Nanoengineered Antibacterial Coatings and Materials: A Perspective
Abstract
1. Introduction
2. Antibacterial Coatings
Plasma Deposition
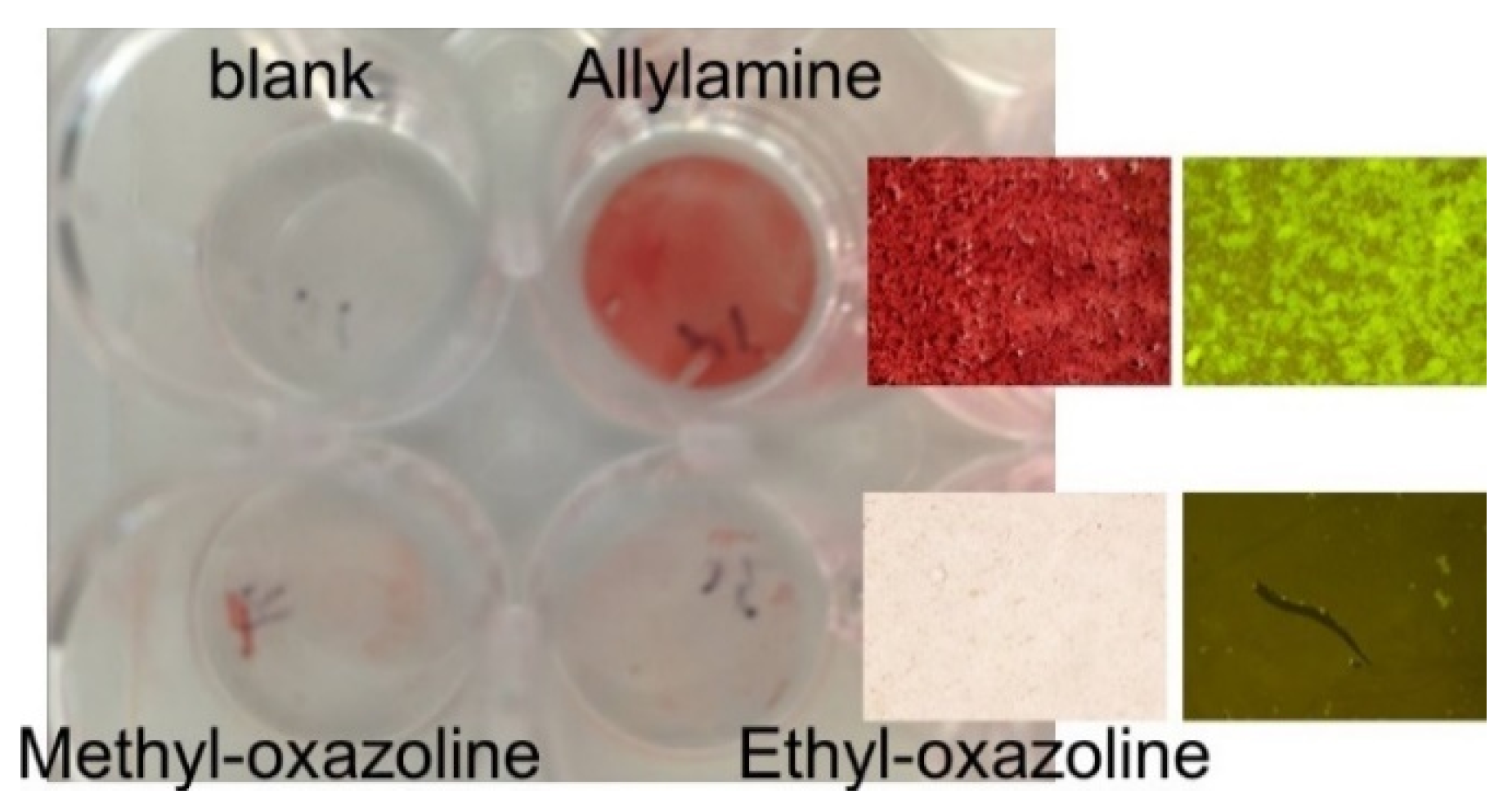
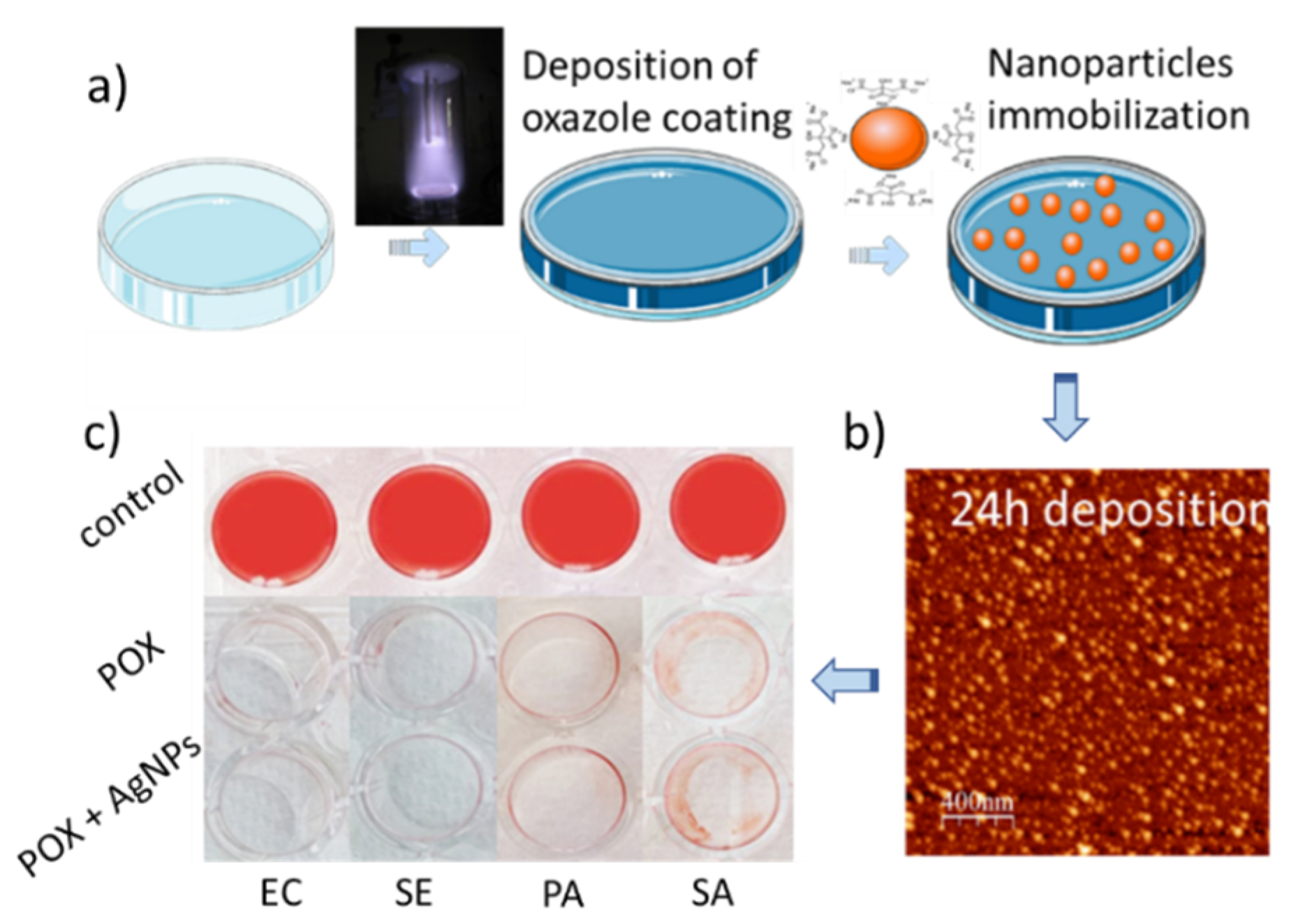
3. Oxazoline Based Coatings That Inhibit Biofilm Growth
4. Contact Killing Surfaces
5. Releasing Surfaces
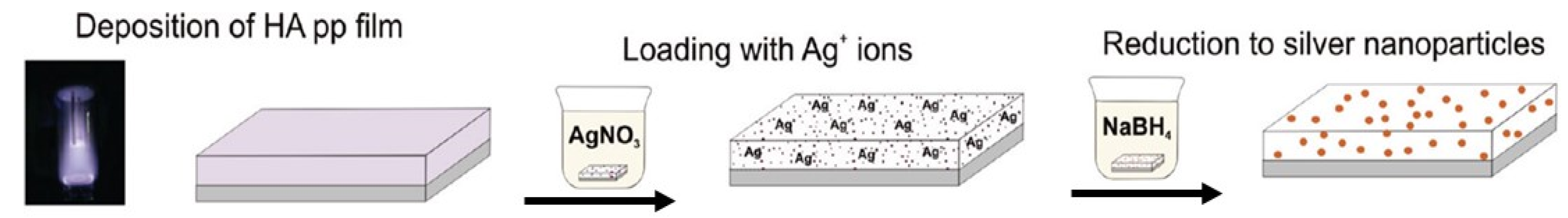
5.1. Silver Containing Coatings and Materials
5.2. Release of Conventional Antibiotics, Nitric Oxide and Antibacterial Polymers and Peptides
6. Responsive Coatings
7. Future Outlook and Challenges
Funding
Conflicts of Interest
References
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K.; Cook, J.; Griesser, H.J. Antibacterial surfaces for biomedical devices. Expert Rev. Med. Devices 2009, 6, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Bozic, K.; Kurtz, S.M.; Lau, E.; Ong, K.; Vail, T.P.; Rubash, H.E.; Berry, D.J. The epidemiology of revision total knee arthroplasty in the United States. J. Arthroplast. 2009, 24, e49. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic burden of periprosthetic joint infection in the United States. J. Arthroplast. 2012, 27, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.J. Use of antimicrobial catheter lock solutions to prevent catheter-related bacteremia. Clin. J. Am. Soc. Nephrol. 2007, 2, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.W.; Dent, H.; Hawley, C.M.; McDonald, S.P.; Rosman, J.B.; Brown, F.G.; Bannister, K.M.; Wiggins, K.J. Associations of dialysis modality and infectious mortality in incident dialysis patients in Australia and New Zealand. Am. J. Kidney Dis. 2009, 53, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Dimick, J.B.; Pelz, R.K.; Consunji, R.; Swoboda, S.M.; Hendrix, C.W.; Lipsett, P.A. Increased resource use associated with catheter-related bloodstream infection in the surgical intensive care unit. Arch. Surg. 2001, 136, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O. Current concepts—Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, R.; Brunetto, P.S.; Woischnig, A.K.; Varisco, M.; Rajacic, Z.; Vosbeck, J.; Terracciano, L.; Fromm, K.M.; Khanna, N. Preventing implant-associated infections by silver coating. Antimicrob. Agents Chemother. 2016, 60, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Current concepts: Prosthetic-joint infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef]
- Vasilev, K.; Griesser, S.S.; Griesser, H.J. Antibacterial surfaces and coatings produced by plasma techniques. Plasma Process. Polym. 2011, 8, 1010–1023. [Google Scholar] [CrossRef]
- Cavallaro, A.; Taheri, S.; Vasilev, K. Responsive and “smart” antibacterial surfaces: Common approaches and new developments (Review). Biointerphases 2014, 9, 029005. [Google Scholar] [CrossRef] [PubMed]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, A.A.; MacGregor-Ramiasa, M.N.; Vasilev, K. Antibiofouling properties of plasma-deposited Oxazoline-based thin films. ACS Appl. Mater. Interfaces 2016, 8, 6354–6362. [Google Scholar] [CrossRef] [PubMed]
- Ramiasa, M.N.; Cavallaro, A.A.; Mierczynska, A.; Christo, S.N.; Gleadle, J.M.; Hayball, J.D.; Vasilev, K. Plasma polymerised polyoxazoline thin films for biomedical applications. Chem. Commun. 2015, 51, 4279–4282. [Google Scholar] [CrossRef] [PubMed]
- Michl, T.D.; Coad, B.R.; Hüsler, A.; Valentin, J.D.P.; Vasilev, K.; Griesser, H.J. Effects of precursor and deposition conditions on prevention of bacterial biofilm growth on Chlorinated plasma polymers. Plasma Process. Polym. 2016, 13, 654–662. [Google Scholar] [CrossRef]
- Michl, T.D.; Coad, B.R.; Doran, M.; Hüsler, A.; Valentin, J.D.P.; Vasilev, K.; Griesser, H.J. Plasma polymerization of 1,1,1-trichloroethane yields a coating with robust antibacterial surface properties. RSC Adv. 2014, 4, 27604–27606. [Google Scholar] [CrossRef]
- Michl, T.D.; Barz, J.; Giles, C.; Haupt, M.; Henze, J.H.; Mayer, J.; Futrega, K.; Doran, M.R.; Oehr, C.; Vasilev, K.; et al. Plasma polymerization of TEMPO yields coatings containing stable Nitroxide radicals for controlling interactions with Prokaryotic and Eukaryotic Cells. ACS Appl. Nano Mater. 2018, 1, 6587–6595. [Google Scholar] [CrossRef]
- Cavallaro, A.; Mierczynska, A.; Barton, M.; Majewski, P.; Vasilev, K. Influence of immobilized quaternary ammonium group surface density on antimicrobial efficacy and cytotoxicity. Biofouling 2016, 32, 13–24. [Google Scholar] [CrossRef]
- Cavallaro, A.; Majewski, P.; Barton, M.; Vasilev, K. Substrate independent approach for immobilisation of quaternary ammonium compounds to surfaces to reduce bio-burden. Mater. Sci. Forum 2014, 783, 1389–1395. [Google Scholar] [CrossRef]
- Vasilev, K.; Poulter, N.; Martinek, P.; Griesser, H.J. Controlled release of levofloxacin sandwiched between two plasma polymerized layers on a solid carrier. ACS Appl. Mater. Interfaces 2011, 3, 4831–4836. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, A.; Vasilev, K. Controlled and sustained release of pharmaceuticals via single step solvent-free encapsulation. Chem. Commun. 2015, 51, 1838–1841. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Michl, T.D.; Coad, B.R.; Doran, M.; Osiecki, M.; Kafshgari, M.H.; Voelcker, N.H.; Hüsler, A.; Vasilev, K.; Griesser, H.J. Nitric oxide releasing plasma polymer coating with bacteriostatic properties and no cytotoxic side effects. Chem. Commun. 2015, 51, 7058–7060. [Google Scholar] [CrossRef] [PubMed]
- Kafshgari, M.H.; Cavallaro, A.; Delalat, B.; Harding, F.J.; McInnes, S.J.P.; Mäkilä, E.; Salonen, J.; Vasilev, K.; Voelcker, N.H. Nitric oxide-releasing porous silicon nanoparticles. Nanoscale Res. Lett. 2014, 9, 333. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh Kafshgari, M.; Delalat, B.; Harding, F.J.; Cavallaro, A.; Mäkilä, E.; Salonen, J.; Vasilev, K.; Voelcker, N.H. Antibacterial properties of nitric oxide-releasing porous silicon nanoparticles. J. Mater. Chem. B 2016, 4, 2051–2058. [Google Scholar] [CrossRef]
- Michl, T.D.; Locock, K.E.S.; Stevens, N.E.; Hayball, J.D.; Vasilev, K.; Postma, A.; Qu, Y.; Traven, A.; Haeussler, M.; Meagher, L.; et al. RAFT-derived antimicrobial polymethacrylates: Elucidating the impact of end-groups on activity and cytotoxicity. Polym. Chem. 2014, 5, 5813–5822. [Google Scholar] [CrossRef]
- Locock, K.E.S.; Michl, T.D.; Valentin, J.D.P.; Vasilev, K.; Hayball, J.D.; Qu, Y.; Traven, A.; Griesser, H.J.; Meagher, L.; Haeussler, M. Guanylated polymethacrylates: A class of potent antimicrobial polymers with low hemolytic activity. Biomacromolecules 2013, 14, 4021–4031. [Google Scholar] [CrossRef] [PubMed]
- Locock, K.E.S.; Michl, T.D.; Stevens, N.; Hayball, J.D.; Vasilev, K.; Postma, A.; Griesser, H.J.; Meagher, L.; Haeussler, M. Antimicrobial polymethacrylates synthesized as mimics of tryptophan-rich cationic peptides. ACS Macro Lett. 2014, 3, 319–323. [Google Scholar] [CrossRef]
- Baier, G.; Cavallaro, A.; Vasilev, K.; Mailänder, V.; Musyanovych, A.; Landfester, K. Enzyme responsive hyaluronic acid nanocapsules containing polyhexanide and their exposure to bacteria to prevent infection. Biomacromolecules 2013, 14, 1103–1112. [Google Scholar] [CrossRef]
- Baier, G.; Cavallaro, A.; Friedemann, K.; Müller, B.; Glasser, G.; Vasilev, K.; Landfester, K. Enzymatic degradation of poly(L-lactide) nanoparticles followed by the release of octenidine and their bactericidal effects. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 131–139. [Google Scholar] [CrossRef]
- Vasilev, K. Nanoengineered plasma polymer films for biomaterial applications. Plasma Chem. Plasma Process. 2014, 34, 545–558. [Google Scholar] [CrossRef]
- Liu, X.; Shi, S.; Feng, Q.; Bachhuka, A.; He, W.; Huang, Q.; Zhang, R.; Yang, X.; Vasilev, K. Surface chemical gradient affects the differentiation of human adipose-derived stem cells via ERK1/2 signaling pathway. ACS Appl. Mater. Interfaces 2015, 7, 18473–18482. [Google Scholar] [CrossRef] [PubMed]
- Mierczynska, A.; Michelmore, A.; Tripathi, A.; Goreham, R.V.; Sedev, R.; Vasilev, K. PH-tunable gradients of wettability and surface potential. Soft Matter 2012, 8, 8399–8404. [Google Scholar] [CrossRef]
- Hazrati, H.D.; Whittle, J.D.; Vasilev, K. A mechanistic study of the plasma polymerization of ethanol. Plasma Process. Polym. 2014, 11, 149–157. [Google Scholar] [CrossRef]
- Thierry, B.; Jasieniak, M.; De Smet, L.C.P.M.; Vasilev, K.; Griesser, H.J. Reactive epoxy-functionalized thin films by a pulsed plasma polymerization process. Langmuir 2008, 24, 10187–10195. [Google Scholar] [CrossRef]
- MacGregor, M.N.; Michelmore, A.; Safizadeh Shirazi, H.; Whittle, J.; Vasilev, K. Secrets of plasma-deposited polyoxazoline functionality lie in the plasma phase. Chem. Mater. 2017, 29, 8047–8051. [Google Scholar] [CrossRef]
- Michl, T.D.; Coad, B.R.; Hüsler, A.; Vasilev, K.; Griesser, H.J. Laboratory scale systems for the plasma treatment and coating of particles. Plasma Process. Polym. 2015, 12, 305–313. [Google Scholar] [CrossRef]
- Michelmore, A.; Bryant, P.M.; Steele, D.A.; Vasilev, K.; Bradley, J.W.; Short, R.D. Role of positive ions in determining the deposition rate and film chemistry of continuous wave hexamethyl disiloxane plasmas. Langmuir 2011, 27, 11943–11950. [Google Scholar] [CrossRef]
- Coad, B.R.; Vasilev, K.; Diener, K.R.; Hayball, J.D.; Short, R.D.; Griesser, H.J. Immobilized streptavidin gradients as bioconjugation platforms. Langmuir 2012, 28, 2710–2717. [Google Scholar] [CrossRef]
- Goreham, R.V.; Mierczynska, A.; Pierce, M.; Short, R.D.; Taheri, S.; Bachhuka, A.; Cavallaro, A.; Smith, L.E.; Vasilev, K. A substrate independent approach for generation of surface gradients. Thin Solid Films. 2013, 528, 106–110. [Google Scholar] [CrossRef]
- Coad, B.R.; Scholz, T.; Vasilev, K.; Hayball, J.D.; Short, R.D.; Griesser, H.J. Functionality of proteins bound to plasma polymer surfaces. ACS Appl. Mater. Interfaces 2012, 4, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K.; Michelmore, A.; Martinek, P.; Chan, J.; Sah, V.; Griesser, H.J.; Short, R.D. Early stages of growth of plasma polymer coatings deposited from nitrogen- and oxygen-containing monomers. Plasma Process. Polym. 2010, 7, 824–835. [Google Scholar] [CrossRef]
- Vasilev, K.; Michelmore, A.; Griesser, H.J.; Short, R.D. Substrate influence on the initial growth phase of plasma-deposited polymer films. Chem. Commun. 2009, 24, 3600–3602. [Google Scholar] [CrossRef] [PubMed]
- Michelmore, A.; Martinek, P.; Sah, V.; Short, R.D.; Vasilev, K. Surface morphology in the early stages of plasma polymer film growth from amine-containing monomers. Plasma Process. Polym. 2011, 8, 367–372. [Google Scholar] [CrossRef]
- Hernandez-Lopez, J.L.; Bauer, R.E.; Chang, W.S.; Glasser, G.; Grebel-Koehler, D.; Klapper, M.; Kreiter, M.; Leclaire, J.; Majoral, J.P.; Mittler, S.; et al. Functional polymers as nanoscopic building blocks. Mater. Sci. Eng. C 2003, 23, 267–274. [Google Scholar] [CrossRef]
- McInnes, S.J.P.; Michl, T.D.; Delalat, B.; Al-Bataineh, S.A.; Coad, B.R.; Vasilev, K.; Griesser, H.J.; Voelcker, N.H. “Thunderstruck”: Plasma-Polymer-Coated Porous Silicon Microparticles As a Controlled Drug Delivery System. ACS Appl. Mater. Interfaces 2016, 8, 4467–4476. [Google Scholar] [CrossRef] [PubMed]
- Wahono, S.K.; Cavallaro, A.; Vasilev, K.; Mierczynska, A. Plasma polymer facilitated magnetic technology for removal of oils from contaminated waters. Environ. Pollut. 2018, 240, 725–732. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Boyer, P.; Vasilev, K.; Smith, P.A. A novel technology for the rapid, selective, magnetic removal of pathogenesis-related proteins from wines. Food Chem. 2017, 232, 508–514. [Google Scholar] [CrossRef]
- Ramiasa-MacGregor, M.; Mierczynska, A.; Sedev, R.; Vasilev, K. Tuning and predicting the wetting of nanoengineered material surface. Nanoscale 2016, 8, 4635–4642. [Google Scholar] [CrossRef]
- Christo, S.N.; Bachhuka, A.; Diener, K.R.; Mierczynska, A.; Hayball, J.D.; Vasilev, K. The Role of Surface Nanotopography and Chemistry on Primary Neutrophil and Macrophage Cellular Responses. Adv. Healthc. Mater. 2016, 5, 956–965. [Google Scholar] [CrossRef]
- Macgregor-Ramiasa, M.N.; Cavallaro, A.A.; Vasilev, K. Properties and reactivity of polyoxazoline plasma polymer films. J. Mater. Chem. B 2015, 3, 6327–6337. [Google Scholar] [CrossRef]
- MacGregor, M.; Sinha, U.; Visalakshan, R.M.; Cavallaro, A.; Vasilev, K. Preserving the reactivity of coatings plasma deposited from oxazoline precursors—An in depth study. Plasma Process. Polym. 2019, 16, 1800130. [Google Scholar] [CrossRef]
- Macgregor, M.; Vasilev, K. Perspective on plasma polymers for applied biomaterials nanoengineering and the recent rise of oxazolines. Materials 2019, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K. Plasma derived oxazoline based coatings for advanced medical technologies. Galvanotechnik 2019, 110, 170–175. [Google Scholar]
- Gonzalez Garcia, L.E.; Macgregor-Ramiasa, M.; Visalakshan, R.M.; Vasilev, K. Protein Interactions with Nanoengineered Polyoxazoline Surfaces Generated via Plasma Deposition. Langmuir 2017, 33, 7322–7331. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Visalakshan, R.M.; Guo, J.; Wei, F.; Zhang, L.; Chen, L.; Lin, Z.; Vasilev, K.; Xiao, Y. Plasma deposited poly-oxazoline nanotextured surfaces dictate osteoimmunomodulation towards ameliorative osteogenesis. Acta Biomater. 2019, 96, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Macgregor-Ramiasa, M.; McNicholas, K.; Ostrikov, K.; Li, J.; Michael, M.; Gleadle, J.M.; Vasilev, K. A platform for selective immuno-capture of cancer cells from urine. Biosens. Bioelectron. 2017, 96, 373–380. [Google Scholar] [CrossRef]
- Visalakshan, R.M.; MacGregor, M.N.; Cavallaro, A.A.; Sasidharan, S.; Bachhuka, A.; Mierczynska-Vasilev, A.M.; Hayball, J.D.; Vasilev, K. Creating Nano-engineered Biomaterials with Well-Defined Surface Descriptors. ACS Appl. Nano Mater. 2018, 1, 2796–2807. [Google Scholar] [CrossRef]
- Visalakshan, R.M.; MacGregor, M.N.; Sasidharan, S.; Ghazaryan, A.; Mierczynska-Vasilev, A.M.; Morsbach, S.; Mailänder, V.; Landfester, K.; Hayball, J.D.; Vasilev, K. Biomaterial Surface Hydrophobicity-Mediated Serum Protein Adsorption and Immune Responses. ACS Appl. Mater. Interfaces 2019. [Google Scholar] [CrossRef]
- Al-Bataineh, S.A.; Cavallaro, A.A.; Michelmore, A.; Macgregor, M.N.; Whittle, J.D.; Vasilev, K. Deposition of 2-oxazoline-based plasma polymer coatings using atmospheric pressure helium plasma jet. Plasma Process. Polym. 2019. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef]
- Ostrikov, K.; MacGregor-Ramiasa, M.; Cavallaro, A.; Vasilev, K. Bactericidal effects of plasma-modified surface chemistry of silicon nanograss. J. Phys. D Appl. Phys. 2016, 49, 304001. [Google Scholar] [CrossRef]
- Alexander, J.W. History of the medical use of silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef]
- Vasilev, K.; Sah, V.R.; Goreham, R.V.; Ndi, C.; Short, R.D.; Griesser, H.J. Antibacterial surfaces by adsorptive binding of polyvinyl-sulphonate-stabilized silver nanoparticles. Nanotechnology 2010, 21, 215102. [Google Scholar] [CrossRef] [PubMed]
- Ploux, L.; Mateescu, M.; Anselme, K.; Vasilev, K. Antibacterial properties of silver-loaded plasma polymer coatings. J. Nanomater. 2012, 2012, 6. [Google Scholar] [CrossRef]
- Lombi, E.; Donner, E.; Taheri, S.; Tavakkoli, E.; Jämting, Å.K.; McClure, S.; Naidu, R.; Miller, B.W.; Scheckel, K.G.; Vasilev, K. Transformation of four silver/silver chloride nanoparticles during anaerobic treatment of wastewater and post-processing of sewage sludge. Environ. Pollut. 2013, 176, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Poulter, N.; Vasilev, K.; Griesser, S.S.; Griesser, H.J. Silver containing biomaterials. In Biomaterials Associated Infection: Immunological Aspects and Antimicrobial Strategies; Springer: Berlin/Heidelberg, Germany, 2013; pp. 355–378. [Google Scholar]
- Taheri, S.; Baier, G.; Majewski, P.; Barton, M.; Förch, R.; Landfester, K.; Vasilev, K. Synthesis and surface immobilization of antibacterial hybrid silver-poly(L-lactide) nanoparticles. Nanotechnology 2014, 25, 305102. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Baier, G.; Majewski, P.; Barton, M.; Förch, R.; Landfester, K.; Vasilev, K. Synthesis and antibacterial properties of a hybrid of silver-potato starch nanocapsules by miniemulsion/polyaddition polymerization. J. Mater. Chem. B 2014, 2, 1838–1845. [Google Scholar] [CrossRef]
- Taheri, S.; Cavallaro, A.; Barton, M.; Whittle, J.D.; Majewski, P.; Smith, L.E.; Vasilev, K. Antibacterial efficacy and cytotoxicity of silver-nanoparticle–based coatings facilitated by a plasma deposited polymer interlayer. Plasma Med. 2014, 4, 101–115. [Google Scholar] [CrossRef]
- Taheri, S.; Cavallaro, A.; Christo, S.N.; Smith, L.E.; Majewski, P.; Barton, M.; Hayball, J.D.; Vasilev, K. Substrate independent silver nanoparticle based antibacterial coatings. Biomaterials 2014, 35, 4601–4609. [Google Scholar] [CrossRef]
- Brunetti, G.; Donner, E.; Laera, G.; Sekine, R.; Scheckel, K.G.; Khaksar, M.; Vasilev, K.; De Mastro, G.; Lombi, E. Fate of zinc and silver engineered nanoparticles in sewerage networks. Water Res. 2015, 77, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Khaksar, M.; Jolley, D.F.; Sekine, R.; Vasilev, K.; Johannessen, B.; Donner, E.; Lombi, E. In situ chemical transformations of silver nanoparticles along the water-sediment continuum. Environ. Sci. Technol. 2015, 49, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Sekine, R.; Khurana, K.; Vasilev, K.; Lombi, E.; Donner, E. Quantifying the adsorption of ionic silver and functionalized nanoparticles during ecotoxicity testing: Test container effects and recommendations. Nanotoxicology 2015, 9, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Cavallaro, A.; Christo, S.N.; Majewski, P.; Barton, M.; Hayball, J.D.; Vasilev, K. Antibacterial Plasma Polymer Films Conjugated with Phospholipid Encapsulated Silver Nanoparticles. ACS Biomater. Sci. Eng. 2015, 1, 1278–1286. [Google Scholar] [CrossRef]
- Taheri, S.; Vasilev, K.; Majewski, P. Silver nanoparticles: Synthesis, antimicrobial coatings, and applications for medical devices. Recent Pat. Mater. Sci. 2015, 8, 166–175. [Google Scholar] [CrossRef]
- Alhmoud, H.; Delalat, B.; Ceto, X.; Elnathan, R.; Cavallaro, A.; Vasilev, K.; Voelcker, N.H. Antibacterial properties of silver dendrite decorated silicon nanowires. RSC Adv. 2016, 6, 65976–65987. [Google Scholar] [CrossRef]
- Chowdhury, N.R.; MacGregor-Ramiasa, M.; Zilm, P.; Majewski, P.; Vasilev, K. ‘Chocolate’ silver nanoparticles: Synthesis, antibacterial activity and cytotoxicity. J. Colloid Interface Sci. 2016, 482, 151–158. [Google Scholar] [CrossRef]
- He, W.; Elkhooly, T.A.; Liu, X.; Cavallaro, A.; Taheri, S.; Vasilev, K.; Feng, Q. Silver nanoparticle based coatings enhance adipogenesis compared to osteogenesis in human mesenchymal stem cells through oxidative stress. J. Mater. Chem. B 2016, 4, 1466–1479. [Google Scholar] [CrossRef]
- Ostrikov, K.; Macgregor-Ramiasa, M.N.; Cavallaro, A.A.; Jacob, M.; Vasilev, K. A comparative assessment of nanoparticulate and metallic silver coated dressings. Recent Pat. Mater. Sci. 2016, 9, 50–57. [Google Scholar] [CrossRef]
- Prasad, K.; Lekshmi, G.S.; Ostrikov, K.; Lussini, V.; Blinco, J.; Mohandas, M.; Vasilev, K.; Bottle, S.; Bazaka, K.; Ostrikov, K. Synergic bactericidal effects of reduced graphene oxide and silver nanoparticles against Gram-positive and Gram-negative bacteria. Sci. Rep. 2017, 7, 1591. [Google Scholar] [CrossRef]
- Schmidt-Braekling, T.; Streitbuerger, A.; Gosheger, G.; Boettner, F.; Nottrott, M.; Ahrens, H.; Dieckmann, R.; Guder, W.; Andreou, D.; Hauschild, G. Silver-coated megaprostheses: Review of the literature. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Roy Chowdhury, N.; Hopp, I.; Zilm, P.; Murray, P.; Vasilev, K. Silver nanoparticle modified surfaces induce differentiation of mouse kidney-derived stem cells. RSC Adv. 2018, 8, 20334–20340. [Google Scholar] [CrossRef]
- González García, L.E.; MacGregor, M.N.; Visalakshan, R.M.; Ninan, N.; Cavallaro, A.A.; Trinidad, A.D.; Zhao, Y.; Hayball, A.J.D.; Vasilev, K. Self-sterilizing antibacterial silver-loaded microneedles. Chem. Commun. 2019, 55, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K.; Sah, V.; Anselme, K.; Ndi, C.; Mateescu, M.; Dollmann, B.; Martinek, P.; Ys, H.; Ploux, L.; Griesser, H.J. Tunable antibacterial coatings that support mammalian cell growth. Nano Lett. 2010, 10, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.R.; Cowin, A.J.; Zilm, P.; Vasilev, K. “Chocolate” gold nanoparticles—One pot synthesis and biocompatibility. Nanomaterials 2018, 8, 496. [Google Scholar] [CrossRef]
- Taheri, S.; Ruiz, J.C.; Michelmore, A.; Macgregor, M.; Förch, R.; Majewski, P.; Vasilev, K. Binding of Nanoparticles to Aminated Plasma Polymer Surfaces is Controlled by Primary Amine Density and Solution pH. J. Phys. Chem. C 2018, 122, 14986–14995. [Google Scholar] [CrossRef]
- Biswas, B.; Warr, L.N.; Hilder, E.F.; Goswami, N.; Rahman, M.M.; Churchman, J.G.; Vasilev, K.; Pan, G.; Naidu, R. Biocompatible functionalisation of nanoclays for improved environmental remediation. Chem. Soc. Rev. 2019, 48, 3740–3770. [Google Scholar] [CrossRef]
- Goswami, N.; Bright, R.; Visalakshan, R.M.; Biswas, B.; Zilm, P.; Vasilev, K. Core-in-cage structure regulated properties of ultra-small gold nanoparticles. Nanoscale Adv. 2019, 1, 2356–2364. [Google Scholar] [CrossRef]
- Ravindran Girija, A.; Balasubramanian, S.; Bright, R.; Cowin, A.J.; Goswami, N.; Vasilev, K. Ultrasmall Gold Nanocluster Based Antibacterial Nanoaggregates for Infectious Wound Healing. ChemNanoMat 2019. [Google Scholar] [CrossRef]
- Simovic, S.; Losic, D.; Vasilev, K. Controlled drug release from porous materials by plasma polymer deposition. Chem. Commun. 2010, 46, 1317–1319. [Google Scholar] [CrossRef]
- Simovic, S.; Diener, K.R.; Bachhuka, A.; Kant, K.; Losic, D.; Hayball, J.D.; Brownc, M.P.; Vasilev, K. Controlled release and bioactivity of the monoclonal antibody rituximab from a porous matrix: A potential in situ therapeutic device. Mater. Lett. 2014, 130, 210–214. [Google Scholar] [CrossRef]
- Müller, S.; Cavallaro, A.; Vasilev, K.; Voelcker, N.H.; Schönherr, H. Temperature-Controlled Antimicrobial Release from Poly(diethylene glycol methylether methacrylate)-Functionalized Bottleneck-Structured Porous Silicon for the Inhibition of Bacterial Growth. Macromol. Chem. Phys. 2016, 217, 2243–2251. [Google Scholar] [CrossRef]



© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilev, K. Nanoengineered Antibacterial Coatings and Materials: A Perspective. Coatings 2019, 9, 654. https://doi.org/10.3390/coatings9100654
Vasilev K. Nanoengineered Antibacterial Coatings and Materials: A Perspective. Coatings. 2019; 9(10):654. https://doi.org/10.3390/coatings9100654
Chicago/Turabian StyleVasilev, Krasimir. 2019. "Nanoengineered Antibacterial Coatings and Materials: A Perspective" Coatings 9, no. 10: 654. https://doi.org/10.3390/coatings9100654
APA StyleVasilev, K. (2019). Nanoengineered Antibacterial Coatings and Materials: A Perspective. Coatings, 9(10), 654. https://doi.org/10.3390/coatings9100654



