.org/10.3390/coatings9100660






