Comparison of Properties of the Hybrid and Bilayer MWCNTs—Hydroxyapatite Coatings on Ti Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Substrate Surfaces
2.2. Preparation of CNTs’ Suspension
2.3. Deposition of Coatings
2.4. Structure and Morphology
2.5. Nanomechanical Studies
2.6. Contact Angle Studies
3. Results and Discussion
3.1. Structure and Morphology
3.2. Mechanical Studies
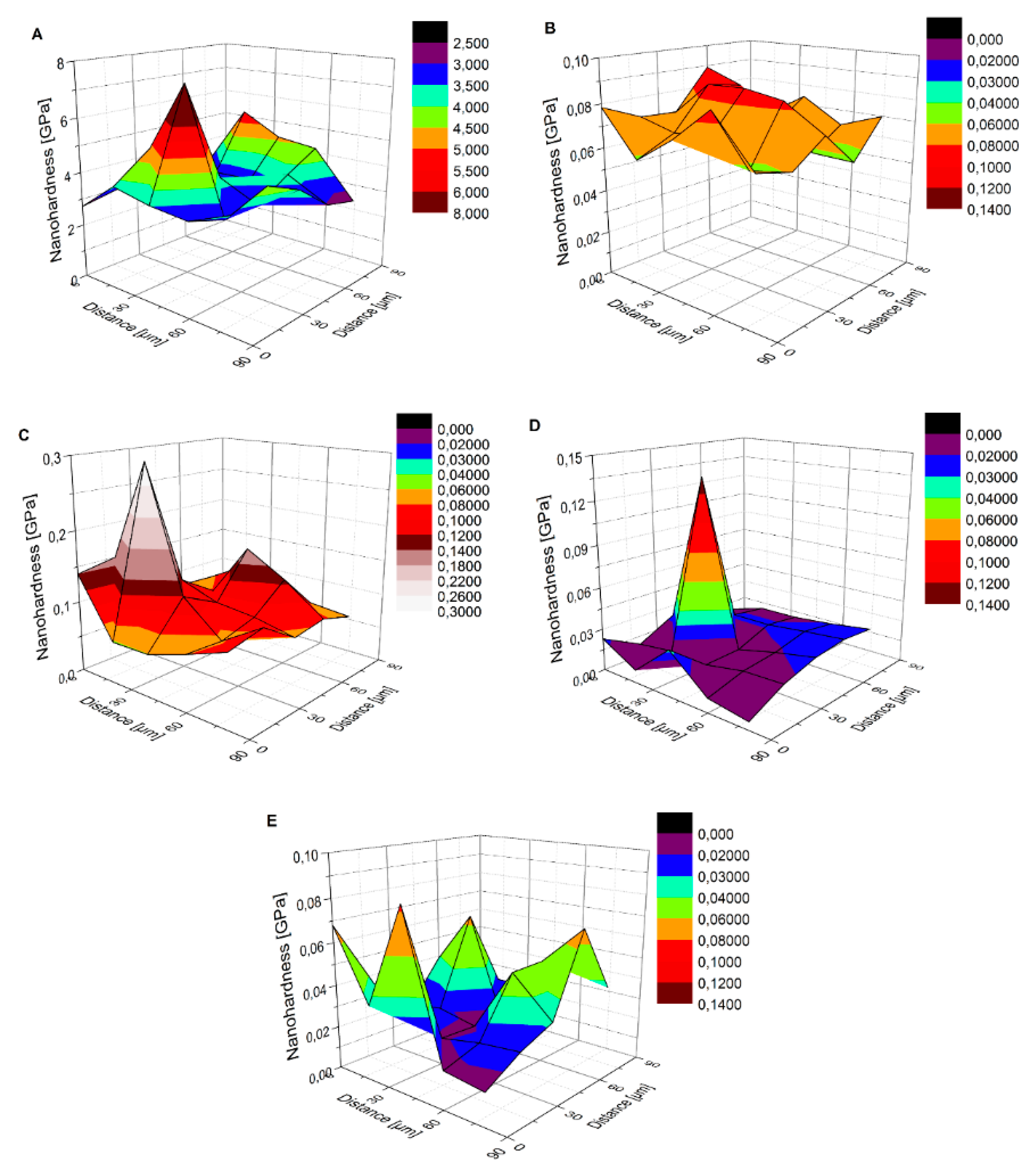
3.2.1. Nanoindentation
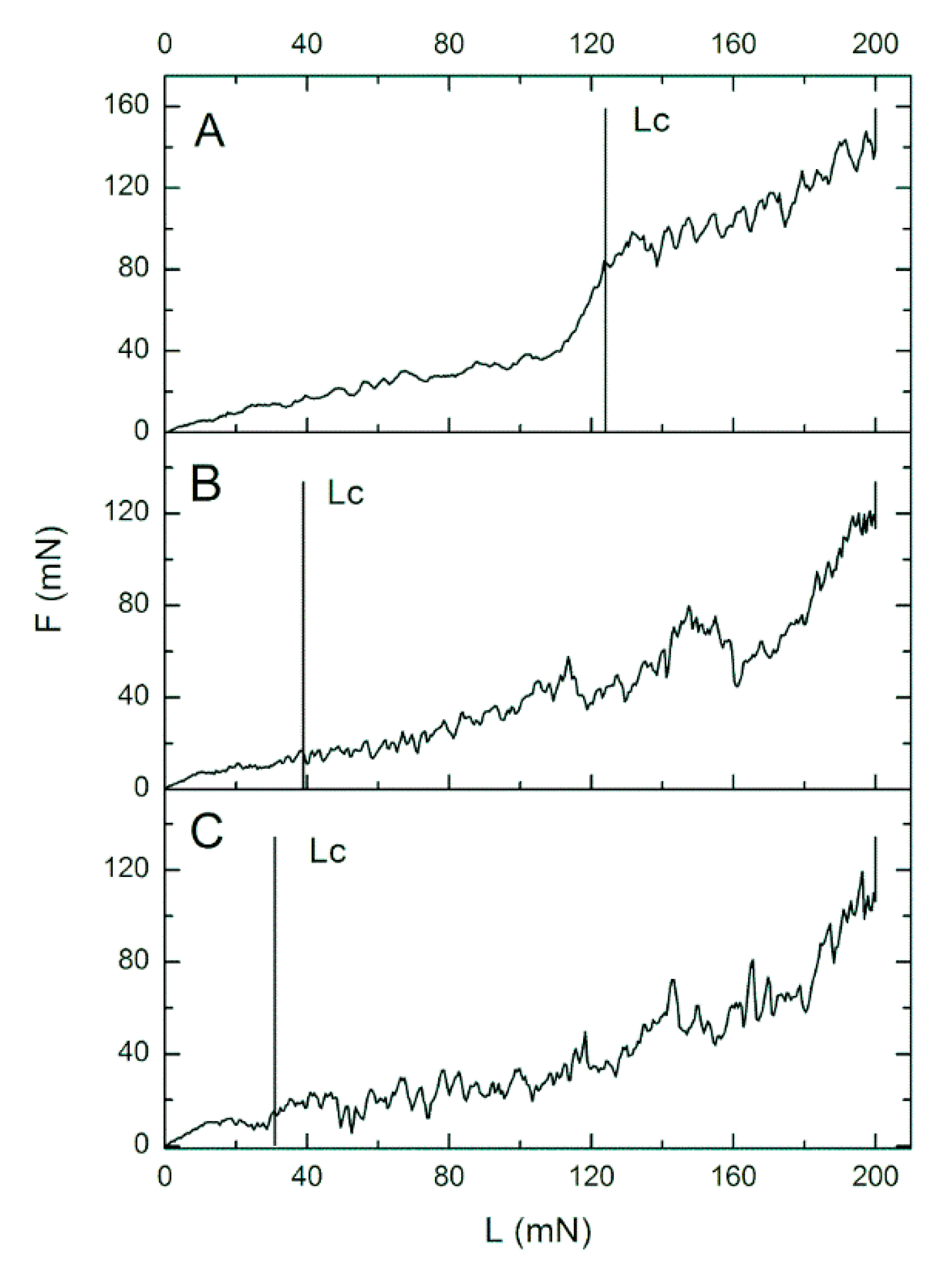
3.2.2. Nanoscratch Test
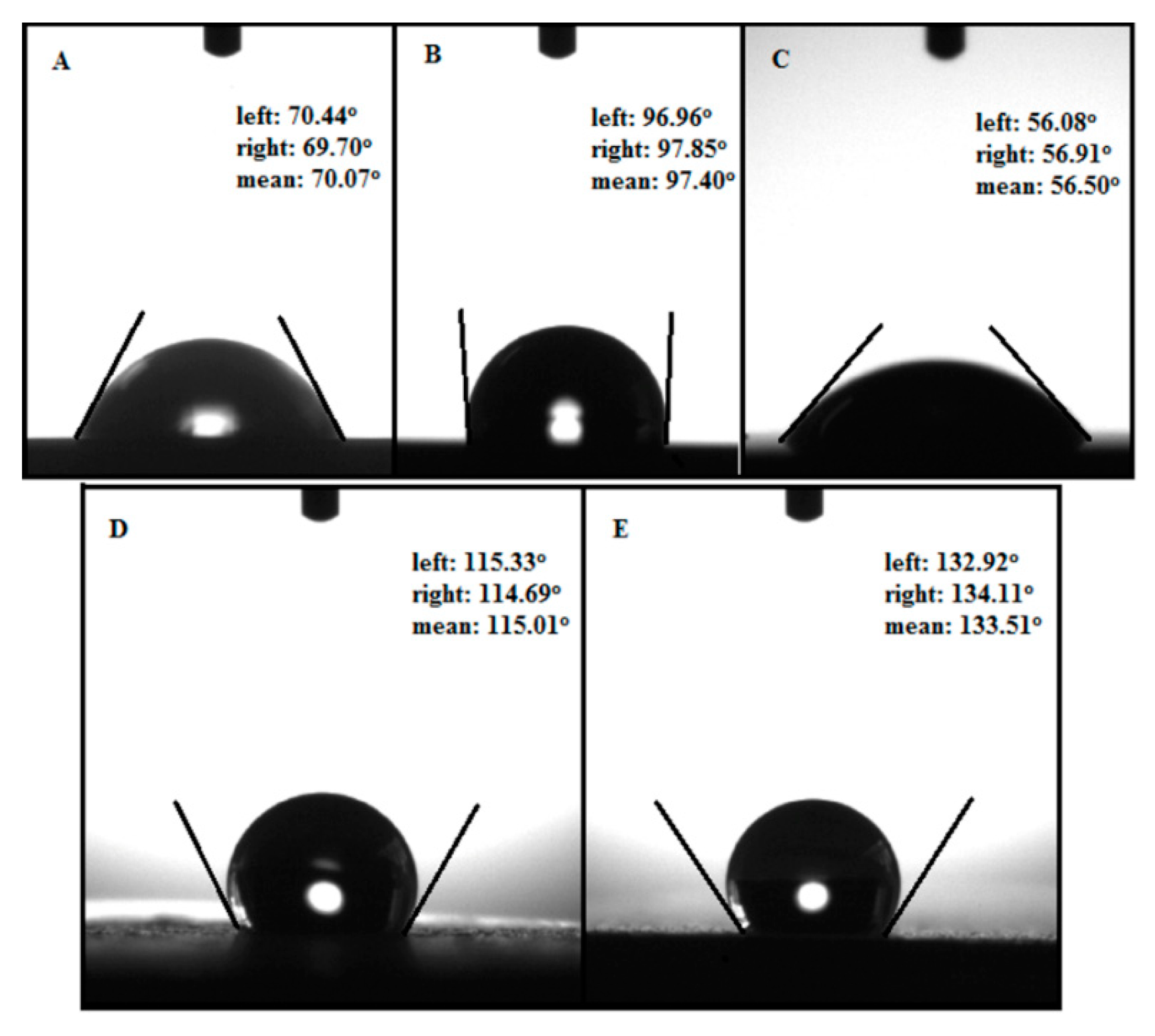
3.3. Contact Angle Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ku, S.H.; Lee, M.; Park, C.B. Carbon-Based Nanomaterials for Tissue Engineering. Adv. Healthc. Mater. 2013, 2, 244–260. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Huang, J.; Fan, Y.; Cui, F. Surface & Coatings Technology Biomedical investigation of CNT based coatings. Surf. Coat. Technol. 2011, 206, 759–766. [Google Scholar]
- Dlugon, E.; Simka, W.; Fraczek-Szczypta, A.; Niemiec, W.; Markowski, J.; Szymanska, M.; Blazewicz, M. Carbon nanotube-based coatings on titanium. Bull. Mater. Sci. 2015, 38, 1339–1344. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Sato, Y.; Zhang, M.; Haniu, H.; Okamoto, M.; Aoki, K.; Takizawa, T.; Yoshida, K.; Sobajima, A.; Kamanaka, T.; et al. In Vitro and In Vivo Evaluation of a Three-Dimensional Porous Multi-Walled Carbon Nanotube Scaffold for Bone Regeneration. Nanomaterials 2017, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.; Ghosh, S.; Agarwal, A. Carbon nanotube reinforced hydroxyapatite composite for orthopedic application: A review. Mater. Sci. Eng. C 2012, 32, 1727–1758. [Google Scholar] [CrossRef]
- Usui, Y.; Aoki, K.; Narita, N.; Murakami, N.; Nakamura, I.; Nakamura, K.; Ishigaki, N.; Yamazaki, H.; Horiuchi, H.; Kato, H.; et al. full papers Carbon Nanotubes with High Bone-Tissue Compatibility and Bone-Formation Acceleration Effects. Small 2008, 8621, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Kalbacova, M.; Kalbac, M. Influence of single-walled carbon nanotube films on metabolic activity and adherence of human osteoblasts. Carbon 2007, 45, 2266–2272. [Google Scholar] [CrossRef]
- Lahiri, D.; Benaduce, A.P.; Rouzaud, F.; Solomon, J.; Keshri, A.K.; Kos, L.; Agarwal, A. Wear behavior and in vitro cytotoxicity of wear debris generated from hydroxyapatite–carbon nanotube composite coating. J. Biomed. Mater. Res. Part A 2010, 0494, 1–12. [Google Scholar] [CrossRef]
- Matsuoka, M.; Akasaka, T.; Totsuka, Y.; Watari, F. Strong adhesion of Saos-2 cells to multi-walled carbon nanotubes. Mater. Sci. Eng. B 2010, 173, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Akasaka, T.; Yokoyama, A.; Matsuoka, M.; Hashimoto, T.; Watari, F. Thin fi lms of single-walled carbon nanotubes promote human osteoblastic cells (Saos-2) proliferation in low serum concentrations. Mater. Sci. Eng. C 2010, 30, 391–399. [Google Scholar] [CrossRef]
- Hirschfeld, J.; Akinoglu, E.M.; Wirtz, D.C.; Hoerauf, A.; Bekeredjian-Ding, I.; Jepsen, S.; Haddouti, E.M.; Limmer, A.; Giersig, M. Long-term release of antibiotics by carbon nanotube-coated titanium alloy surfaces diminish biofilm formation by Staphylococcus epidermidis. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Prasad, M.; Song, I.; Ho, M.; Sung, T.; Watari, F.; Uo, M. Electrophoretic deposition of carbon nanotubes – hydroxyapatite nanocomposites on titanium substrate. Mater. Sci. Eng. C 2010, 30, 1043–1049. [Google Scholar] [CrossRef]
- Długoń, E.; Niemiec, W.; Fra̧czek-Szczypta, A.; Jeleń, P.; Sitarz, M.; Błazewicz, M. Spectroscopic studies of electrophoretically deposited hybrid HAp/CNT coatings on titanium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 872–875. [Google Scholar] [CrossRef]
- Abrishamchian, A.; Hooshmand, T.; Mohammadi, M.; Najafi, F. Preparation and characterization of multi-walled carbon nanotube/hydroxyapatite nanocomposite film dip coated on Ti-6Al-4V by sol-gel method for biomedical applications: An in vitro study. Mater. Sci. Eng. C 2013, 33, 2002–2010. [Google Scholar] [CrossRef]
- Gopi, D.; Shinyjoy, E.; Kavitha, L. Influence of ionic substitution in improving the biological property of carbon nanotubes reinforced hydroxyapatite composite coating on titanium for orthopedic applications. Ceram. Int. 2015, 41, 5454–5463. [Google Scholar] [CrossRef]
- Gopi, D.; Shinyjoy, E.; Sekar, M.; Surendiran, M.; Kavitha, L.; Sampath Kumar, T.S. Development of carbon nanotubes reinforced hydroxyapatite composite coatings on titanium by electrodeposition method. Corros. Sci. 2013, 73, 321–330. [Google Scholar] [CrossRef]
- Pei, X.; Zeng, Y.; He, R.; Li, Z.; Tian, L.; Wang, J.; Wan, Q.; Li, X.; Bao, H. Single-walled carbon nanotubes/hydroxyapatite coatings on titanium obtained by electrochemical deposition. Appl. Surf. Sci. 2014, 295, 71–80. [Google Scholar] [CrossRef]
- Zhong, Z.; Qin, J.; Ma, J. Electrophoretic deposition of biomimetic zinc substituted hydroxyapatite coatings with chitosan and carbon nanotubes on titanium. Ceram. Int. 2015, 41, 8878–8884. [Google Scholar] [CrossRef]
- Zanello, L.P.; Zhao, B.; Hu, H.; Haddon, R.C. Bone cell proliferation on carbon nanotubes. Nano Lett. 2006, 6, 562–567. [Google Scholar] [CrossRef]
- Prodana, M.; Duta, M.; Ionita, D.; Bojin, D.; Stan, M.S.; Dinischiotu, A.; Demetrescu, I. A new complex ceramic coating with carbon nanotubes, hydroxyapatite and TiO 2 nanotubes on Ti surface for biomedical applications. Ceram. Int. 2015, 41, 6318–6325. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, D.; Vijayalakshmi, K. Substantial effect of magnesium incorporation on hydroxyapatite/carbon nanotubes coatings on metallic implant surfaces for better anticorrosive protection and antibacterial ability. J. Anal. Appl. Pyrolysis 2018, 135, 15–21. [Google Scholar] [CrossRef]
- Mukherjee, S.; Nandi, S.K.; Kundu, B.; Chanda, A.; Sen, S.; Das, P.K. Enhanced bone regeneration with carbon nanotube reinforced hydroxyapatite in animal model. J. Mech. Behav. Biomed. Mater. 2016, 60, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A.; Nabi, F. Nanotoxicity: Dimensional and morphological concerns. Adv. Phys. Chem. 2011, 2011. [Google Scholar]
- Teleanu, D.; Chircov, C.; Grumezescu, A.; Teleanu, R. Neurotoxicity of Nanomaterials: An Up-to-Date Overview. Nanomaterials 2019, 9, 96. [Google Scholar] [CrossRef]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon nanotubes: Evaluation of toxicity at biointerfaces. J. Pharm. Anal. 2019. [Google Scholar] [CrossRef]
- Nawrotek, K.; Tylman, M.; Rudnicka, K.; Gatkowska, J.; Balcerzak, J. Tubular electrodeposition of chitosan-carbon nanotube implants enriched with calcium ions. J. Mech. Behav. Biomed. Mater. 2016, 60, 256–266. [Google Scholar] [CrossRef]
- Guan, K.; Zhang, L.; Zhu, F.; Sheng, H.; Li, H. Surface modification for carbon/carbon composites with Mg-CaP coating reinforced by SiC nanowire-carbon nanotube hybrid for biological application. Appl. Surf. Sci. 2019, 489, 856–866. [Google Scholar] [CrossRef]
- Khazeni, D.; Saremi, M.; Soltani, R. Development of HA-CNTs composite coating on AZ31 Magnesium alloy by cathodic electrodeposition. Part 2: Electrochemical and in-vitro behavior. Ceram. Int. 2019, 45, 11186–11194. [Google Scholar] [CrossRef]
- Alsagri, A.S.; Nasir, S.; Gul, T.; Islam, S.; Nisar, K.S.; Shah, Z.; Khan, I. MHD thin film flow and thermal analysis of blood with CNTs nanofluid. Coatings 2019, 9, 175. [Google Scholar] [CrossRef]
- Przekora, A.; Benko, A.; Nocun, M.; Wyrwa, J.; Blazewicz, M.; Ginalska, G. Titanium coated with functionalized carbon nanotubes—A promising novel material for biomedical application as an implantable orthopaedic electronic device. Mater. Sci. Eng. C 2014, 45, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.B.; Peairs, M.J.; Venton, B.J. Analytica Chimica Acta Review: Carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta 2010, 662, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Benko, A.; Nocuń, M.; Berent, K.; Gajewska, M.; Klita, Ł.; Wyrwa, J.; Błażewicz, M. Diluent changes the physicochemical and electrochemical properties of the electrophoretically-deposited layers of carbon nanotubes. Appl. Surf. Sci. 2017, 403, 206–217. [Google Scholar] [CrossRef]
- Sivaraj, D.; Vijayalakshmi, K. Novel synthesis of bioactive hydroxyapatite/f-multiwalled carbon nanotube composite coating on 316L SS implant for substantial corrosion resistance and antibacterial activity. J. Alloys Compd. 2019, 1340–1346. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Khalil-Allafi, J. Characterization, mechanical and in vitro biological behavior of hydroxyapatite-titanium-carbon nanotube composite coatings deposited on NiTi alloy by electrophoretic deposition. Surf. Coatings Technol. 2019, 363, 179–190. [Google Scholar] [CrossRef]
- Mohajernia, S.; Pour-Ali, S.; Hejazi, S.; Saremi, M.; Kiani-Rashid, A.R. Hydroxyapatite coating containing multi-walled carbon nanotubes on AZ31 magnesium: Mechanical-electrochemical degradation in a physiological environment. Ceram. Int. 2018, 44, 8297–8305. [Google Scholar] [CrossRef]
- Park, J.E.; Jang, Y.S.; Bae, T.S.; Lee, M.H. Multi-walled carbon nanotube coating on alkali treated TiO2 nanotubes surface for improvement of biocompatibility. Coatings 2018, 8, 159. [Google Scholar] [CrossRef]
- Fraczek-szczypta, A.; Jantas, D.; Ciepiela, F.; Grzonka, J.; Bernasik, A.; Marzec, M. Diamond & Related Materials Carbon nanomaterials coatings – Properties and in fl uence on nerve cells response. Diam. Relat. Mater. 2018, 84, 127–140. [Google Scholar]
- Farrokhi-Rad, M.; Menon, M. Effect of Dispersants on the Electrophoretic Deposition of Hydroxyapatite-Carbon Nanotubes Nanocomposite Coatings. J. Am. Ceram. Soc. 2016, 99, 2947–2955. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Su, Y.; Guo, Q.; Zhang, L. Preparation and properties of in-situ growth of carbon nanotubes reinforced hydroxyapatite coating for carbon/carbon composites. Mater. Sci. Eng. C 2017, 70, 805–811. [Google Scholar] [CrossRef]
- Singh, I.; Kaya, C.; Shaffer, M.S.; Thomas, B.C.; Boccaccini, A.R. Bioactive ceramic coatings containing carbon nanotubes on metallic substrates by electrophoretic deposition. J. Mater. Sci. 2006, 41, 8144–8151. [Google Scholar] [CrossRef]
- Constanda, S.; Stan, M.S.; Ciobanu, C.S.; Motelica-Heino, M.; Guégan, R.; Lafdi, K.; Dinischiotu, A.; Predoi, D. Carbon Nanotubes-Hydroxyapatite Nanocomposites for an Improved Osteoblast Cell Response. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Yi, C.; Bagchi, S.; Dmuchowski, C.M.; Gou, F.; Chen, X.; Park, C.; Chew, H.B.; Ke, C. Direct nanomechanical characterization of carbon nanotube - titanium interfaces. Carbon N. Y. 2018, 132, 548–555. [Google Scholar] [CrossRef]
- Sasani, N.; Vahdati Khaki, J.; Mojtaba Zebarjad, S. Characterization and nanomechanical properties of novel dental implant coatings containing copper decorated-carbon nanotubes. J. Mech. Behav. Biomed. Mater. 2014, 37, 125–132. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Zhang, L.; Liu, Q.; Wang, Y.; Zhang, W.; Zheng, J. Preparation of Nano-Hydroxyapatite Coated Carbon Nanotube Reinforced Hydroxyapatite Composites. Coatings 2018, 8, 357. [Google Scholar] [CrossRef]
- Fathyunes, L.; Khalil-Allafi, J.; Moosavifar, M. Development of graphene oxide/calcium phosphate coating by pulse electrodeposition on anodized titanium: Biocorrosion and mechanical behavior. J. Mech. Behav. Biomed. Mater. 2019, 90, 575–586. [Google Scholar] [CrossRef]
- Cuppone, M.; Seedhom, B.B.; Berry, E.; Ostell, A.E. The Longitudinal Young’s Modulus of Cortical Bone in the Midshaft of Human Femur and its Correlation with CT Scanning Data. Calcif. Tissue Int. 2004, 74, 302–309. [Google Scholar]
- Sansotera, M.; Talaeemashhadi, S.; Gambarotti, C.; Pirola, C.; Longhi, M.; Ortenzi, M.A.; Navarrini, W.; Bianchi, C.L. Comparison of branched and linear perfluoropolyether chains functionalization on hydrophobic, morphological and conductive properties of multi-walled carbon nanotubes. Nanomaterials 2018, 8, 176. [Google Scholar] [CrossRef]
- Türk, S.; Altınsoy, I.; Çelebi Efe, G.; Ipek, M.; Özacar, M.; Bindal, C. 3D porous collagen/functionalized multiwalled carbon nanotube/chitosan/hydroxyapatite composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2018, 92, 757–768. [Google Scholar] [CrossRef]
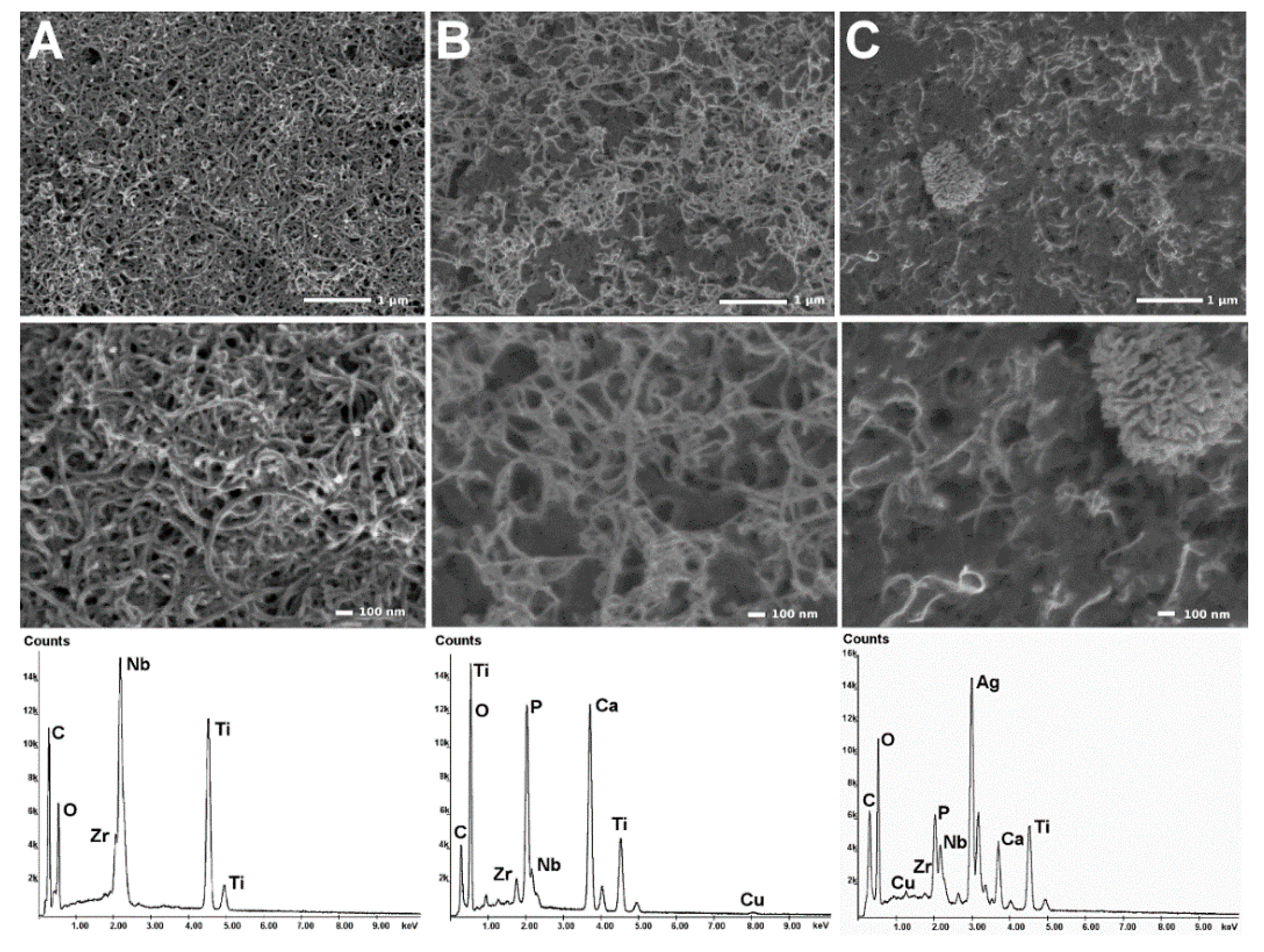
| Element | Nb | Zr | Fe | C | H | O | S | Hf | Ti |
|---|---|---|---|---|---|---|---|---|---|
| wt. pct. | 13.18 | 13.49 | 0.085 | 0.035 | 0.004 | 0.078 | <0.001 | 0.055 | rem. |
| Coating | Synthesis Stage | Concentration of MWCNTs [%] | Duration of EPD [min] | EDP Voltage [V] | Temperature [°C] |
|---|---|---|---|---|---|
| 0.27CNT | EPD of MWCNTs | 0.27 | 2 | 11 | ambient |
| H0.27CNT | EPD of nanoHAp | – | 2 | 30 | ambient |
| Sintering | – | 120 | – | 800 | |
| EPD of MWCNTs | 0.27 | 2 | 30 | ambient | |
| m0.4CNT | EPD of MWCNTs, nanoHAp, nanosilver, nanocopper | 0.4 | 2 | 30 | ambient |
| Sintering | – | 120 | – | 800 |
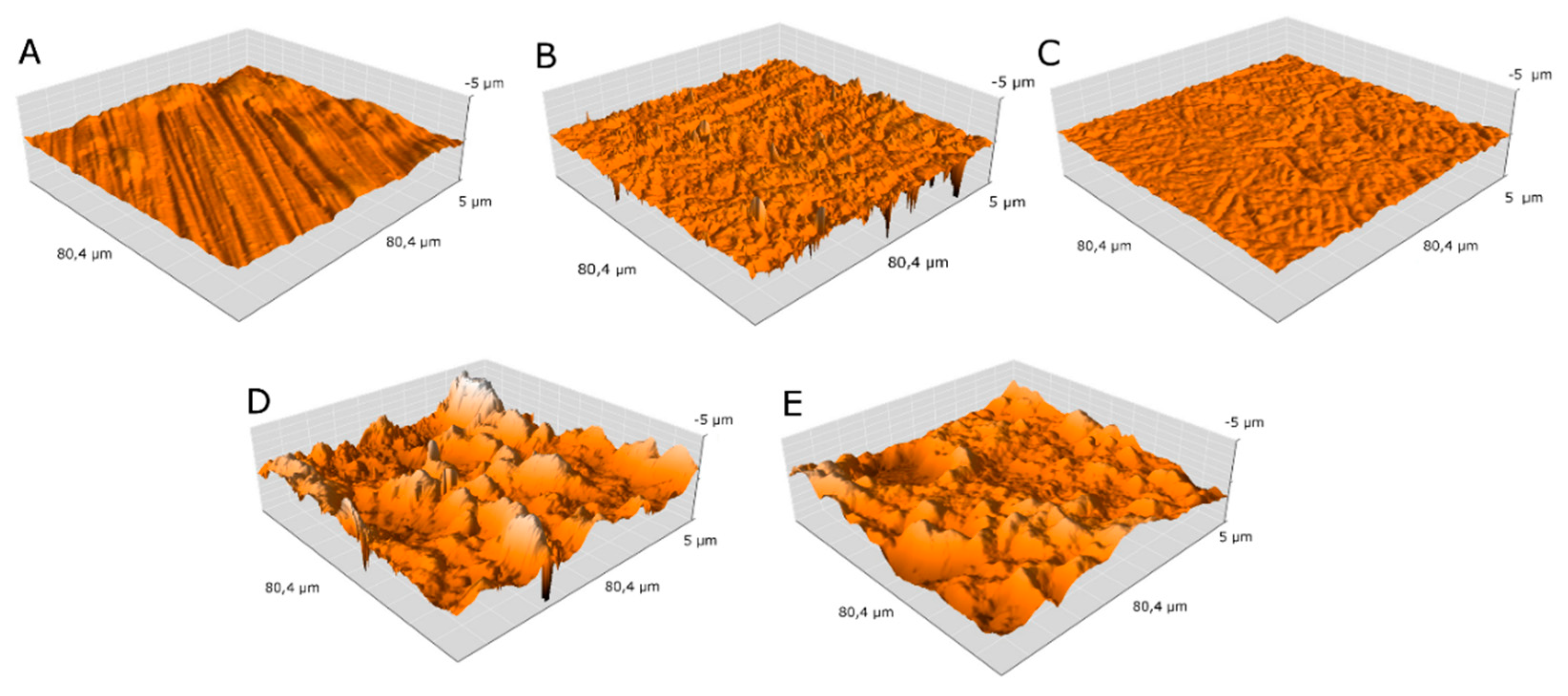
| Sample | Roughness Sa [μm] |
|---|---|
| MR | 0.203 |
| MRe | 0.256 |
| 0.27CNT | 0.098 |
| H0.27CNT | 0.980 |
| M0.4CNT | 0.618 |
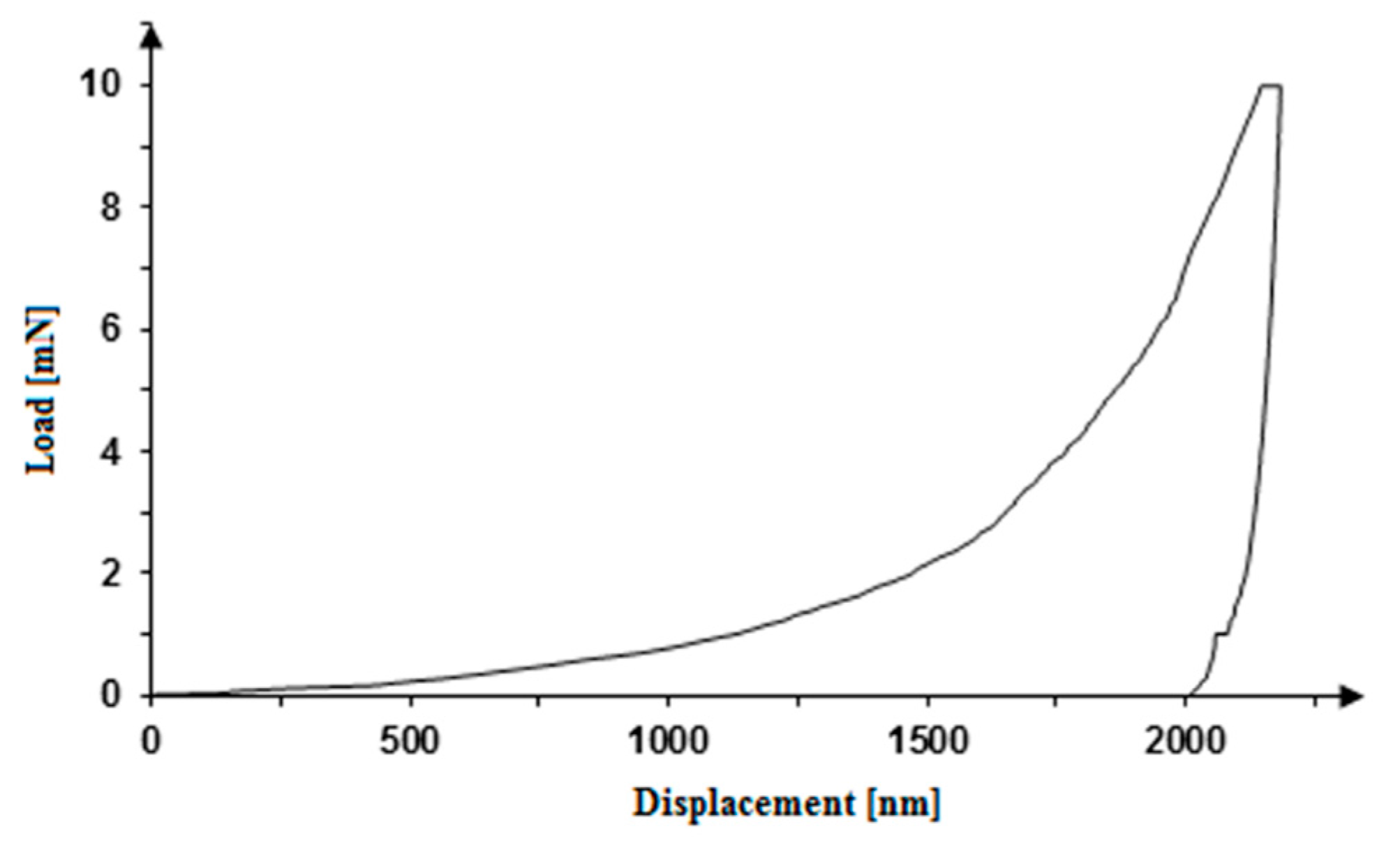
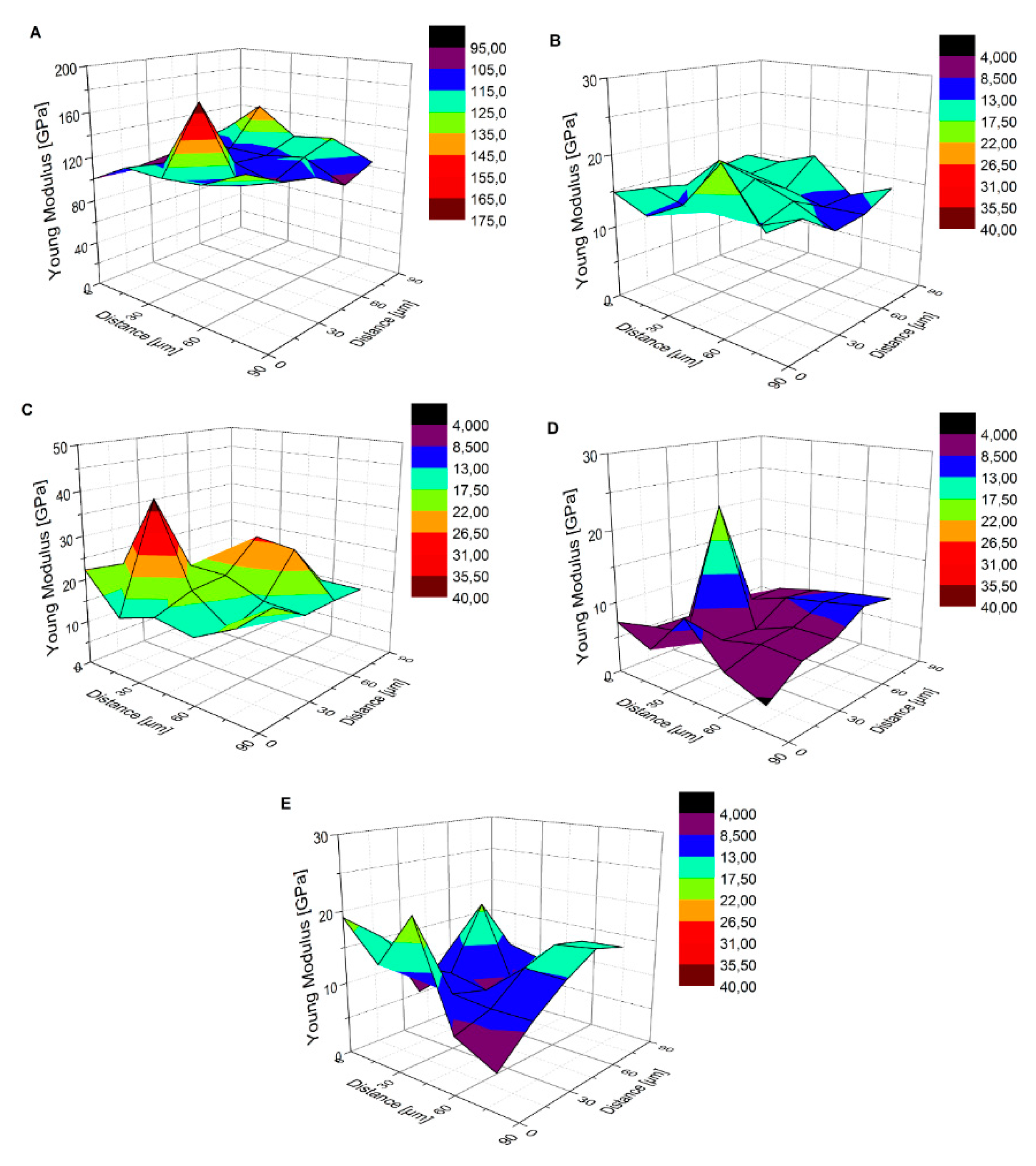
| Sample | Nanohardness [GPa] | Reduced Young’s Modulus [GPa] | Young’s Modulus [GPa] | Maximum Indent Depth [nm] |
|---|---|---|---|---|
| I | 3.758 ± 1.045 | 116.91 ± 16.32 | 83.32 ± 11.63 | 330.92 ± 36.61 |
| MRe | 0.071 ± 0.010 | 14.57 ± 2.21 | 9.44 ± 1.43 | 2358.11 ± 170.08 |
| 0.27CNT | 0.101 ± 0.049 | 18.59 ± 5.66 | 14.17 ± 4.32 | 2069.67 ± 352.57 |
| H0.27CNT | 0.022 ± 0.015 | 7.46 ± 3.65 | 5.63 ± 2.76 | 4264.18 ± 1150.11 |
| m0.4CNT | 0.035 ± 0.019 | 11.72 ± 4.31 | 8.88 ± 3.26 | 3210.02 ± 817.53 |
| Sample | Critical Friction (Fc) [mN] | Critical Load (Lc) [mN] |
|---|---|---|
| 0.27CNT | 89.42 ± 36.19 | 116.50 ± 32.07 |
| H0.27CNT | 39.96 ± 18.07 | 92.06 ± 34.3 |
| m0.4CNT | 29.56 ± 6.92 | 60.38 ± 10.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majkowska-Marzec, B.; Rogala-Wielgus, D.; Bartmański, M.; Bartosewicz, B.; Zieliński, A. Comparison of Properties of the Hybrid and Bilayer MWCNTs—Hydroxyapatite Coatings on Ti Alloy. Coatings 2019, 9, 643. https://doi.org/10.3390/coatings9100643
Majkowska-Marzec B, Rogala-Wielgus D, Bartmański M, Bartosewicz B, Zieliński A. Comparison of Properties of the Hybrid and Bilayer MWCNTs—Hydroxyapatite Coatings on Ti Alloy. Coatings. 2019; 9(10):643. https://doi.org/10.3390/coatings9100643
Chicago/Turabian StyleMajkowska-Marzec, Beata, Dorota Rogala-Wielgus, Michał Bartmański, Bartosz Bartosewicz, and Andrzej Zieliński. 2019. "Comparison of Properties of the Hybrid and Bilayer MWCNTs—Hydroxyapatite Coatings on Ti Alloy" Coatings 9, no. 10: 643. https://doi.org/10.3390/coatings9100643
APA StyleMajkowska-Marzec, B., Rogala-Wielgus, D., Bartmański, M., Bartosewicz, B., & Zieliński, A. (2019). Comparison of Properties of the Hybrid and Bilayer MWCNTs—Hydroxyapatite Coatings on Ti Alloy. Coatings, 9(10), 643. https://doi.org/10.3390/coatings9100643








