Impact of K+ Doping on Modulating Majority Charge Carrier Type and Quality of Perovskite Thin Films by Two-step Solution Method for Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Characterization
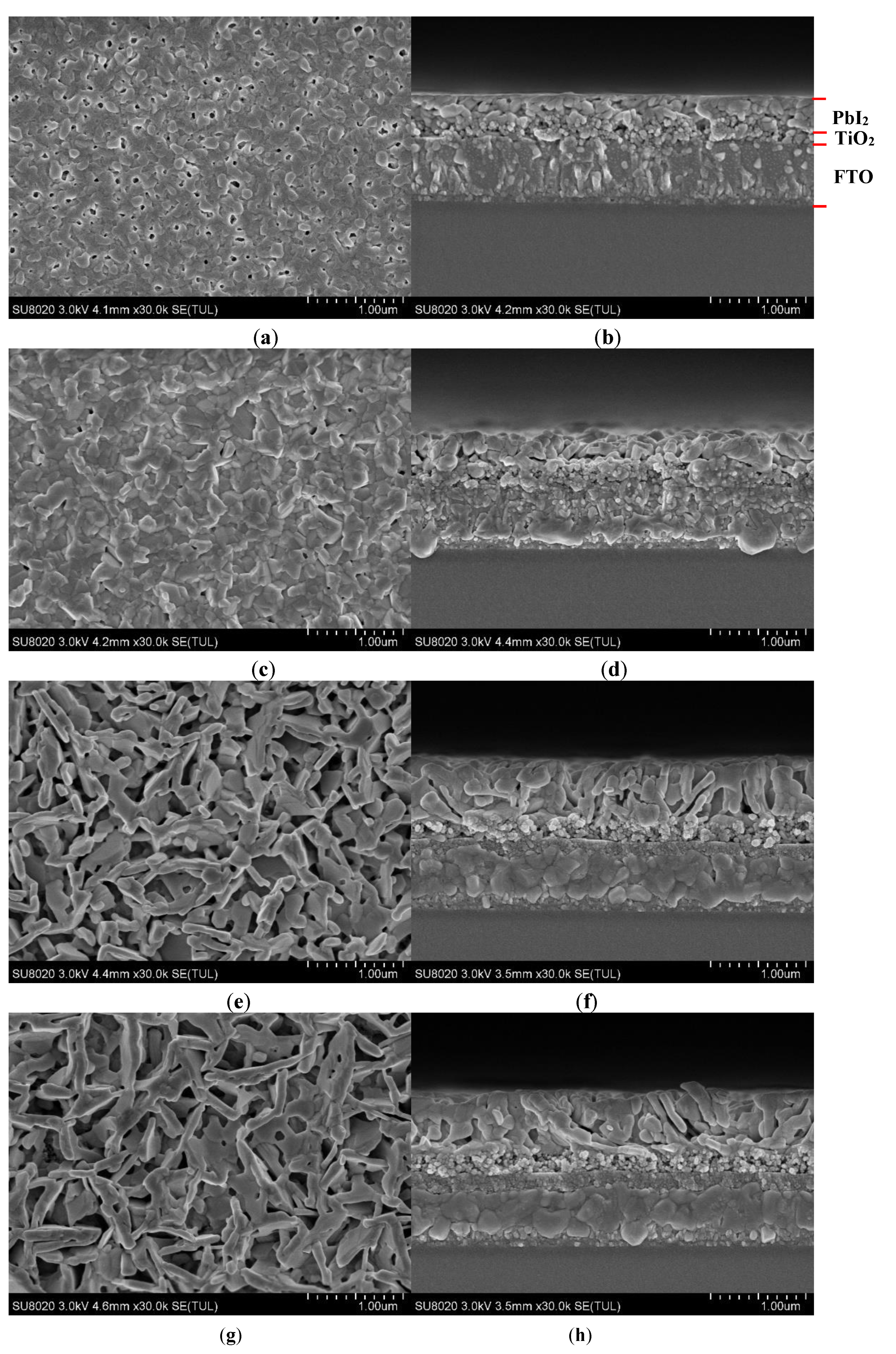
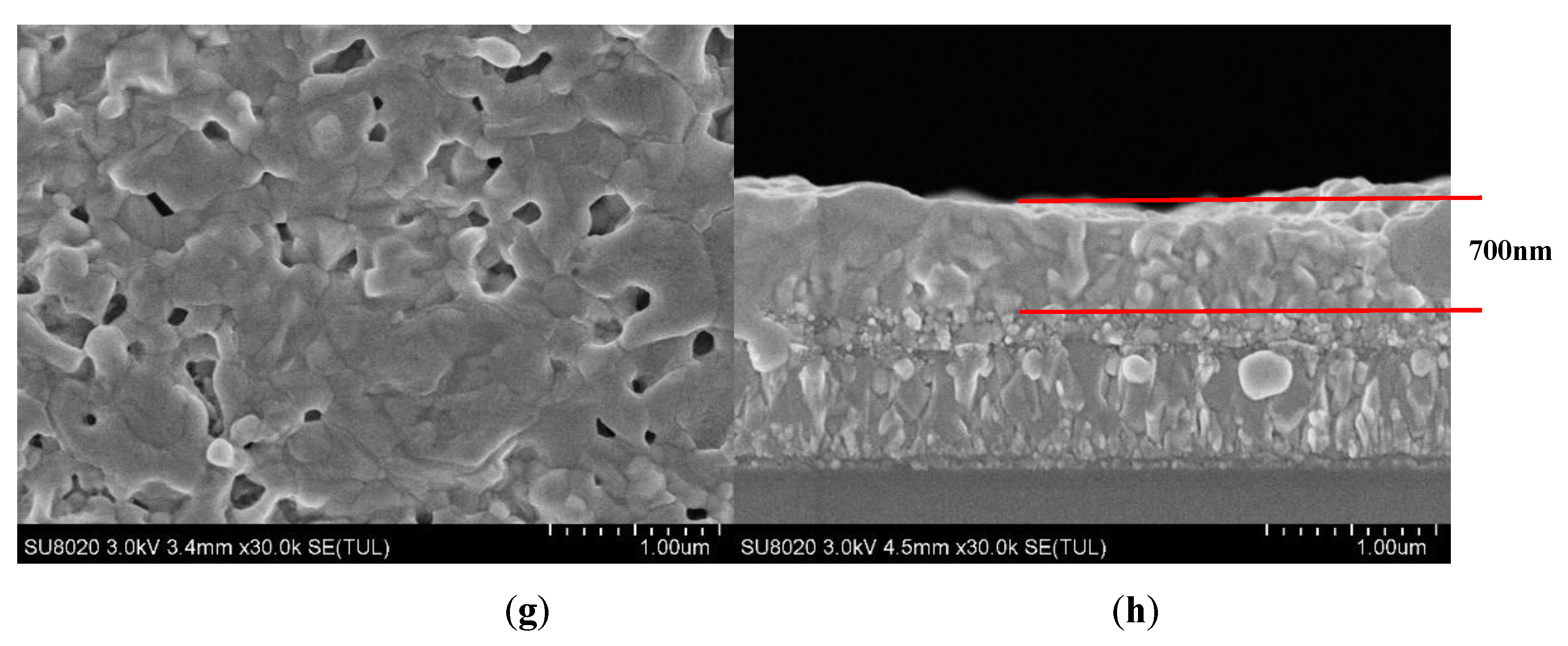
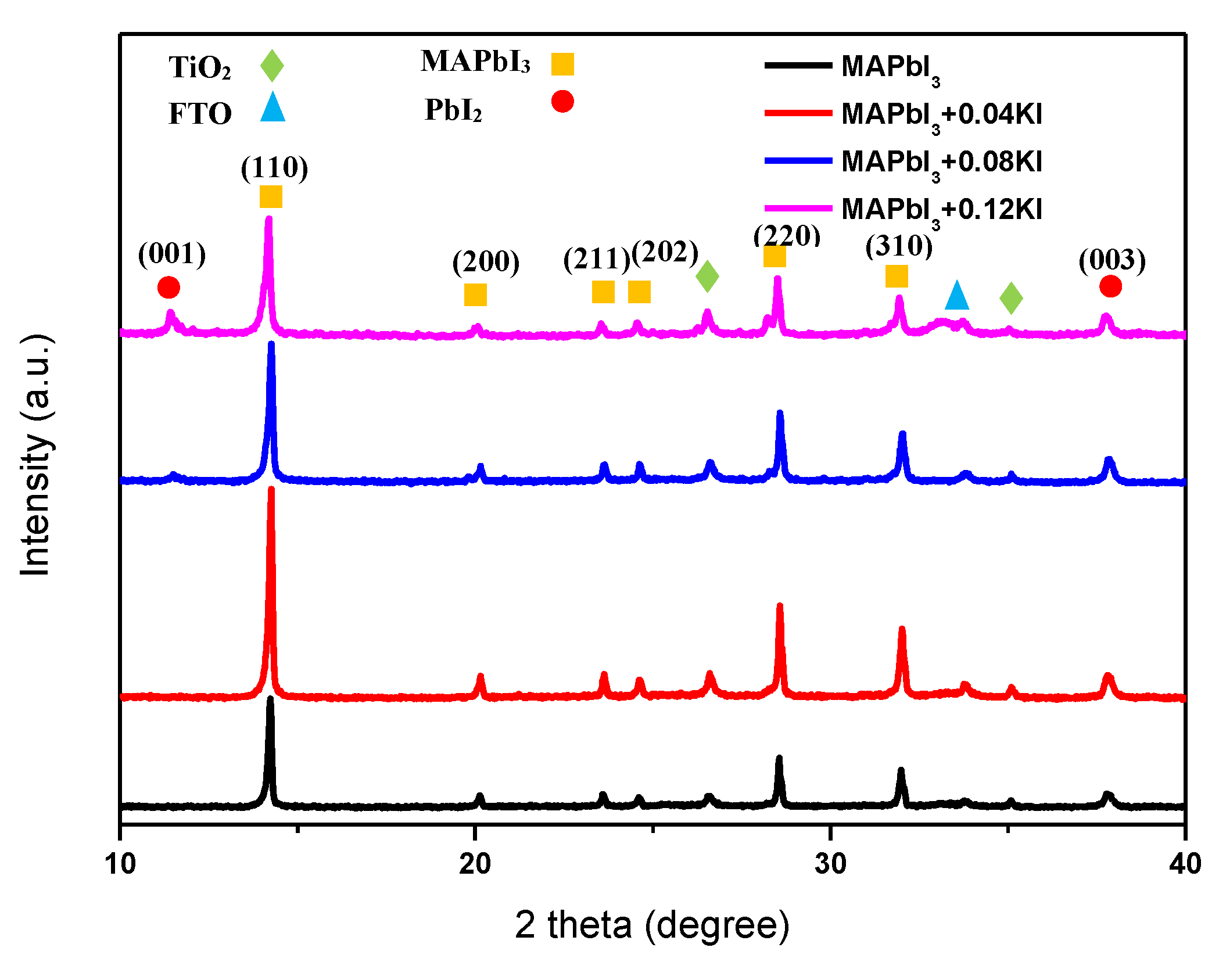
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.B.; Duan, H.S.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Eperon, G.E.; Burlakov, V.M.; Docampo, P.; Goriely, A.; Snaith, H.J. Morphological Control for High Performance, Solution-Processed Planar Heterojunction Perovskite Solar Cells. Adv. Funct. Mater. 2014, 24, 151–157. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, Y.; Xie, H.; Lyu, L.; Liu, X.; Gao, Y.; Huang, J. Qualifying composition dependent p and n self-doping in CH3NH3PbI3. Appl. Phys. Lett. 2014, 105, 163508. [Google Scholar] [CrossRef]
- Cui, P.; Wei, D.; Ji, J.; Huang, H.; Jia, E.D.; Dou, S.Y. Planar p–n homojunction perovskite solar cells with efficiency exceeding 21.3%. Nat. Energy 2019, 4, 150–159. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; You, J. Enhanced electron extraction using SnO2 for high efficiency planar-structure HC (NH2)2PbI3-based perovskite solar cells. Nat. Energy 2017, 2, 16177. [Google Scholar] [CrossRef]
- Jia, S.; Wang, J.; Zhu, L. Enhancing the photovoltaic performance of perovskite solar cells by potassium ions doping. J. Mater. Sci. Mater. Electron. 2019, 30, 2057–2066. [Google Scholar] [CrossRef]
- Ling, T.; Zou, X.; Cheng, J.; Yang, Y.; Ren, H.; Chen, D. Modulating Surface Morphology Related to Crystallization Speed of Perovskite Grain and Semiconductor Properties of Optical Absorber Layer under Controlled Doping of Potassium Ions for Solar Cells. Materials 2018, 11, 1605. [Google Scholar] [CrossRef]
- Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Cacovich, S.; Stavrakas, C.; Philippe, B.; Richter, J.M.; Lilliu, S. Maximizing and stabilizing luminescence from halide perovskites with potassium passivation. Nature 2018, 555, 497–501. [Google Scholar] [CrossRef]
- Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Pearson, A.J.; Divitini, G.; Cacovich, S.; Philippe, B.; Stranks, S.D. Potassium- and Rubidium-Passivated Alloyed Perovskite Films: Optoelectronic Properties and Moisture Stability. ACS Energy Lett. 2018, 3, 2671–2678. [Google Scholar] [CrossRef]
- Son, D.Y.; Kim, S.G.; Seo, J.Y.; Lee, S.H.; Shin, H.; Lee, D.; Park, N.G. Universal Approach toward Hysteresis-Free Perovskite Solar Cell via Defect Engineering. J. Am. Chem. Soc. 2018, 140, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.Y.; Zou, X.P.; Cheng, J.; Ling, T.; Bai, X.; Chen, D. Facile solution spin-coating SnO2 thin film covering cracks of TiO2 hole blocking layer for perovskite solar cells. Coatings 2018, 8, 314. [Google Scholar] [CrossRef]
- Yao, Y.; Zou, X.; Cheng, J.; Chen, D.; Chang, C.; Ling, T.; Ren, H. Impact of Delay Time before Annealing MAI-PbI2-DMSO Intermediate Phase on Perovskite Film Quality and Photo-Physical Properties. Crystals 2019, 9, 151. [Google Scholar] [CrossRef]
- Yang, Y.; Zou, X.; Pei, Y.; Bai, X.; Jin, W.; Chen, D. Impact of doping of NaI monovalent cation halide on the structural, morphological, optical and optoelectronic properties of MAPbI3 perovskite. J. Mater. Sci. Mater. Electron. 2018, 29, 205–210. [Google Scholar] [CrossRef]
- Cheng, J.; Qiang, Y.; Zhou, C.; Shi, H.; Liu, H.; Geng, C.; Xie, Y. Impactive improvement of the photovoltaic performance of carbon-based perovskites solar cells by grinding process and its capacitor model. J. Power Sources 2019, 422, 131–137. [Google Scholar] [CrossRef]
- Tan, H.; Jain, A.; Voznyy, O.; Lan, X.; De Arquer, F.P.G.; Fan, J.Z.; Fan, F. Efficient and stable solution-processed planar perovskite solar cells via contact passivation. Science 2017, 355, 722–726. [Google Scholar] [CrossRef]
- Kim, B.G.; Jang, W.; Cho, J.S.; Wang, D.H. Tailoring solubility of methylammonium lead halide with non-stoichiometry molar ratio in perovskite solar cells: Morphological and electrical relationships for high current generation. Sol. Energy Mater. Sol. Cells 2019, 192, 24–35. [Google Scholar] [CrossRef]
- Ogunniran, K.O.; Murugadoss, G.; Thangamuthu, R.; Karthikeyan, J.; Murugan, P.J.S.E.M. Integration of phenylammoniumiodide (PAI) as a surface coating molecule towards ambient stable MAPbI3 perovskite for solar cell application. Sol. Energy Mater. Sol. Cells 2019, 191, 316–328. [Google Scholar] [CrossRef]
- Tang, Z.; Tanaka, S.; Ito, S.; Ikeda, S.; Taguchi, K.; Minemoto, T. Investigating relation of photovoltaic factors with properties of perovskite films based on various solvents. Nano Energy 2015, 21, 51–61. [Google Scholar] [CrossRef]
- Huang, Y.C.; Tsao, C.S.; Cho, Y.J.; Chen, K.C.; Chiang, K.M.; Hsiao, S.Y.; Chen, C.W.; Su, C.J.; Jeng, U.S. Insight into evolution, processing and performance of multi-length-scale structures in planar heterojunction perovskite solar cells. Sci. Rep. 2015, 5, 13657. [Google Scholar] [CrossRef]
- Khadka, D.B.; Shirai, Y.; Yanagida, M.; Ryan, J.W.; Miyano, K. Exploring the impacts of interfacial carrier transport layers on device performance and optoelectronic properties of planar perovskite solar cells. J. Mater. Chem. C 2017, 5, 8819–8827. [Google Scholar] [CrossRef]
- Mojtaba, A.J.; Ibrahim, D.M.; Aditya, S.; Senanayak, S.P.; Fabrizio, G.; Mohammed, Z.S.; Michael, G. Impact of a mesoporous titania-perovskite interface on the performance of hybrid organic-inorganic perovskite solar cells. J. Phys. Chem. Lett. 2016, 7, 3264–3269. [Google Scholar]

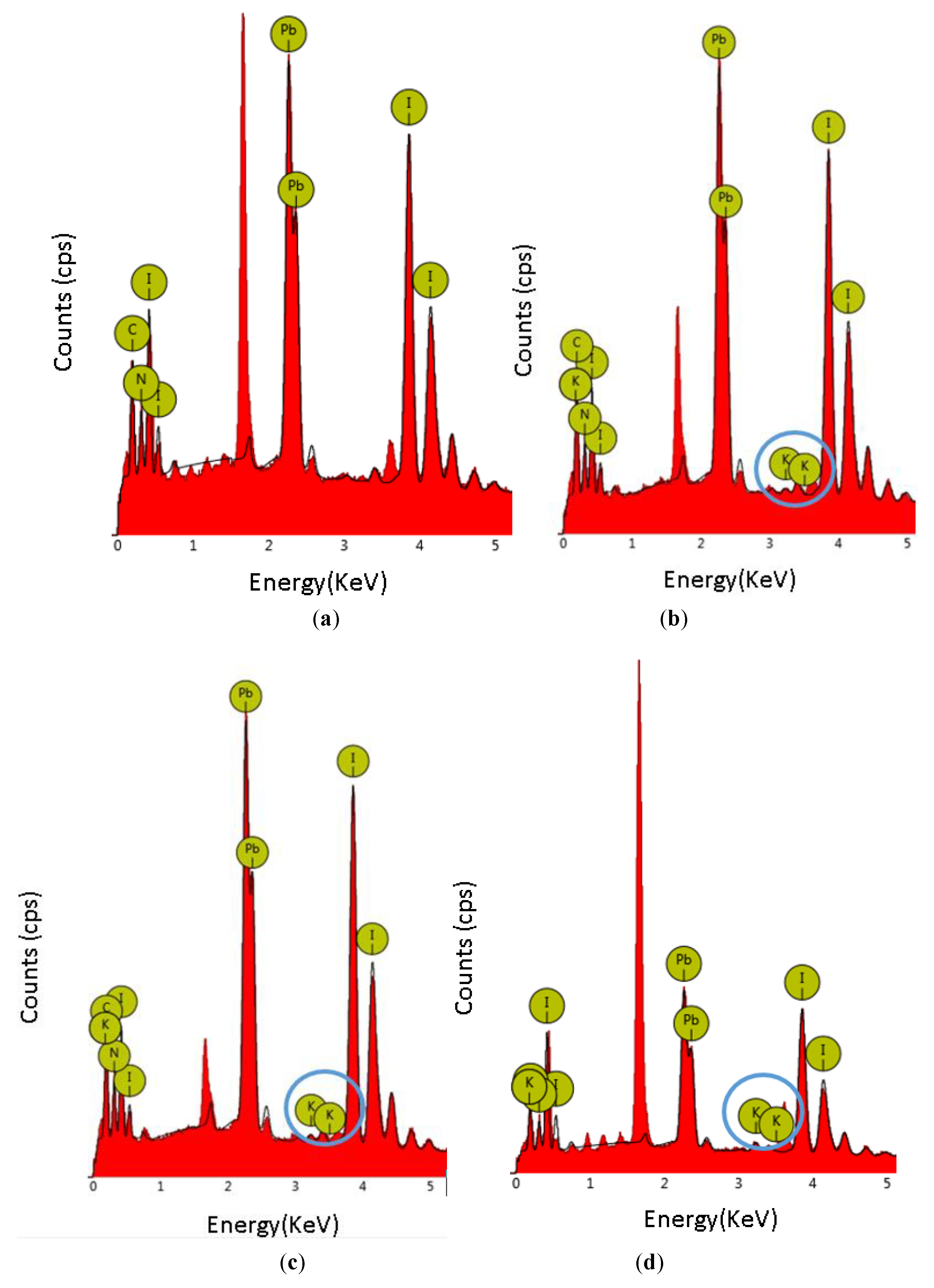
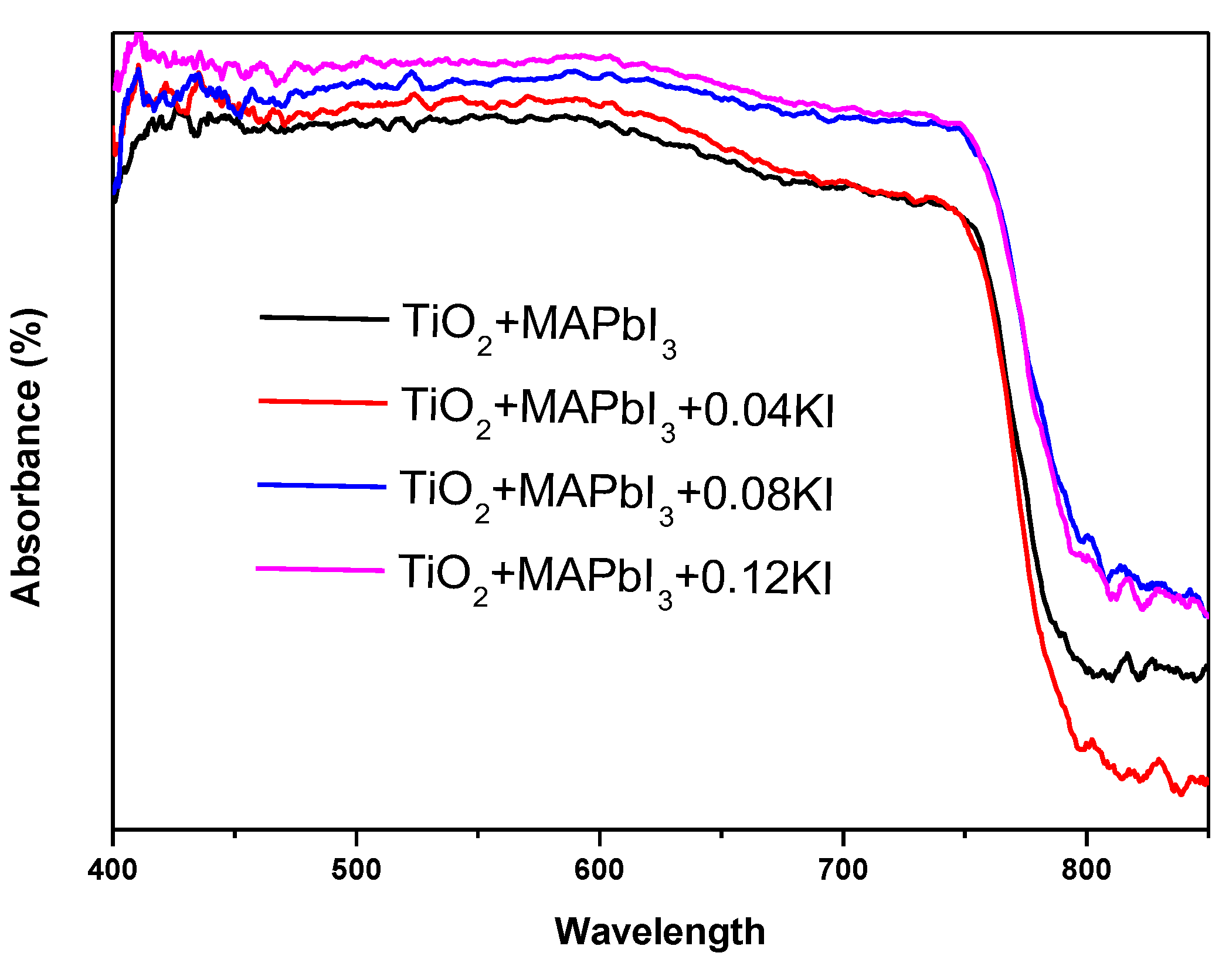
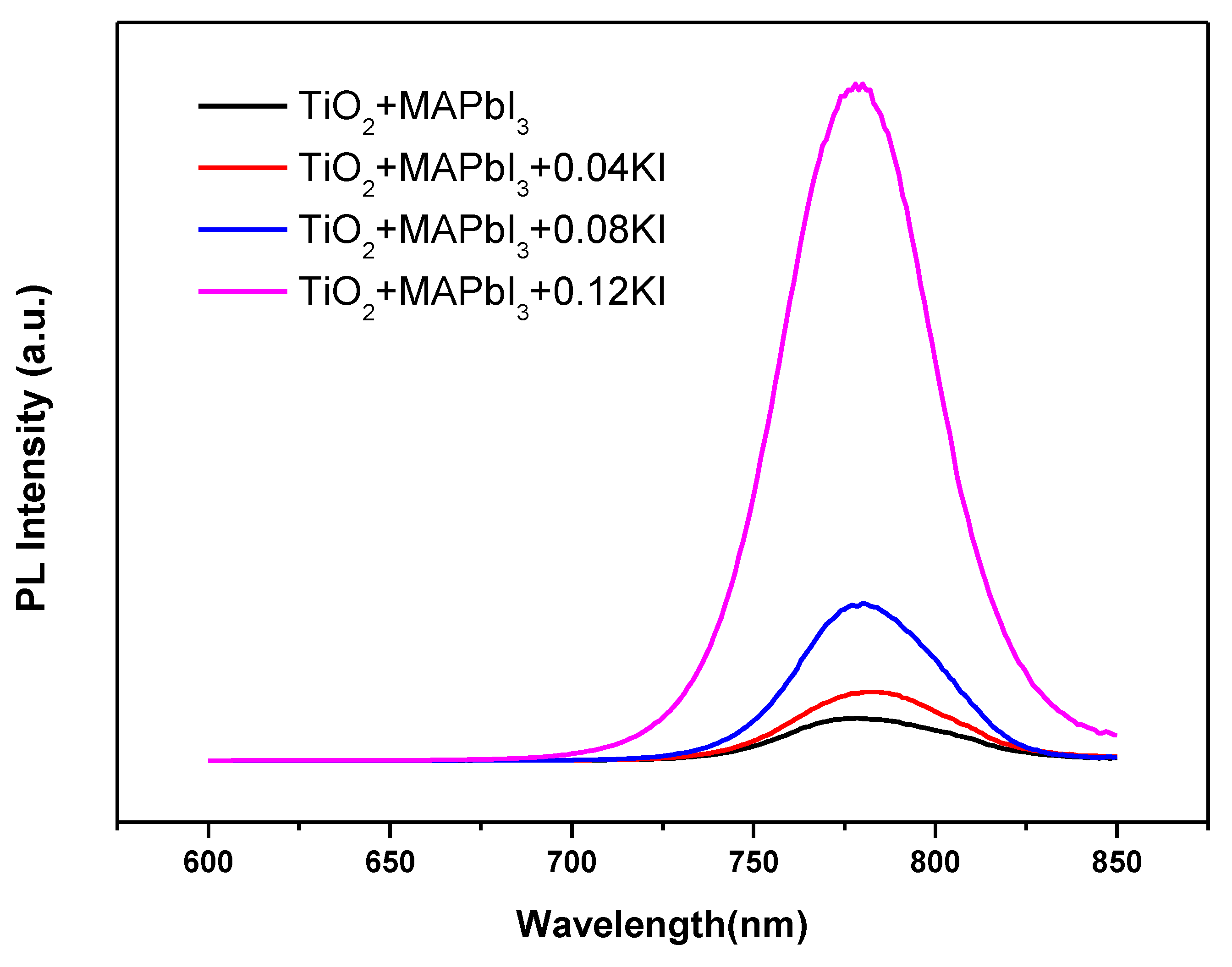
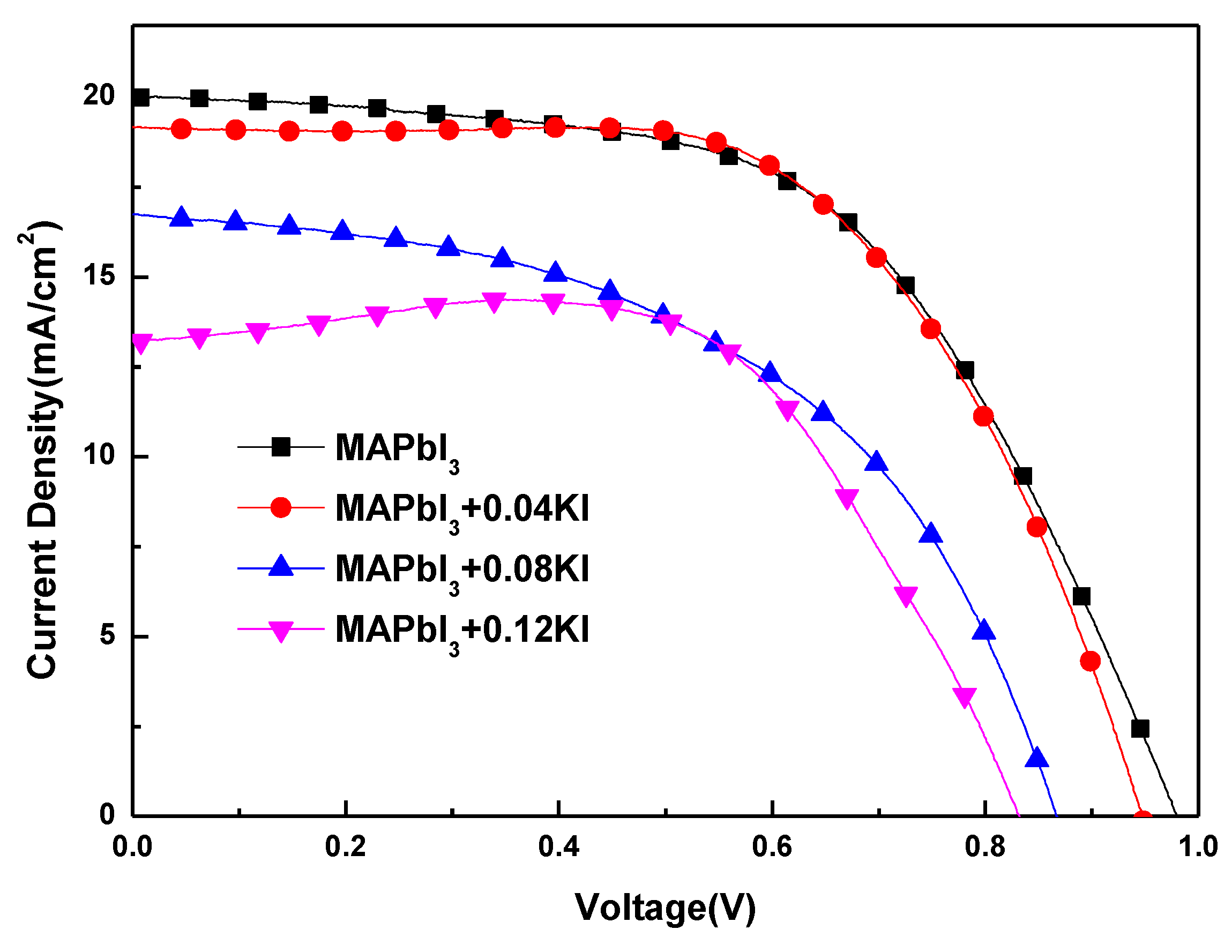
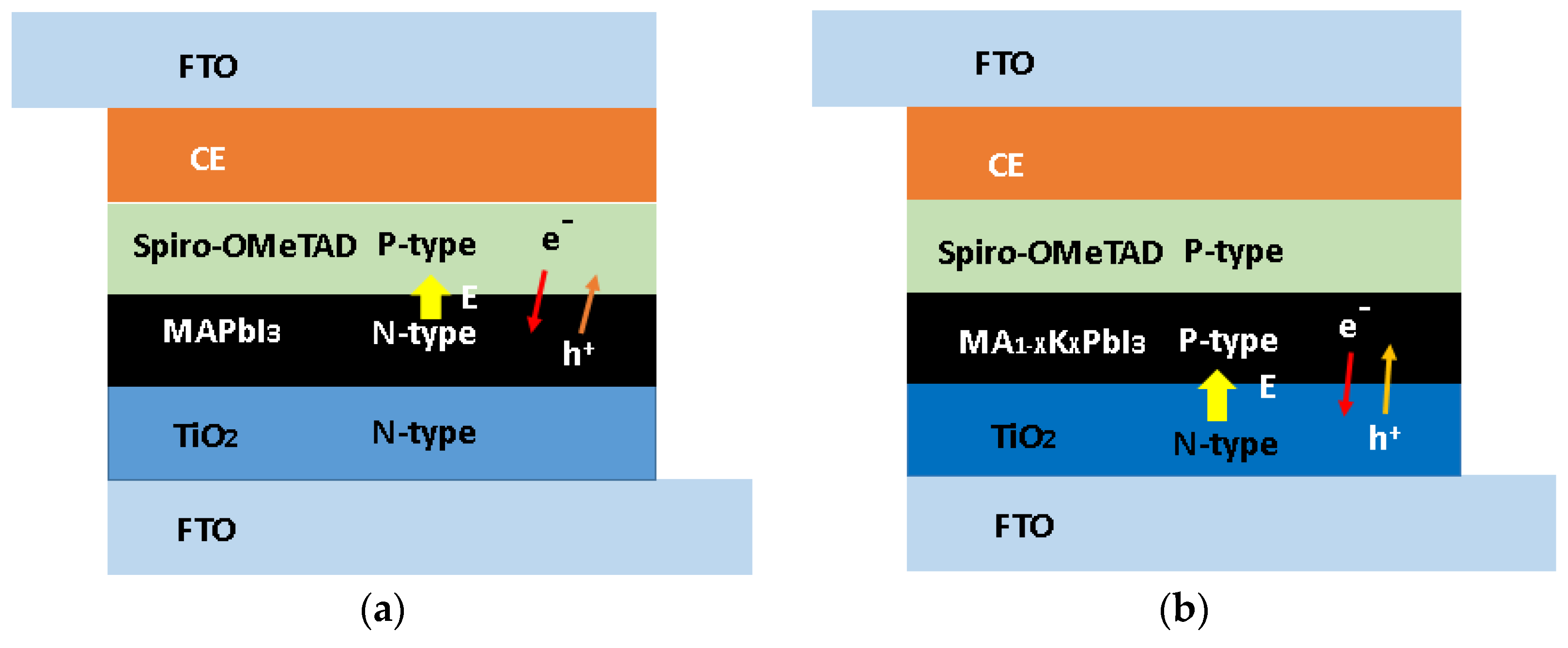
| Sample | Atomic Percentage | ||||
|---|---|---|---|---|---|
| K | N | C | I | Pb | |
| MAPbI3 | 0 | 25.24 | 31.80 | 25.22 | 17.75 |
| MAPbI3+0.04KI | 0.29 | 21.19 | 33.61 | 26.00 | 18.91 |
| MAPbI3+0.08KI | 0.42 | 23.13 | 31.20 | 26.76 | 18.48 |
| MAPbI3+0.12KI | 1.03 | 27.21 | 30.18 | 25.06 | 16.51 |
| Samples | Hall Parameters | ||
|---|---|---|---|
| Mob (cm2/Vs) | N (/cm3) | Types | |
| MAPbI3 | 494 | −5.4 × 1010 | n |
| MAPbI3+0.04KI | 56.6 | +2.3 × 1012 | p |
| MAPbI3+0.08KI | 176 | +4.6 × 1013 | p |
| MAPbI3+0.12KI | 66.4 | +7.3 × 1011 | p |
| Samples | Voc (V) | Parameters Jsc (mA/cm2) | FF (%) | η (%) |
|---|---|---|---|---|
| MAPbI3 | 0.98 | 19.99 | 56.63 | 11.09 |
| MAPbI3+0.04KI | 0.95 | 19.16 | 60.92 | 11.05 |
| MAPbI3+0.08KI | 0.87 | 16.73 | 50.74 | 7.36 |
| MAPbI3+0.12KI | 0.83 | 13.22 | 65.89 | 7.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Zou, X.; Cheng, J.; Ling, T.; Chang, C.; Chen, D. Impact of K+ Doping on Modulating Majority Charge Carrier Type and Quality of Perovskite Thin Films by Two-step Solution Method for Solar Cells. Coatings 2019, 9, 647. https://doi.org/10.3390/coatings9100647
Yao Y, Zou X, Cheng J, Ling T, Chang C, Chen D. Impact of K+ Doping on Modulating Majority Charge Carrier Type and Quality of Perovskite Thin Films by Two-step Solution Method for Solar Cells. Coatings. 2019; 9(10):647. https://doi.org/10.3390/coatings9100647
Chicago/Turabian StyleYao, Yujun, Xiaoping Zou, Jin Cheng, Tao Ling, Chuangchuang Chang, and Dan Chen. 2019. "Impact of K+ Doping on Modulating Majority Charge Carrier Type and Quality of Perovskite Thin Films by Two-step Solution Method for Solar Cells" Coatings 9, no. 10: 647. https://doi.org/10.3390/coatings9100647
APA StyleYao, Y., Zou, X., Cheng, J., Ling, T., Chang, C., & Chen, D. (2019). Impact of K+ Doping on Modulating Majority Charge Carrier Type and Quality of Perovskite Thin Films by Two-step Solution Method for Solar Cells. Coatings, 9(10), 647. https://doi.org/10.3390/coatings9100647




