Influence of Bath Additives on the Thermal Stability of the Nanostructure and Hardness of Ni Films Processed by Electrodeposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Processing of Ni Films by Electrodeposition
- The same electrolyte described above was used to produce sample SAA but with the addition of 1 g/L saccharin.
- The electrolyte which was used to produce sample TSC contained only nickel sulfate and trisodium citrate. The pH was 6.0 (adjusted with NaOH) in order to achieve maximum cathode current efficiency and a stability of the electrolyte [33].
2.2. Heat Treatment
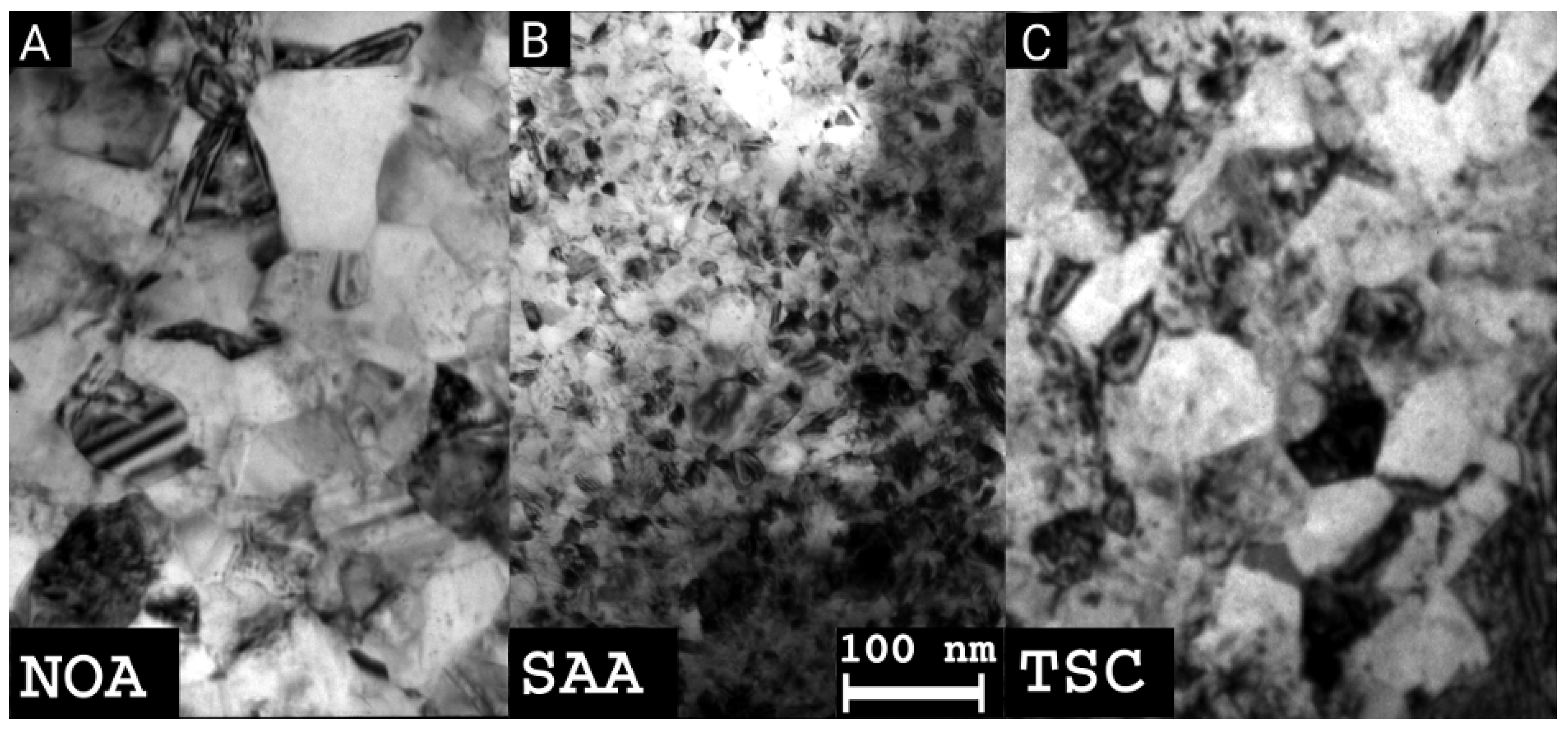
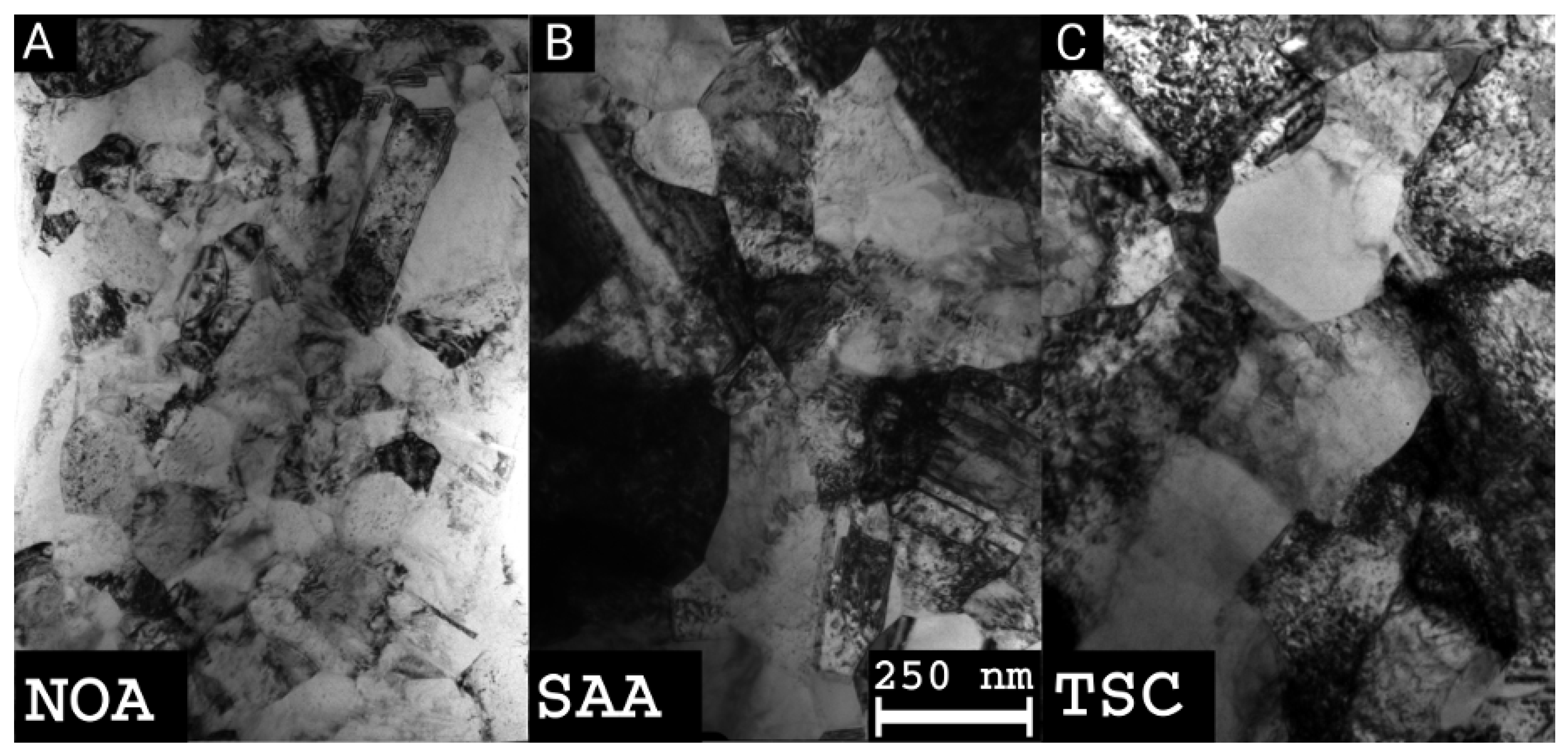
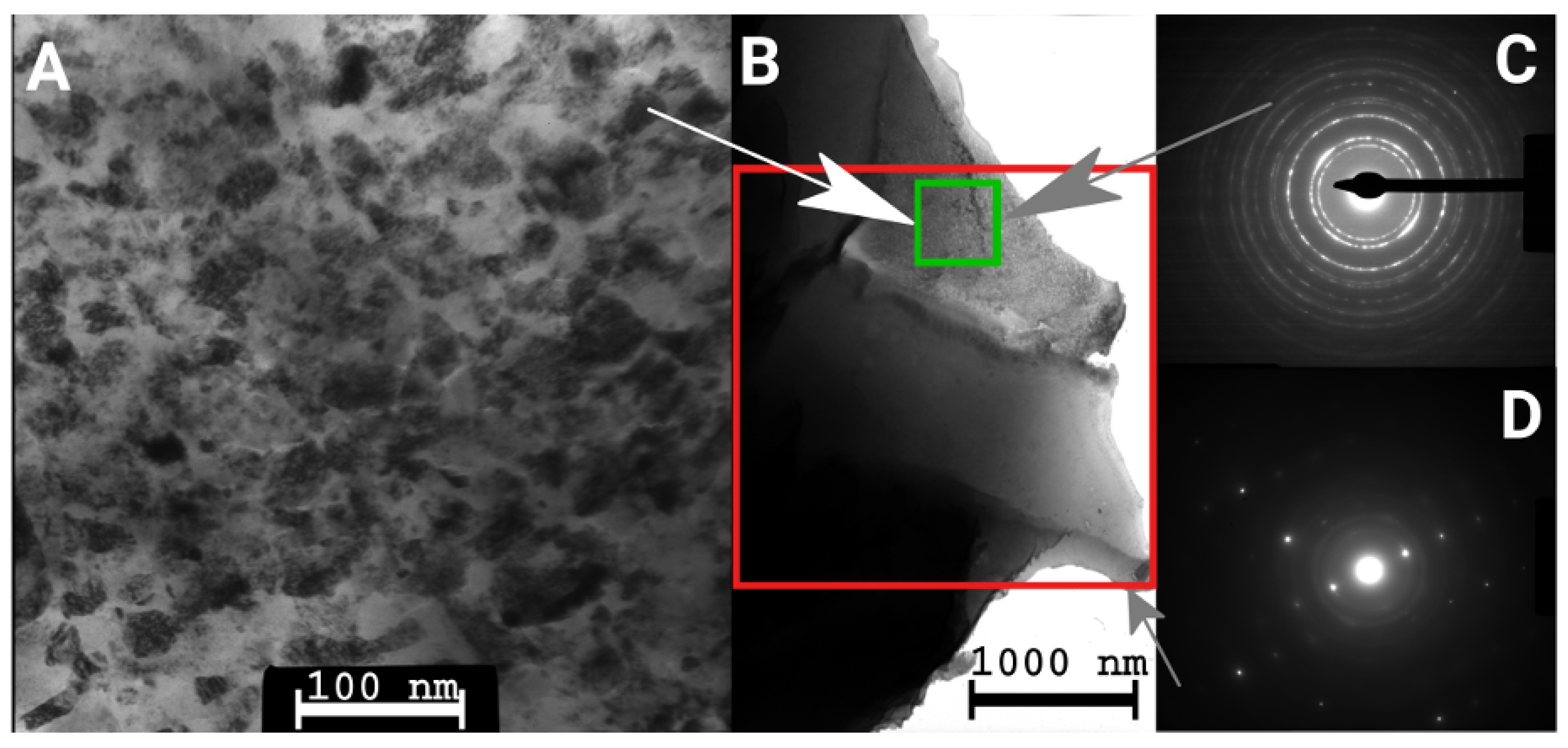
2.3. Transmission Electron Microscopy
2.4. Scanning Electro Microscopy
2.5. X-ray Diffraction
2.6. Hardness Tests
3. Results
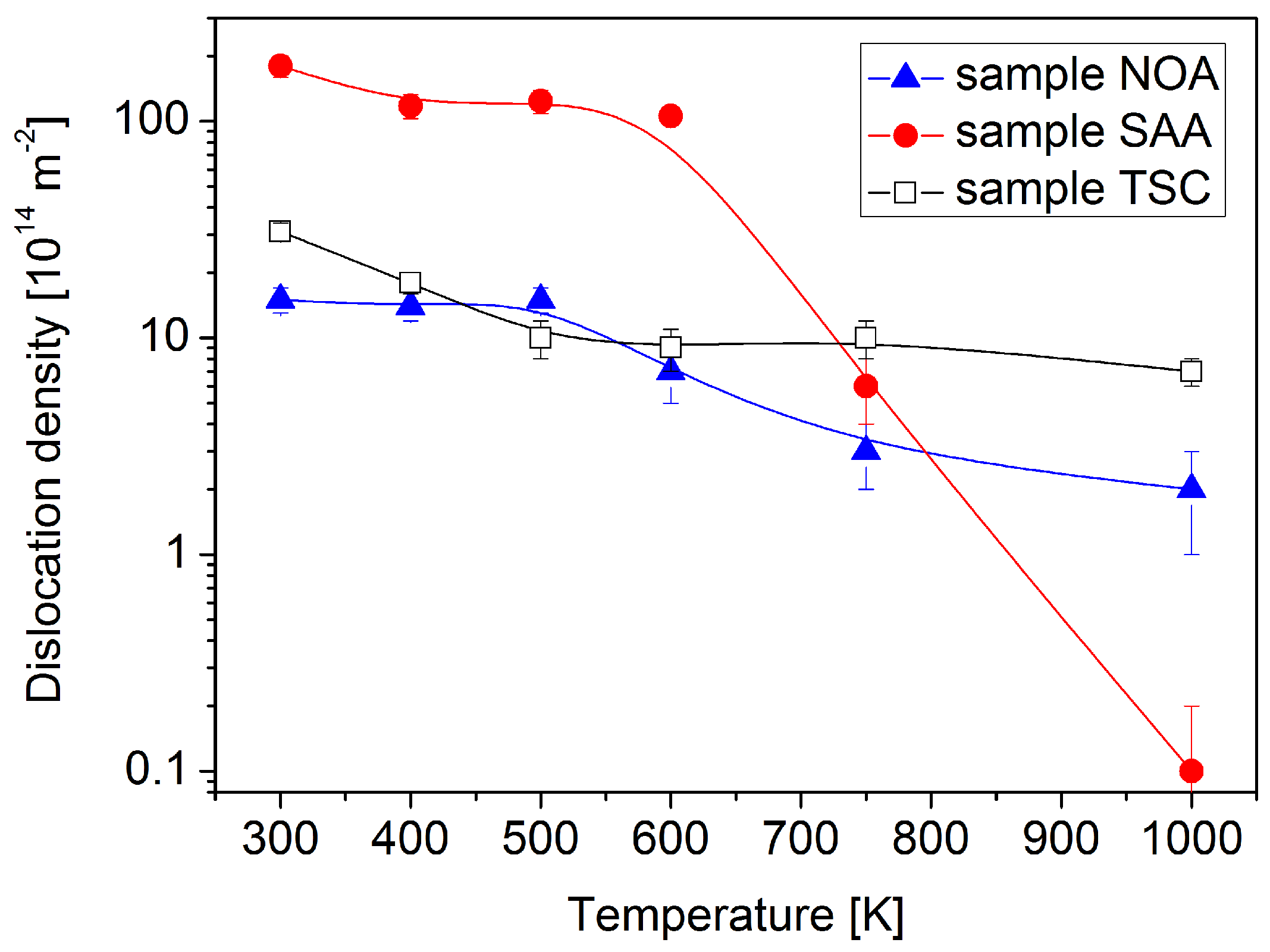
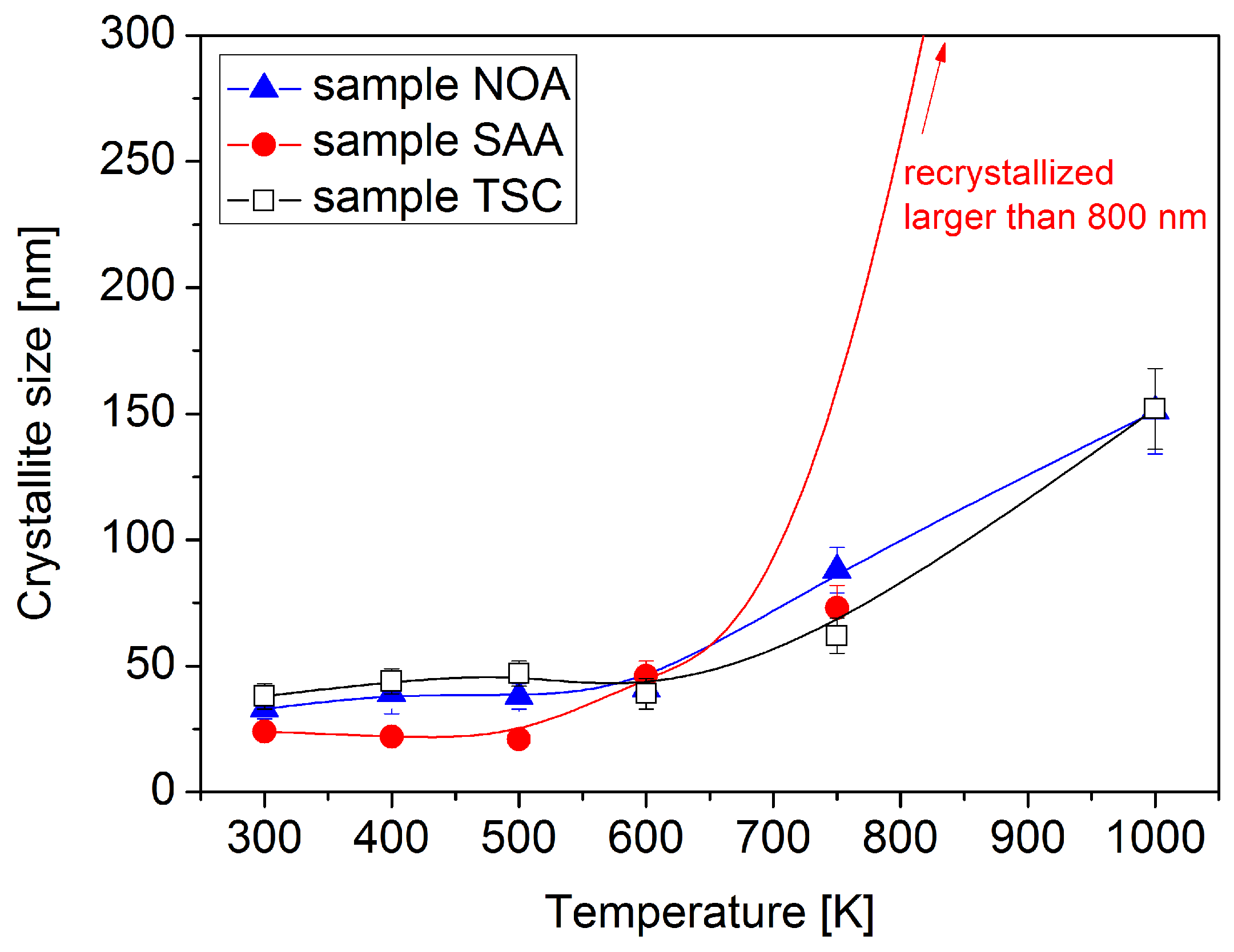
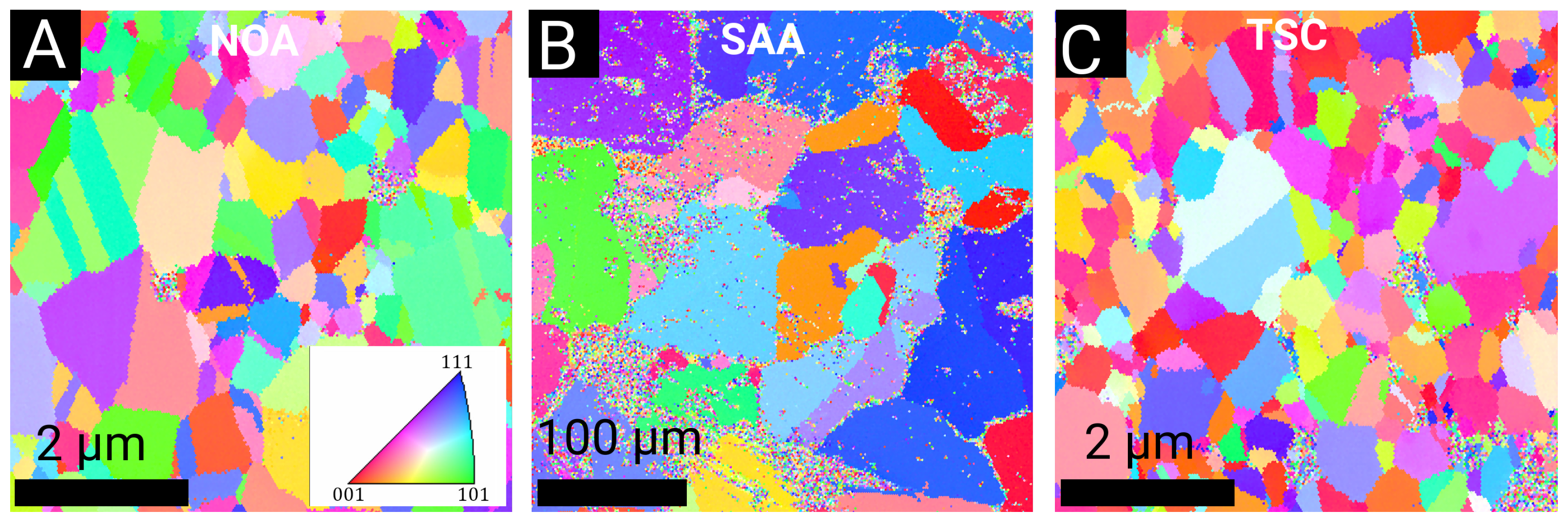
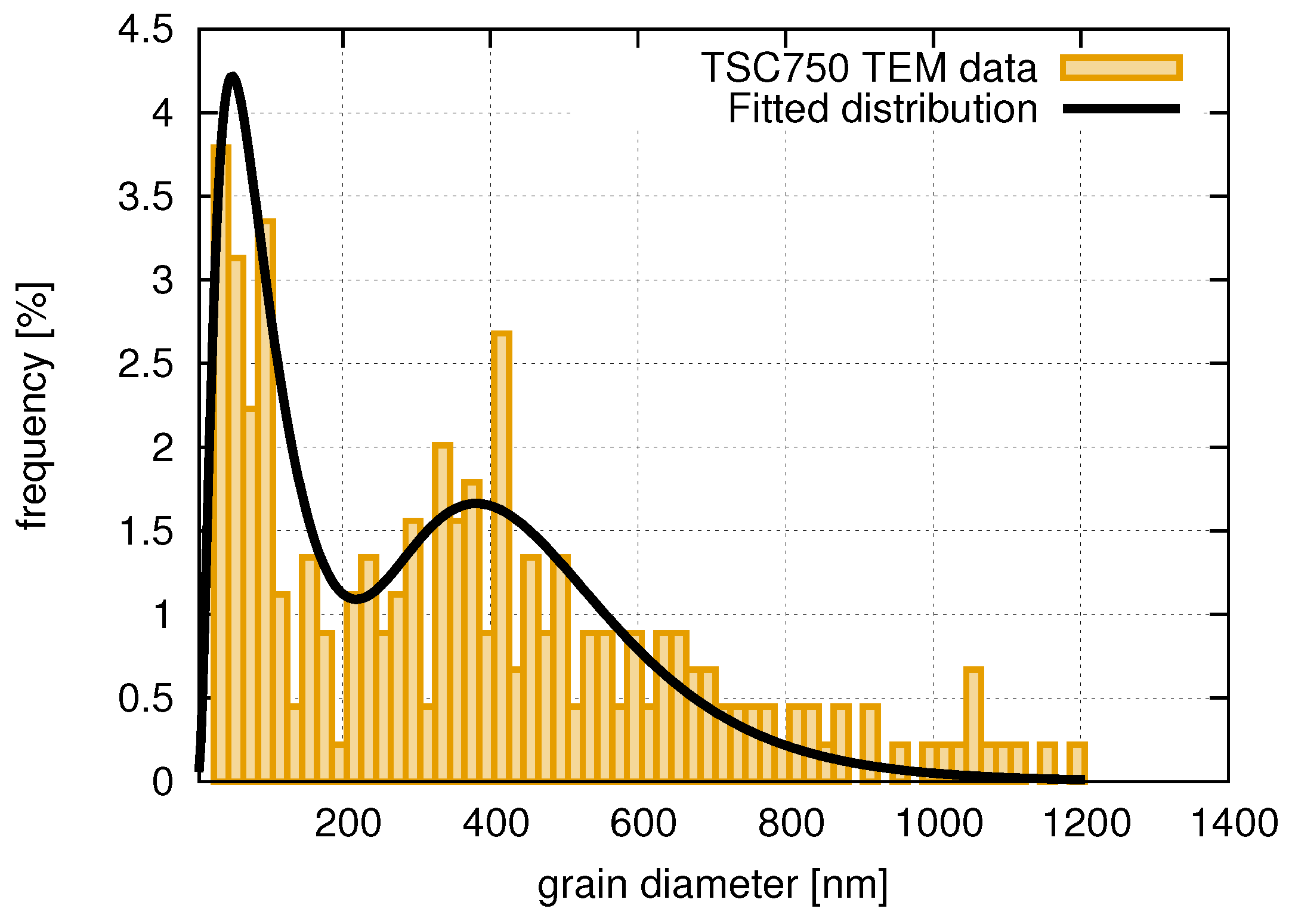
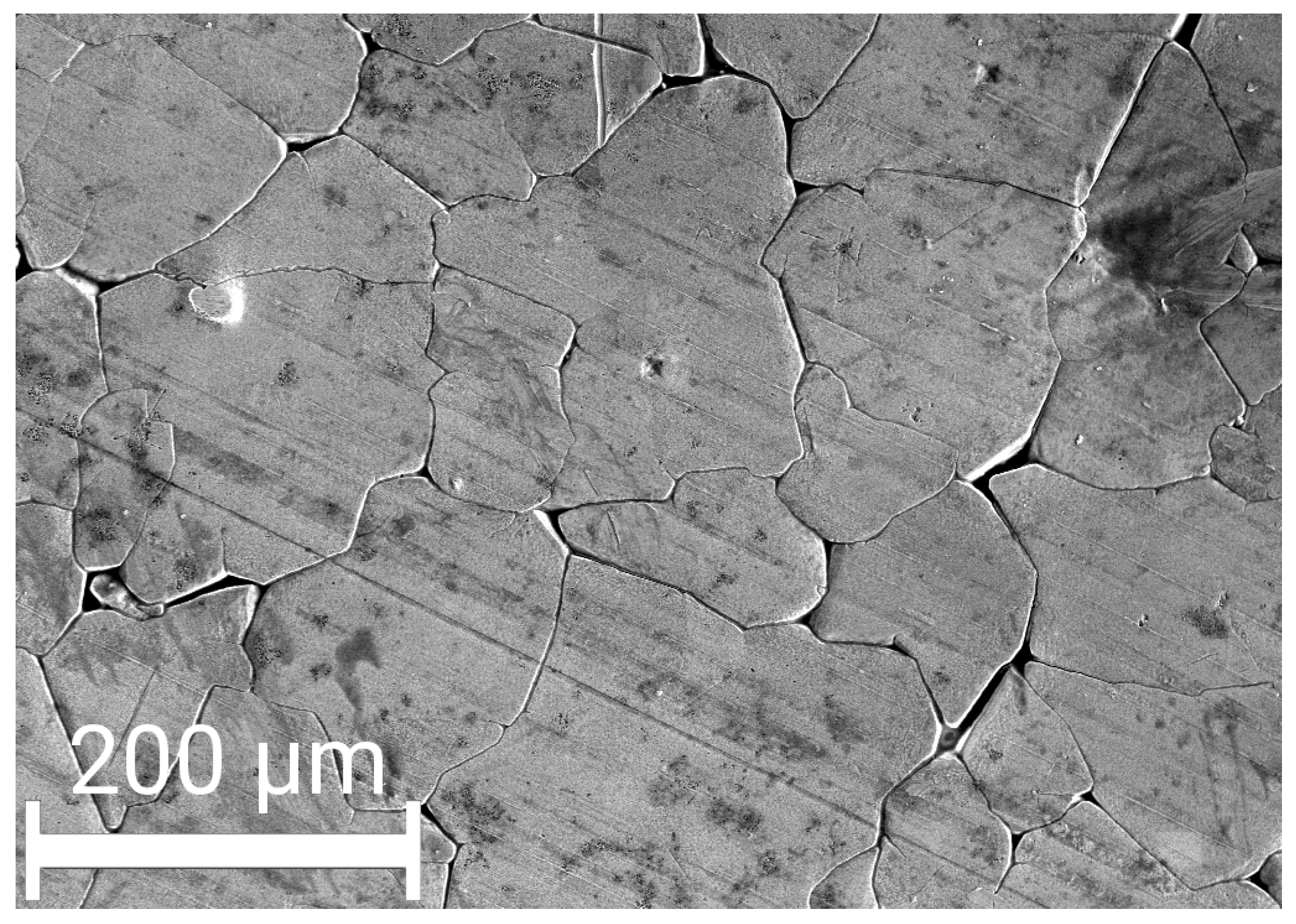
3.1. Evolution of the Crystallite and Grain Sizes, as Well as the Defect Density, during Annealing
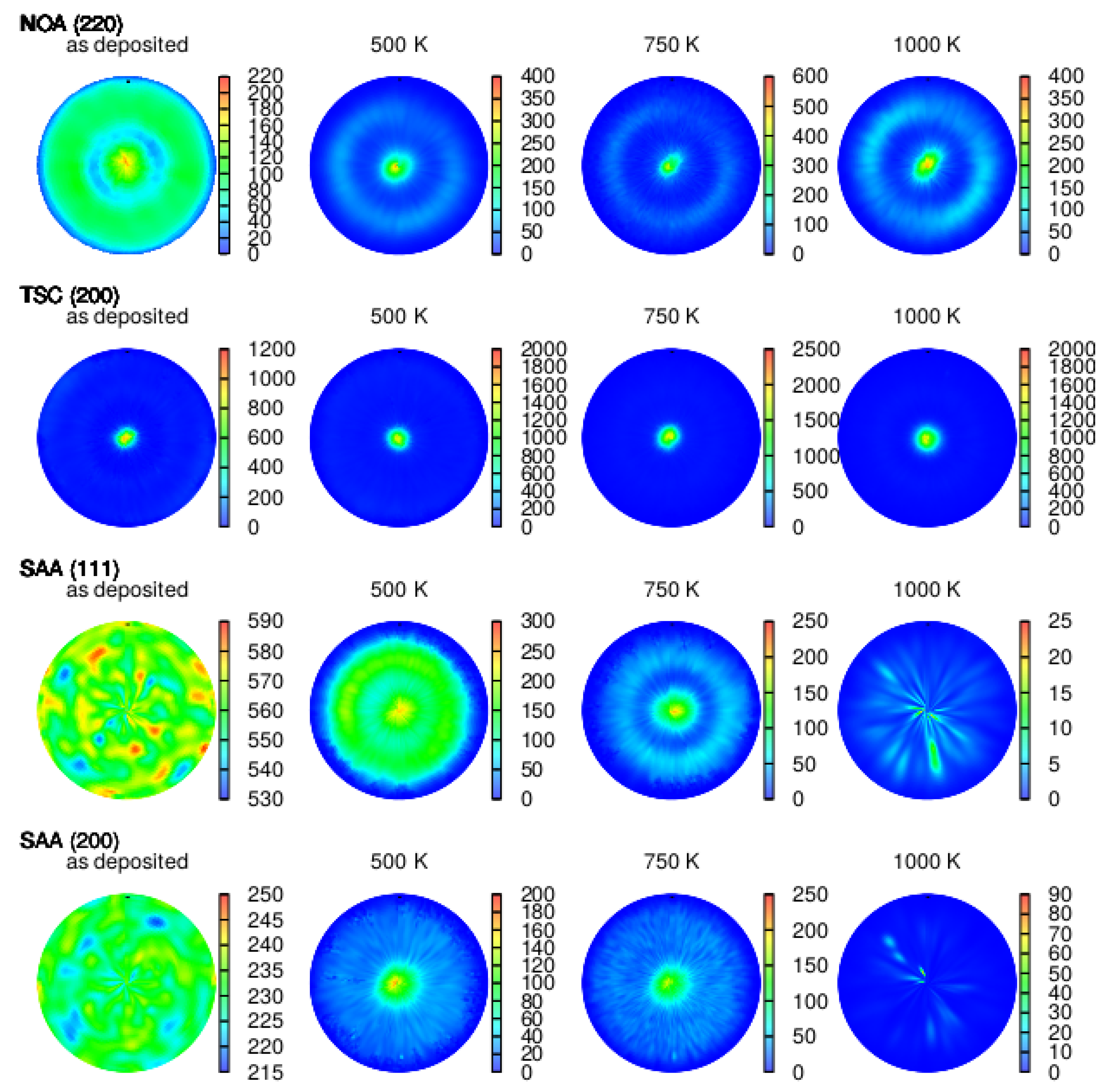
3.2. Texture
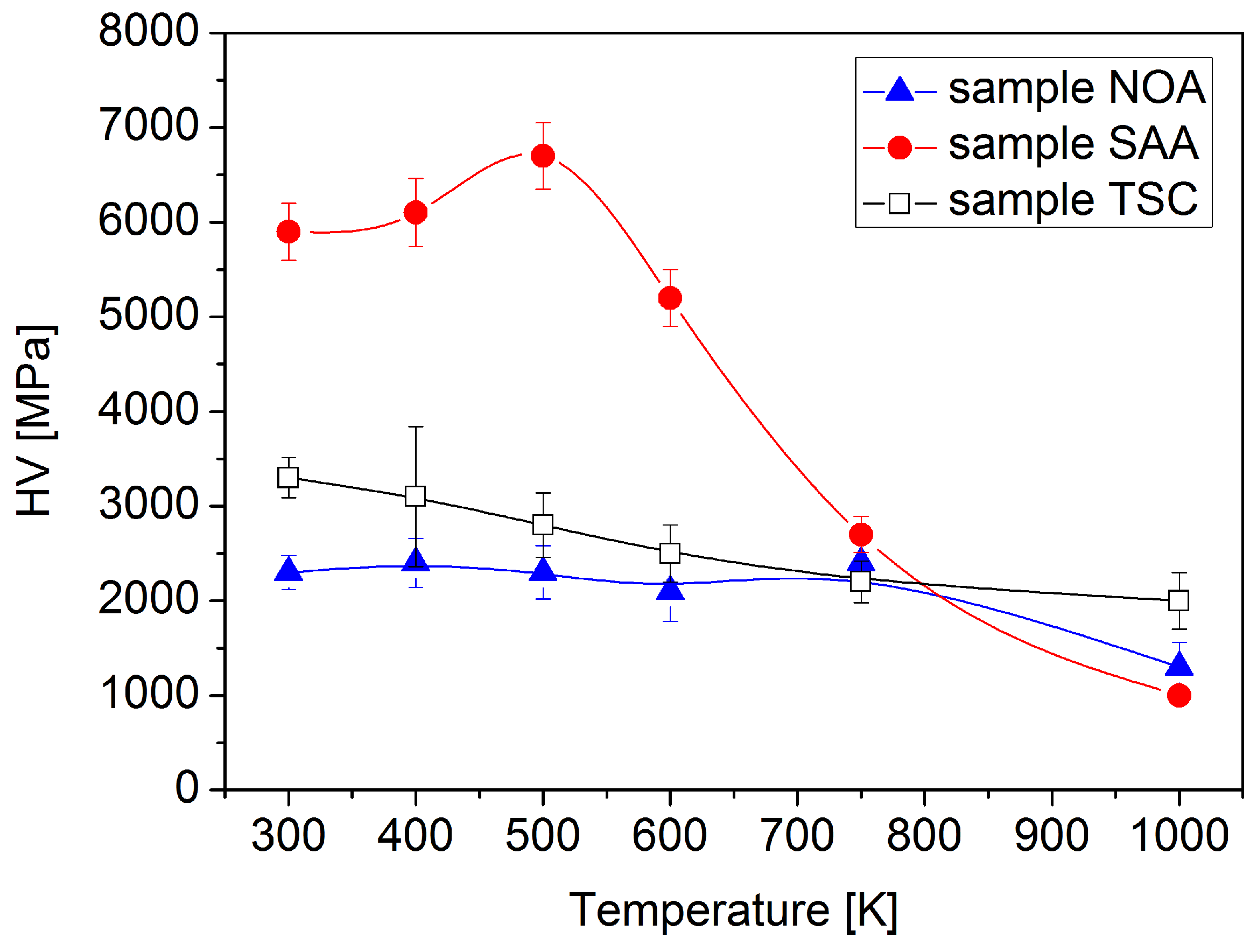
3.3. Hardness
4. Discussion
4.1. Effect of Additives on the Thermal Stability of the Nanostructure
4.2. Effect of Additives on Texture Evolution
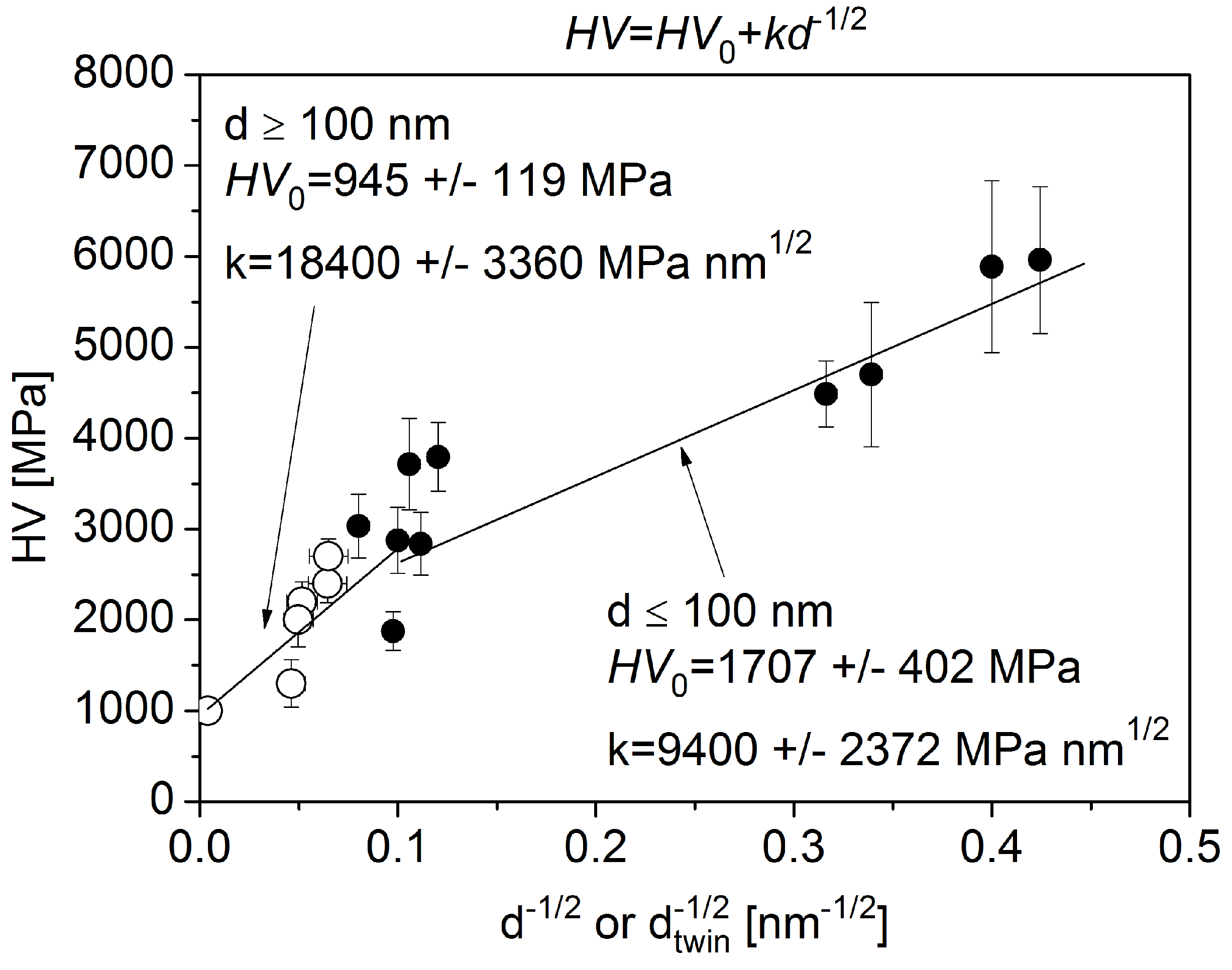
4.3. Correlation between the Microstructure and the Hardness for the Annealed Layers
5. Conclusions
- Both saccharin and trisodium citrate increased the lattice defect density, but this effect was more pronounced in the former case. In addition, saccharin also reduced the grain size. Thus, the addition of saccharin strongly increased the stored energy in Ni films, which resulted in a higher thermodynamic driving force for the destabilization of the microstructure during annealing, leading to a recrystallization at 1000 K. The recrystallization in sample SAA yielded a decrease by three orders of magnitude in the defect density and a similar increase in the grain size. Trisodium citrate also increased the energy stored in dislocations; however, this effect was exceeded by the microstructure stabilization effect of the sharp texture. Therefore, sample TSC showed a better thermal stability as compared to the additive-free layer.
- The recrystallization in sample SAA was inhomogeneous, i.e., between the large recrystallized grains nanocrystalline regions remained after annealing at 1000 K. The sulfur concentration in the nanocrystalline regions was higher than in the recrystallized large grains. This observation suggests that these impurities diffused from the recrystallized grains into the neighboring fine-grained regions where they stabilized the remaining nanostructure even at 1000 K.
- The relative remaining hardness at 1000 K was 56%, 17%, and 61% for the samples NOA, SAA, and TSC, respectively. Thus, the hardness evolution during annealing also showed that the addition of saccharin and trisodium citrate decreased and increased the thermal stability of electrodeposited Ni films, respectively. The first effect is more pronounced than the second one. The Hall–Petch slope for the annealed samples was higher than that for the as-deposited layers due to the higher grain size. Nevertheless, the decrease of the hardness during annealing can be ascribed mainly to the increase of the grain size. On the other hand, some hardness changes cannot be explained by the variation of the grain size. For instance, the hardness of sample SAA increased during annealing up to 500 K, although the crystallite size did not change and the defect density decreased. This annealing-induced hardening can be explained by the reduced grain boundary sliding due to grain boundary relaxation and impurity segregation during annealing. Annealing-induced hardening was not observed for samples NOA and TSC due to their larger grain sizes.
Author Contributions
Funding
Conflicts of Interest
References
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Kumar, K.S.; Swygenhoven, H.V.; Suresh, S. Mechanical behavior of nanocrystalline metals and alloys. Acta Mater. 2003, 51, 5743–5774. [Google Scholar] [CrossRef]
- Kumar, K.S.; Suresh, S.; Chisholm, M.F.; Horton, J.A.; Wang, P. Deformation of electrodeposited nanocrystalline nickel. Acta Mater. 2003, 51, 387–405. [Google Scholar] [CrossRef]
- Torre, F.D.; Swygenhoven, H.V.; Victoria, M. Nanocrystalline electrodeposited Ni: Microstructure and tensile properties. Acta Mater. 2002, 50, 3957–3970. [Google Scholar] [CrossRef]
- Budrovic, Z.; Swygenhoven, H.V.; Derlet, P.M.; Petegem, S.V.; Schmitt, B. Plastic Deformation with Reversible Peak Broadening in Nanocrystalline Nickel. Science 2004, 304, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, R.; Moser, B.; Dao, M.; Chollacoop, N.; Suresh, S. Some critical experiments on the strain-rate sensitivity of nanocrystalline nickel. Acta Mater. 2003, 51, 5159–5172. [Google Scholar] [CrossRef]
- Kolonits, T.; Jenei, P.; Tóth, B.G.; Czigány, Z.; Gubicza, J.; Péter, L.; Bakonyi, I. Characterization of Defect Structure in Electrodeposited Nanocrystalline Ni Films. J. Electrochem. Soc. 2016, 163, D107–D114. [Google Scholar] [CrossRef]
- Kolonits, T.; Jenei, P.; Péter, L.; Bakonyi, I.; Czigány, Z.; Gubicza, J. Effect of bath additives on the microstructure, lattice defect density and hardness of electrodeposited nanocrystalline Ni films. Surf. Coat. Technol. 2018, 349, 611–621. [Google Scholar] [CrossRef]
- Gertsman, V.Y.; Birringer, R. On the room-temperature grain growth in nanocrystalline copper. Scr. Metall. Mater. 1994, 30, 577–581. [Google Scholar] [CrossRef]
- Klement, U.; Erb, U.; El-Sherik, A.M.; Aust, K.T. Thermal stability of nanocrystalline Ni. Mater. Sci. Eng. A 1995, 203, 177–186. [Google Scholar] [CrossRef]
- Dake, J.M.; Krill, C.E. Sudden loss of thermal stability in Fe-based nanocrystalline alloys. Scr. Mater. 2012, 66, 390–393. [Google Scholar] [CrossRef]
- Ames, M.; Markmann, J.; Karos, R.; Michels, A.; Tschope, A.; Birringer, R. Unraveling the nature of room temperature grain growth in nanocrystalline materials. Acta Mater. 2008, 56, 4255–4266. [Google Scholar] [CrossRef]
- Burzyńska, L.; Rudnik, E.; Koza, J.; Błaż, L.; Szymański, W. Electrodeposition and heat treatment of nickel/silicon carbide composites. Surf. Coat. Technol. 2008, 202, 2545–2556. [Google Scholar] [CrossRef]
- Broszeit, E. Mechanical, thermal and tribological properties of electro- and chemodeposited composite coatings. Thin Solid Films 1982, 95, 133–142. [Google Scholar] [CrossRef]
- Gyftou, P.; Stroumbouli, M.; Pavlatou, E.A.; Spyrellis, N. Electrodeposition of Ni/SiC Composites by Pulse Electrolysis. Trans. IMF 2002, 80, 88–91. [Google Scholar] [CrossRef]
- Wang, S.C.; Wei, W.C.J. Characterization of electroplated Ni/SiC and Ni/Al2O3 composite coatings bearing nanoparticles. J. Mater. Res. 2003, 18, 1566–1574. [Google Scholar] [CrossRef]
- Boylan, K.; Ostrander, D.; Erb, U.; Palumbo, G.; Aust, K.T. An in situ TEM study of the thermal stability of nanocrystalline NiP. Scr. Metall. Mater. 1991, 25, 2711–2716. [Google Scholar] [CrossRef]
- Chookajorn, T.; Murdoch, H.A.; Schuh, C.A. Design of stable nanocrystalline alloys. Science 2012, 337, 951–954. [Google Scholar] [CrossRef]
- Darling, K.A.; VanLeeuwen, B.K.; Semones, J.E.; Koch, C.C.; Scattergood, R.O.; Kecskes, L.J.; Mathaudhu, S.N. Stabilized nanocrystalline iron-based alloys: Guiding efforts in alloy selection. Mater. Sci. Eng. A 2011, 528, 4365–4371. [Google Scholar] [CrossRef]
- Choi, P.; da Silva, M.; Klement, U.; Al-Kassab, T.; Kirchheim, R. Thermal stability of electrodeposited nanocrystalline Co-1.1 at.% P. Acta Mater. 2005, 53, 4473–4481. [Google Scholar] [CrossRef]
- Chookajorn, T.; Schuh, C.A. Nanoscale segregation behavior and high-temperature stability for nanocrystalline W–20 at.% Ti. Acta Mater. 2014, 73, 128–138. [Google Scholar] [CrossRef]
- Darling, K.A.; VanLeeuween, B.K.; Koch, C.C.; Scattergood, R.O. Thermal stability of nanocrystalline Fe–Zr alloys. Mater. Sci. Eng. A 2010, 527, 3572–3580. [Google Scholar] [CrossRef]
- Wang, Y.M.; Cheng, S.; Wei, Q.M.; Ma, E.; Nieh, T.G.; Hamza, A. Effects of annealing and impurities on tensile properties of electrodeposited nanocrystalline Ni. Scr. Mater. 2004, 51, 1023–1028. [Google Scholar] [CrossRef]
- Heuer, J.K.; Okamoto, P.R.; Lam, N.Q.; Stubbins, J.F. Relationship between segregation-induced intergranular fracture and melting in the nickel–sulfur system. Appl. Phys. Lett. 2000, 76, 3403–3405. [Google Scholar] [CrossRef]
- Hibbard, G.D.; McCrea, J.L.; Palumbo, G.; Aust, K.T.; Erb, U. An initial analysis of mechanisms leading to late stage abnormal grain growth in nanocrystalline Ni. Scr. Mater. 2002, 47, 83–87. [Google Scholar] [CrossRef]
- Xiao, C.; Mirshams, R.A.; Whang, S.H.; Yin, W.M. Tensile behavior and fracture in nickel and carbon doped nanocrystalline nickel. Mater. Sci. Eng. A 2001, 301, 35–43. [Google Scholar] [CrossRef]
- Lücke, K.; Detert, K. A quantitative theory of grain-boundary motion and recrystallization in metals in the presence of impurities. Acta Metall. 1957, 5, 628–637. [Google Scholar] [CrossRef]
- Michels, A.; Krill, C.E.; Ehrhardt, H.; Birringer, R.; Wu, D.T. Modeling the influence of grain-size-dependent solute drag on the kinetics of grain growth in nanocrystalline materials. Acta Mater. 1999, 47, 2143–2152. [Google Scholar] [CrossRef]
- Hillert, M. Inhibition of grain growth by second-phase particles. Acta Metall. 1998, 36, 3177–3181. [Google Scholar] [CrossRef]
- Gottstein, G.; Shvindlerman, L.S. Grain Boundary Migration in Metals: Thermodynamics, Kinetics, Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Hondros, E.D.; Seah, M.P.; Hofmann, S.; Lejcek, P. Interfacial and surface microchemistry. In Physical Metallurgy, 4th ed.; Cahn, R.W., Haasen, P., Eds.; North-Holland: Amsterdam, The Netherlands, 1996; pp. 1202–1289. [Google Scholar]
- Tóth, B.G.; Péter, L.; Révész, A.; Pádár, J.; Bakonyi, I. Temperature dependence of the electrical resistivity and the anisotropic magnetoresistance (AMR) of electrodeposited Ni-Co alloys. Eur. Phys. J. B 2010, 75, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Green, T.A.; Russell, A.E.; Roy, S. The Development of a Stable Citrate Electrolyte for the Electrodeposition of Copper-Nickel Alloys. J. Electrochem. Soc. 1998, 150, 875–881. [Google Scholar] [CrossRef]
- Barna, A. Topographic kinetics and practice of low angle ion beam thinning. Mater. Res. Soc. Symp. Proc. 1992, 254, 3–22. [Google Scholar] [CrossRef]
- Ribárik, G.; Gubicza, J.; Ungár, T. Correlation between strength and microstructure of ball-milled Al–Mg alloys determined by X-ray diffraction. Mater. Sci. Eng. A 2004, 387, 343–347. [Google Scholar] [CrossRef]
- Balogh, L.; Ribárik, G.; Ungár, T. Stacking faults and twin boundaries in fcc crystals determined by X-ray diffraction profile analysis. J. Appl. Phys. 2006, 100, 023512. [Google Scholar] [CrossRef]
- Pearson, K.X. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Lond. Edinb. Dublin Philos. Mag. J. Sci. Ser. 1900, 50, 157–175. [Google Scholar] [CrossRef]
- Novikov, V.Y. On computer simulation of texture development in grain growth. Acta Metall. 1979, 27, 1461–1466. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Lücke, K. A theory of texture controlled grain growth-I. Derivation and general discussion of the model. Acta Metall. 1986, 34, 905–914. [Google Scholar] [CrossRef]
- Eichelkraut, H.; Abbruzzese, G.; Lücke, K. A theory of texture controlled grain growth-II. Numerical and analytical treatment of grain growth in the presence of two texture components. Acta Metall. 1988, 36, 55–68. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Lucke, K.; Eichelkraut, H. Computer simulation of texture-controlled grain growth. Trans. Iron Steel Inst. Jpn. 1988, 28, 818–825. [Google Scholar] [CrossRef]
- Li, D.Y.; Szpunar, J.A. Textural evolution in electrodeposits under the influence of adsorbed foreign species: Part I textural evolution in iron electrodeposits affected by hydrogen co-deposition. J. Mater. Sci. 1997, 32, 5513–5523. [Google Scholar] [CrossRef]
- Wu, X.; Gao, Q.; Li, Z. Effects of additives on morphology and hydrogen evolution activities of nickel films prepared by electrodepositing. Int. J. Electrochem. Sci. 2015, 10, 8823–8833. [Google Scholar]
- Neurock, M.; van Santen, R.A. Theory of carbon-sulfur bond activation by small metal sulfide particles. J. Am. Chem. Soc. 1994, 116, 4427–4439. [Google Scholar] [CrossRef]
- Reddy, A.K.N. Preferred orientations in nickel electro-deposits I. The mechanism of development of textures in nickel electro-deposits. J. Electroanal. Chem. 1963, 6, 141–152. [Google Scholar]
- Lu, L.; Shen, Y.; Chen, X.; Qian, L.; Lu, K. Ultrahigh strength and high electrical conductivity in copper. Science 2004, 304, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Erb, U. Electrodeposited nanocrystals: Synthesis, properties and industrial applications. Nanostruct. Mater. 1995, 6, 533–538. [Google Scholar] [CrossRef]
- Jeong, D.H.; Gonzalez, F.; Palumbo, G.; Aust, K.T.; Erb, U. The effect of grain size on the wear properties of electrodeposited nanocrystalline nickel coatings. Scr. Mater. 2001, 44, 493–499. [Google Scholar] [CrossRef]
- Shaw, L.L.; Ortiz, A.L.; Villegas, J.C. Hall–Petch relationship in a nanotwinned nickel alloy. Scr. Mater. 2008, 58, 951–954. [Google Scholar] [CrossRef]
- Tabor, D. The hardness and strength of metals. J. Inst. Met. 1951, 79, 1–18. [Google Scholar]
- Hu, J.; Shi, Y.N.; Sauvage, X.; Sha, G.; Lu, K. Grain boundary stability governs hardening and softening in extremely fine nanograined metals. Science 2017, 355, 1292–1296. [Google Scholar] [CrossRef]
- Kapoor, G.; Péter, L.; Fekete, E.; Lábár, J.L.; Gubicza, J. The influence of Mo addition on the microstructure and its thermal stability for electrodeposited Ni films. Mater. Charact. 2018, 145, 563–572. [Google Scholar] [CrossRef]
- Zheng, X.; Hu, J.; Li, J.; Shi, Y. Achieving ultrahigh hardness in electrodeposited nanograined Ni-based binary alloys. Nanomaterials 2019, 9, 546. [Google Scholar] [CrossRef] [PubMed]
- Gubicza, J. Annealing-induced hardening in ultrafine-grained and nanocrystalline materials. Adv. Eng. Mater 2019, 1900507. [Google Scholar] [CrossRef]
| Component/Solution | NOA | SAA | TSC | Role |
|---|---|---|---|---|
| NiSO4·6H2O | 155 | 155 | 185 | Ni source |
| H3BO3 | 15 | 15 | - | pH-buffer |
| NaSO4·10H2O | 96 | 96 | - | Supporting electrolyte |
| H3NO3S | 10 | 10 | - | wetting agent |
| Saccharin | - | 1 | - | additive |
| C7H5NO3S | ||||
| Trisodium citrate | - | - | 65 | additive |
| C6H5O7Na3·2H2O |
| Sample | Temperature (K) | d (nm) | <x> (nm) | ρ (1014 m−2) | β (%) | HV (MPa) |
|---|---|---|---|---|---|---|
| NOA | as-deposited | 105 ± 26 | 33 ± 4 | 15 ± 2 | 0.0 | 2300 ± 180 |
| NOA | 400 | - | 39 ± 8 | 14 ± 2 | 0.0 | 2400 ± 260 |
| NOA | 500 | - | 38 ± 5 | 15 ± 2 | 0.0 | 2300 ± 280 |
| NOA | 600 | - | 41 ± 8 | 7 ± 2 | 0.0 | 2100 ± 320 |
| NOA | 750 | 240 ± 26 | 88 ± 9 | 3 ± 1 | 0.0 | 2400 ± 210 |
| NOA | 1000 | 470 ± 52 | 151 ± 17 | 2 ± 1 | 0.0 | 1300 ± 260 |
| SAA | as-deposited | 32 ± 2 | 24 ± 3 | 180 ± 20 | 3.6 | 5900 ± 300 |
| SAA | 400 | - | 22 ± 3 | 118 ± 15 | 3.1 | 6100 ± 360 |
| SAA | 500 | - | 21 ± 2 | 124 ± 15 | 1.0 | 6700 ± 350 |
| SAA | 600 | - | 46 ± 6 | 106 ± 10 | 0.2 | 5200 ± 300 |
| SAA | 750 | 237 ± 30 | 73 ± 9 | 6 ± 2 | 0.0 | 2700 ± 190 |
| SAA | 1000 | 60000 ± 20000 | >800 | <0.1 | <0.1 | 1000 ± 50 |
| TSC | as-deposited | 90 ± 11 | 38 ± 5 | 31 ± 3 | 0.0 | 3300 ± 210 |
| TSC | 400 | - | 44 ± 5 | 18 ± 2 | 0.0 | 3100 ± 740 |
| TSC | 500 | - | 47 ± 5 | 10 ± 2 | 0.0 | 2800 ± 340 |
| TSC | 600 | - | 39 ± 6 | 9 ± 2 | 0.0 | 2500 ± 300 |
| TSC | 750 | 377 ± 58 | 62 ± 7 | 10 ± 2 | 0.0 | 2200 ± 220 |
| TSC | 1000 | 405 ± 49 | 152 ± 16 | 7 ± 1 | 0.0 | 2000 ± 300 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolonits, T.; Czigány, Z.; Péter, L.; Bakonyi, I.; Gubicza, J. Influence of Bath Additives on the Thermal Stability of the Nanostructure and Hardness of Ni Films Processed by Electrodeposition. Coatings 2019, 9, 644. https://doi.org/10.3390/coatings9100644
Kolonits T, Czigány Z, Péter L, Bakonyi I, Gubicza J. Influence of Bath Additives on the Thermal Stability of the Nanostructure and Hardness of Ni Films Processed by Electrodeposition. Coatings. 2019; 9(10):644. https://doi.org/10.3390/coatings9100644
Chicago/Turabian StyleKolonits, Tamás, Zsolt Czigány, László Péter, Imre Bakonyi, and Jeno Gubicza. 2019. "Influence of Bath Additives on the Thermal Stability of the Nanostructure and Hardness of Ni Films Processed by Electrodeposition" Coatings 9, no. 10: 644. https://doi.org/10.3390/coatings9100644
APA StyleKolonits, T., Czigány, Z., Péter, L., Bakonyi, I., & Gubicza, J. (2019). Influence of Bath Additives on the Thermal Stability of the Nanostructure and Hardness of Ni Films Processed by Electrodeposition. Coatings, 9(10), 644. https://doi.org/10.3390/coatings9100644









