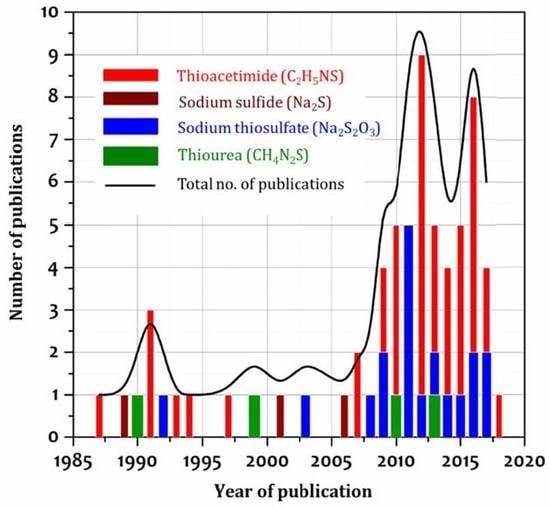
Effect of Thioacetamide Concentration on the Preparation of Single-Phase SnS and SnS2 Thin Films for Optoelectronic Applications
Abstract
1. Introduction
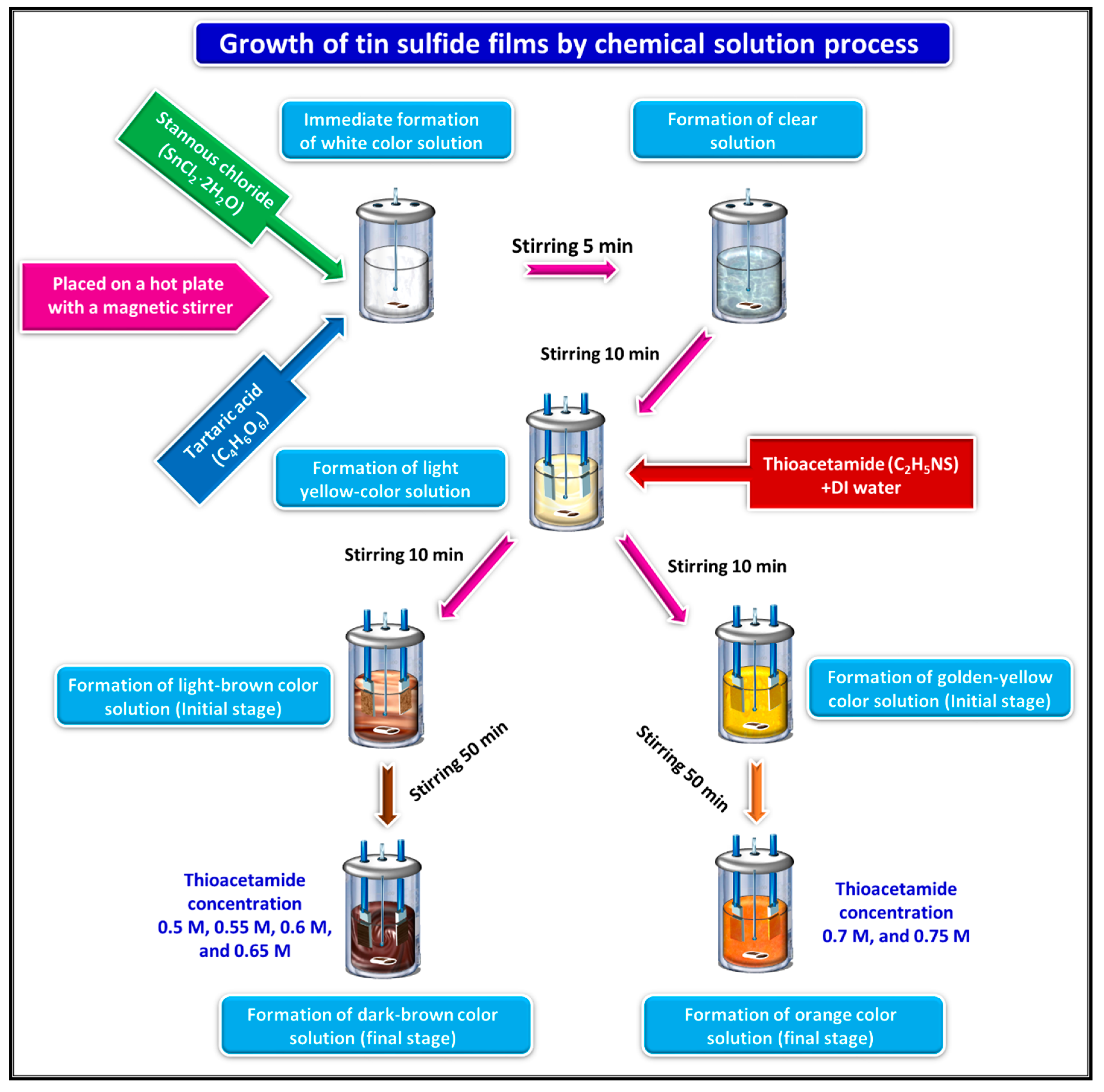
2. Experimental Section
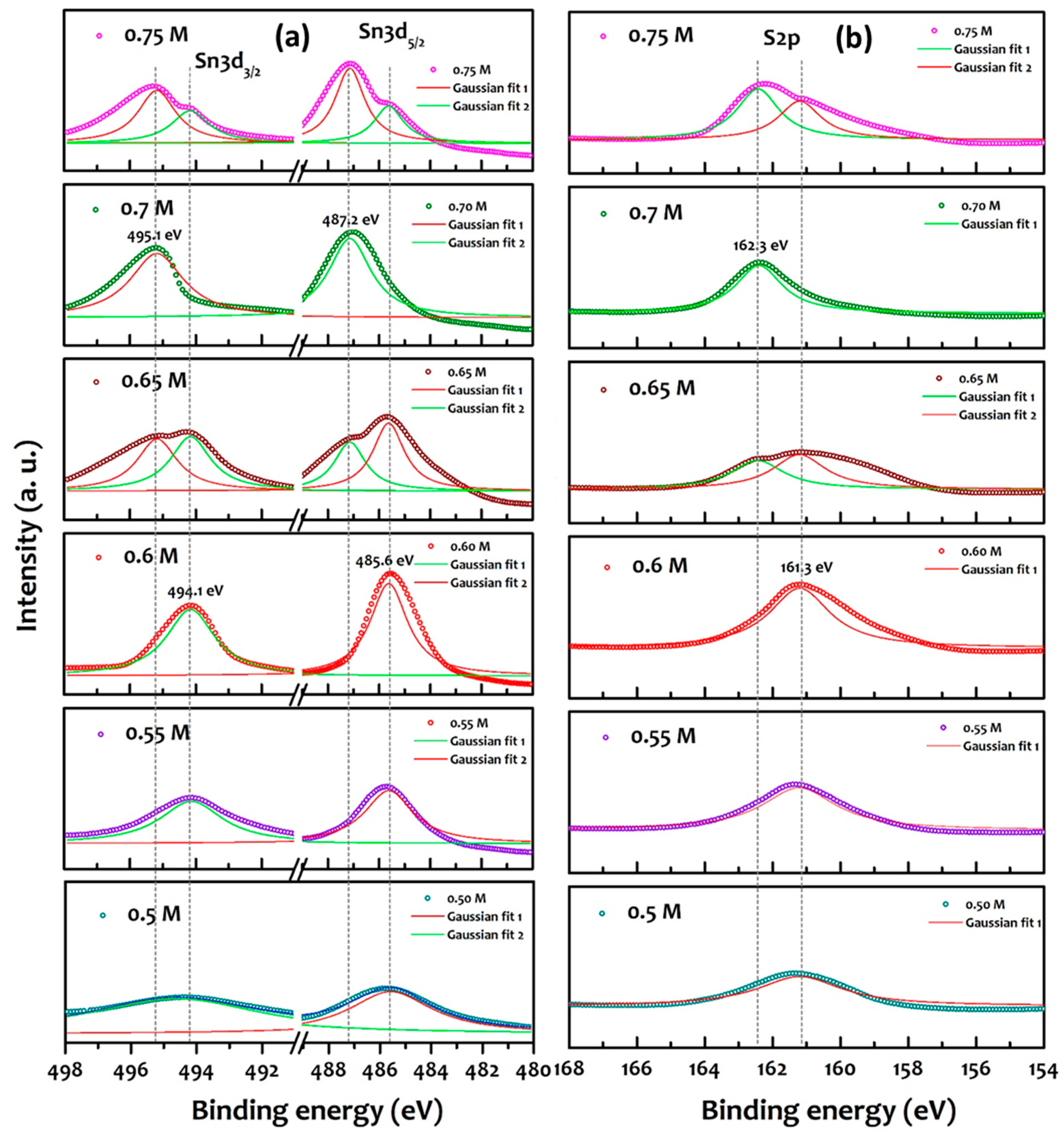
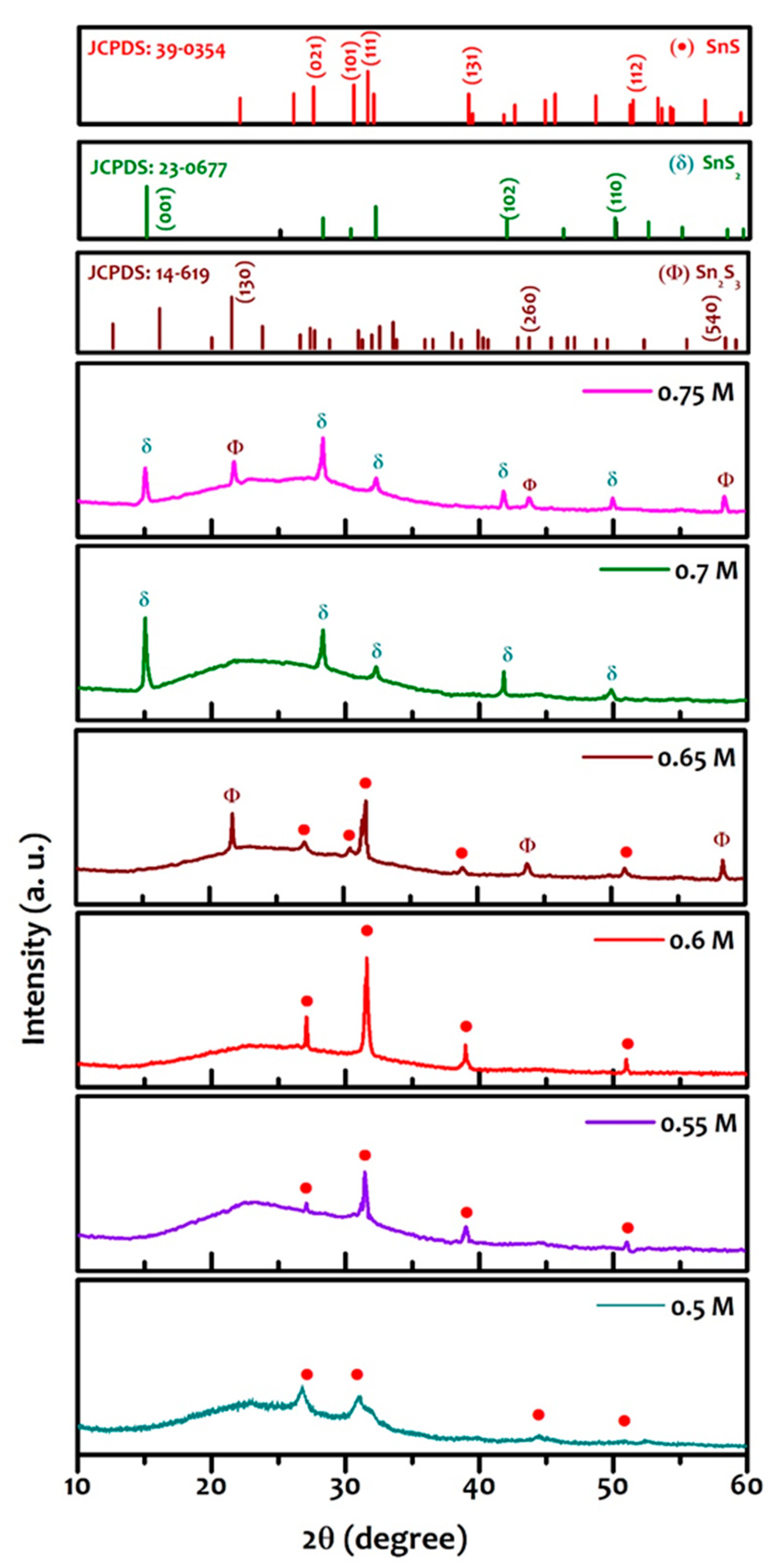
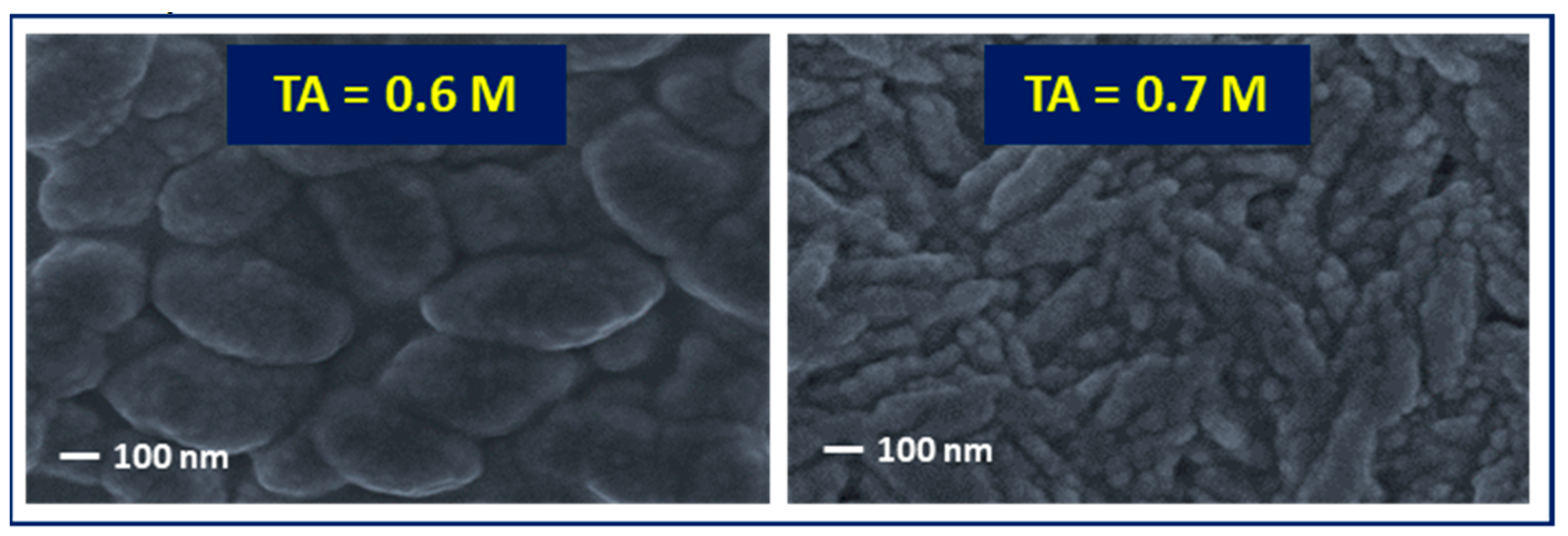
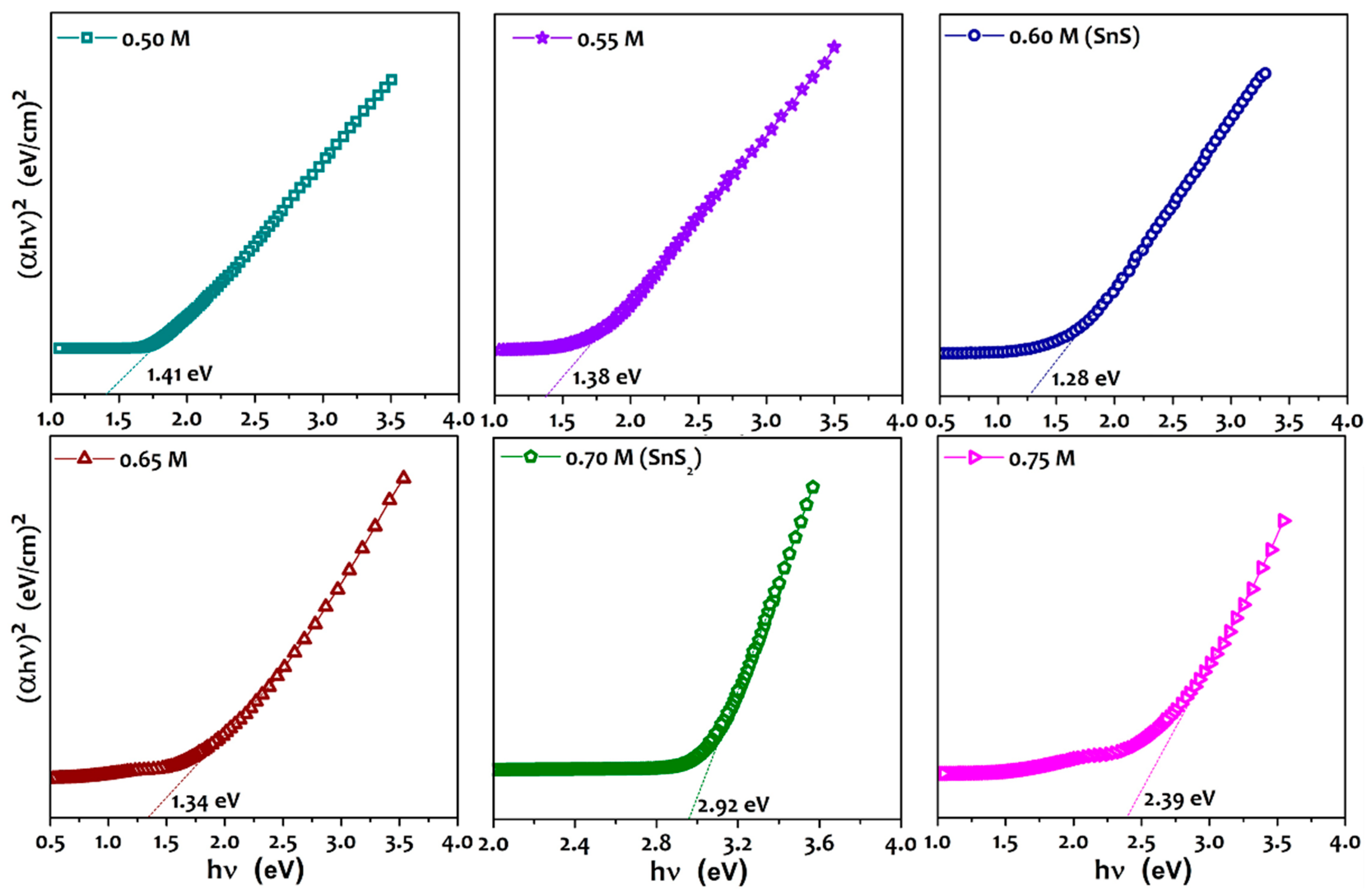
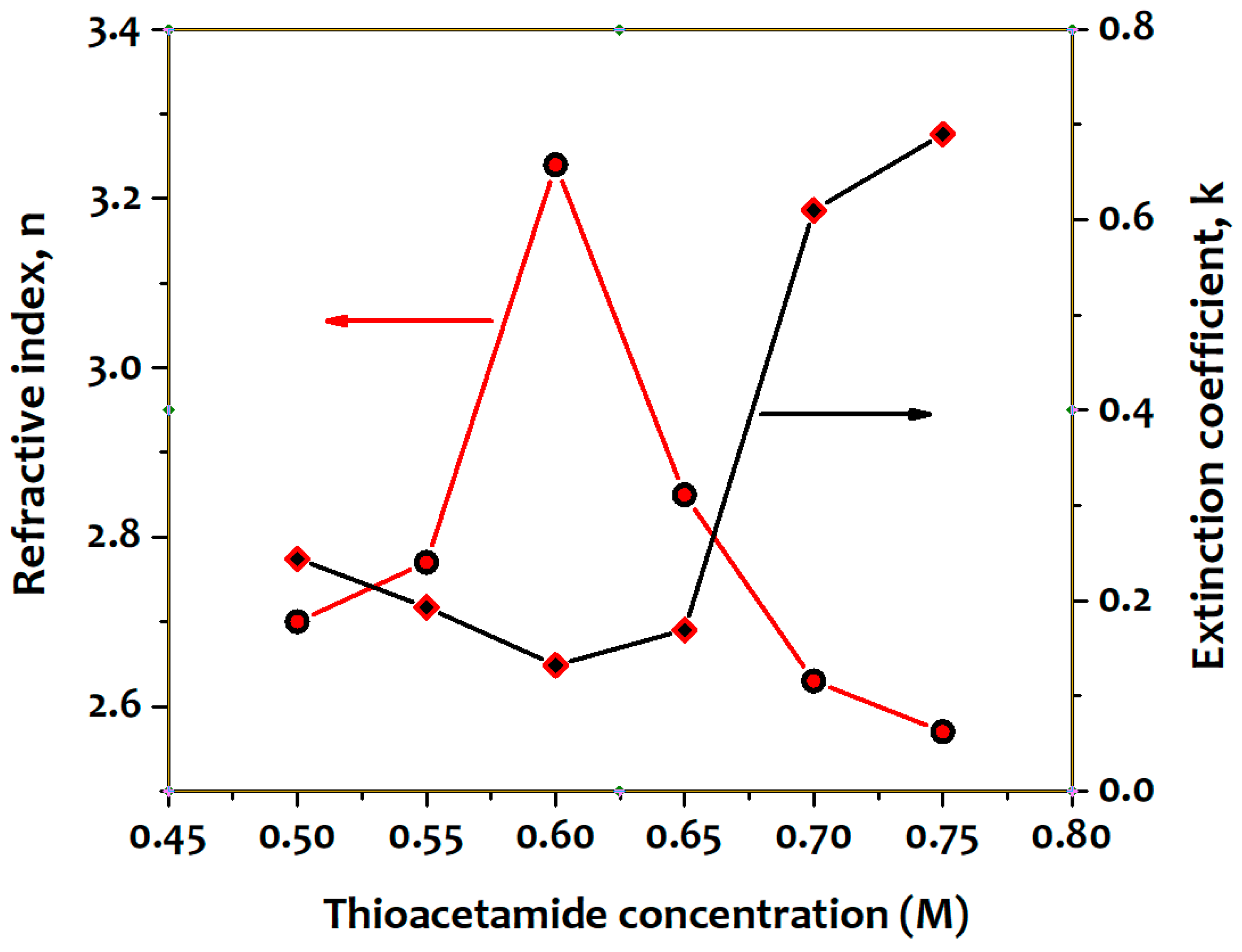
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Minnam Reddy, V.R.; Gedi, S.; Pejjai, B.; Kotte, T.R.; Guillaum, Z.; Park, C. Influence of different substrates on the properties of sulfurized sns films. Sci. Adv. Mater. 2016, 8, 247–251. [Google Scholar] [CrossRef]
- Gedi, S.; Minnam Reddy, V.R.; Pejjai, B.; Park, C.; Jeon, C.W.; Kotte, T.R. Studies on chemical bath deposited SnS 2 films for Cd-free thin film solar cells. Ceram. Int. 2017, 43, 3713–3719. [Google Scholar] [CrossRef]
- Jung, H.-R.; Kim, K.N.; Lee, W.-J. Heterostructured Co0.5Mn0.5Fe2O4-polyaniline nanofibers: highly efficient photocatalysis for photodegradation of methyl orange. Korean J. Chem. Eng. 2019, 36, 807–815. [Google Scholar] [CrossRef]
- Sehar, S.; Naz, I.; Perveen, I.; Ahmed, S. Superior dye degradation using SnO2-ZnO hybrid heterostructure catalysts. Korean J. Chem. Eng. 2019, 36, 56–62. [Google Scholar] [CrossRef]
- Trinh, T.K.; Truong, N.T.N.; Pham, V.T.H.; Kim, H.; Park, C. Effect of sulfur annealing on the morphological, structural, optical and electrical properties of iron pyrite thin films formed from FeS2 nano-powder. Korean J. Chem. Eng. 2018, 35, 1525–1531. [Google Scholar]
- Chen, X.; Hou, Y.; Zhang, B.; Yang, X.H.; Yang, H.G. Low-cost SnS(x) counter electrodes for dye-sensitized solar cells. Chem. Commun. (Camb). 2013, 49, 5793–5795. [Google Scholar] [CrossRef]
- Ettema, A.R.H.F.; de Groot, R.A.; Haas, C.; Turner, T.S. Electronic structure of SnS deduced from photoelectron spectra and band-structure calculations. Phys. Rev. B 1992, 46, 7363–7373. [Google Scholar] [CrossRef]
- Minnam Reddy, V.R.; Gedi, S.; Park, C.; Robert, W.M.; Kotte, T.R. Development of sulphurized SnS thin film solar cells. Curr. Appl. Phys. 2015, 15, 588–598. [Google Scholar] [CrossRef]
- Nguyen Tam Nguyen, T.; Hoang, H.H.T.; Trinh, T.K.; Pham, V.T.H.; Smith, R.P.; Chinho, P. Effect of post-synthesis annealing on properties of SnS nanospheres and its solar cell performance. Korean J. Chem. Eng. 2017, 34, 1208–1213. [Google Scholar]
- Caballero, R.; Conde, V.; Leon, M. SnS thin films grown by sulfurization of evaporated Sn layers: Effect of sulfurization temperature and pressure. Thin Solid Films 2016, 612, 202–207. [Google Scholar] [CrossRef]
- Lee, A.B.; Aron, W. Band alignment in SnS thin-film solar cells: Possible origin of the low conversion efficiency. Appl. Phys. Lett. 2013, 102, 132111. [Google Scholar]
- Umme, F.; Khan, M.A.; Chinho, P. Elucidation of morphological and optoelectronic properties of highly crystalline chalcopyrite (CuInSe2) nanoparticles synthesized via hot injection route. Korean J. Chem. Eng. 2012, 29, 1453–1458. [Google Scholar]
- Lee, H.; Jeong, D.S.; Mun, T.; Pejjai, B.; Minnam Reddy, V.R.; Anderson, T.J.; Park, C. Formation and characterization of CuInSe2 thin films from binary CuSe and In2Se3 nanocrystal-ink spray. Korean J. Chem. Eng. 2016, 33, 2486–2491. [Google Scholar] [CrossRef]
- Lee, D.; Yong, K. Non-vacuum deposition of CIGS absorber films for low-cost thin film solar cells. Korean J. Chem. Eng. 2013, 30, 1347–1358. [Google Scholar] [CrossRef]
- Lee, J.; Chen, H.; Koh, K.; Chang, C.L.; Kim, C.M.; Kim, S.H. Nanoassembly of CdTe nanowires and Au nanoparticles: pH dependence and reversibility of photoluminescence. Korean J. Chem. Eng. 2009, 26, 417–421. [Google Scholar] [CrossRef]
- Greta, L.; Shun Li, S.; Neal R, K.; Tim, A.; Zi Kui, L. Thermodynamics of the S-Sn system: Implication for synthesis of earth abundant photovoltaic absorber materials. Sol. Energy 2016, 125, 314–323. [Google Scholar]
- Ristov, M.; Sinadinovski, G.; Grozdanov, I.; Mitreski, M. Chemical deposition of Tin(II) sulphide thin films. Thin Solid Films 1989, 173, 53–58. [Google Scholar] [CrossRef]
- Solar Frontier Achieves World Record Thin-Film Solar Cell Efficiency of 23.35%. Available online: http://www.solar-frontier.com/eng/news/2019/0117_press.html (accessed on 17 January 2019).
- Elisa De, R. Cadmium telluride solar cells: Selenium diffusion unveiled. Nat. Energy 2016, 1, 16143–16146. [Google Scholar]
- Rui, K.; Takeshi, Y.; Shunsuke, A.; Atsushi, H.; Kong Fai, T.; Takuya, K.; Hiroki, S. New world record Cu(In,Ga)(Se,S)2 thin film solar cell efficiency beyond 22%. In Proceedings of the 2016 IEEE 43rd Photovoltaic Specialists Conference (PVSC), Portland, OR, USA, 5–10 June 2016; pp. 1287–1291. [Google Scholar]
- Mohamed, S.M.; Ibrahim, K.; Hmood, A.; Naser, M.A.; Falah, I.M.; Shrook, A.A. High performance near infrared photodetector based on cubic crystal structure SnS thin film on a glass substrate. Mater. Lett. 2017, 200, 10–13. [Google Scholar]
- Ran, F.Y.; Xiao, Z.; Hiramatsu, H.; Ide, K.; Hosono, H.; Kamiya, T. SnS thin films prepared by H 2 S-free process and its p -type thin film transistor. AIP Adv. 2016, 6, 015112. [Google Scholar] [CrossRef]
- Yao, K.; Li, J.; Shan, S.; Jia, Q. One-step synthesis of urchinlike SnS/SnS2heterostructures with superior visible-light photocatalytic performance. Catal. Commun. 2017, 101, 51–56. [Google Scholar] [CrossRef]
- Huang, P.C.; Shen, Y.M.; Brahma, S.; Shaikh, M.O.; Huang, J.L.; Wang, S.C. SnSx (x = 1, 2) nanocrystals as effective catalysts for photoelectrochemical water splitting. Catalysts 2017, 7, 252. [Google Scholar] [CrossRef]
- Afsar, M.F.; Rafiq, M.A.; Tok, A.I.Y. Two-dimensional SnS nanoflakes: synthesis and application to acetone and alcohol sensors. RSC Adv. 2017, 7, 21556–21566. [Google Scholar] [CrossRef]
- Ou, J.Z.; Ge, W.; Carey, B.; Daeneke, T.; Rotbart, A.; Shan, W.; Wang, Y.; Fu, Z.; Chrimes, A.F.; Wlodarski, W.; et al. Physisorption-based charge transfer in two-dimensional SnS2 for selective and reversible NO2 gas sensing. ACS Nano 2015, 9, 10313–10323. [Google Scholar] [CrossRef]
- Chung, R.J.; Wang, A.N.; Peng, S.Y. An enzymatic glucose sensor composed of carbon-coated nano tin sulfide. Nanomaterials 2017, 7, 39. [Google Scholar] [CrossRef]
- Mudusu, D.; Nandanapalli, K.R.; Dugasani, S.R.; Kang, J.W.; Park, S.H.; Tu, C.W. Growth of single-crystalline cubic structured tin(II) sulfide (SnS) nanowires by chemical vapor deposition. RSC Adv. 2017, 7, 41452–41459. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.; Wei, Z. Photoresponsive field-effect transistors based on multilayer SnS 2 nanosheets. J. Semicond. 2017, 38, 034001. [Google Scholar] [CrossRef]
- Manukumar, K.N.; Nagaraju, G.; Kishore, B.; Madhu, C.; Munichandraiah, N. Ionic liquid-assisted hydrothermal synthesis of SnS nanoparticles: Electrode materials for lithium batteries, photoluminescence and photocatalytic activities. J. Energy Chem. 2018, 27, 806–812. [Google Scholar] [CrossRef]
- Li, J.; Xue, C.; Wang, Y.; Jiang, G.; Liu, W.; Zhu, C. Cu2SnS3 solar cells fabricated by chemical bath deposition-annealing of SnS/Cu stacked layers. Sol. Energy Mater. Sol. Cells 2016, 144, 281–288. [Google Scholar] [CrossRef]
- Zhou, B.; Li, S.; Li, W.; Li, J.; Zhang, X.; Lin, S.; Chen, Z.; Pei, Y. Thermoelectric properties of SnS with na-doping. ACS Appl. Mater. Interfaces 2017, 9, 34033–34041. [Google Scholar] [CrossRef] [PubMed]
- Jayalakshmi, M.; Mohan Rao, M.; Choudary, B.M. Identifying nano SnS as a new electrode material for electrochemical capacitors in aqueous solutions. Electrochem. Commun. 2004, 6, 1119–1122. [Google Scholar] [CrossRef]
- Chauhan, H.; Singh, M.K.; Hashmi, S.A.; Deka, S. Synthesis of surfactant-free SnS nanorods by a solvothermal route with better electrochemical properties towards supercapacitor applications. RSC Adv. 2015, 5, 17228–17235. [Google Scholar] [CrossRef]
- Gedi, S.; Minnam Reddy, V.R.; Kotte, T.R.; Park, Y.; Kim, W.K. Effect of C4H6O6 concentration on the properties of SnS thin films for solar cell applications. Appl. Surf. Sci. 2019, 465, 802–815. [Google Scholar] [CrossRef]
- Gedi, S.; Minnam Reddy, V.R.; Alhammadi, S.; Guddeti, P.R.; Kotte, T.R.; Park, C.; Kim, W.K. Influence of deposition temperature on the efficiency of SnS solar cells. Sol. Energy 2019, 184, 305–314. [Google Scholar] [CrossRef]
- Huang, C.C.; Lin, Y.J.; Chuang, C.Y.; Liu, C.J.; Yang, Y.W. Conduction-type control of SnSx films prepared by the sol-gel method for different sulfur contents. J. Alloys Compd. 2013, 553, 208–211. [Google Scholar] [CrossRef]
- Minnam Reddy, V.R.; Pejjai, B.; Kotte, T.R.; Robert, W.M. X-ray photoelectron spectroscopy and X-ray diffraction studies on tin sulfide films grown by sulfurization process. J. Renew. Sustain. Energy 2013, 5, 031613. [Google Scholar] [CrossRef]
- Sugaki, A.; Kitakaze, A.; Kitazawa, H. Synthesized tin and tin-silver sulfide minerals: Synthetic sulfide minerals (XIII). Sci. Reports 1985, 3, 199–211. [Google Scholar]
- Mosburg, S.; Ross, D.R.; Bethke, P.M.; Toul-min, P. X-ray powder data for herzenbergite, teallite and tin trisulfide. US Geol. Surv. Prof. Pap. C 1961, 424, 347. [Google Scholar]
- Guenter, J.R.; Oswald, H. New polytype form of tin (IV) sulfide. Nat. Sci. 1968, 55, 171–177. [Google Scholar]
- Warren, B.E. X-ray Diffraction; Courier Corporation: Chelmsford, MA, USA, 1990; ISBN 0486663175. [Google Scholar]
- Tallapally, V.; Nakagawara, T.A.; Demchenko, D.O.; Özgür, Ü.; Arachchige, I.U. Ge1−x Snx alloy quantum dots with composition-tunable energy gaps and near-infrared photoluminescence. Nanoscale 2018, 10, 20296–20305. [Google Scholar] [CrossRef]
- Gedi, S.; Minnam Reddy, V.R.; Park, C.; Jeon, C.W.; Kotte, T.R. Comprehensive optical studies on SnS layers synthesized by chemical bath deposition. Opt. Mater. (Amst). 2015, 42, 468–475. [Google Scholar] [CrossRef]
- Biswajit, G.; Rupanjali, B.; Pushan, B.; Subrata, D. Structural and optoelectronic properties of vacuum evaporated SnS thin films annealed in argon ambient. Appl. Surf. Sci. 2011, 257, 3670–3676. [Google Scholar]
- Nandanapalli, K.R.; Kotte, T.R. Preparation and characterisation of sprayed tin sulphide films grown at different precursor concentrations. Mater. Chem. Phys. 2007, 102, 13–18. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gedi, S.; Minnam Reddy, V.R.; Alhammadi, S.; Moon, D.; Seo, Y.; Kotte, T.R.R.; Park, C.; Kim, W.K. Effect of Thioacetamide Concentration on the Preparation of Single-Phase SnS and SnS2 Thin Films for Optoelectronic Applications. Coatings 2019, 9, 632. https://doi.org/10.3390/coatings9100632
Gedi S, Minnam Reddy VR, Alhammadi S, Moon D, Seo Y, Kotte TRR, Park C, Kim WK. Effect of Thioacetamide Concentration on the Preparation of Single-Phase SnS and SnS2 Thin Films for Optoelectronic Applications. Coatings. 2019; 9(10):632. https://doi.org/10.3390/coatings9100632
Chicago/Turabian StyleGedi, Sreedevi, Vasudeva Reddy Minnam Reddy, Salh Alhammadi, Doohyung Moon, Yeongju Seo, Tulasi Ramakrishna Reddy Kotte, Chinho Park, and Woo Kyoung Kim. 2019. "Effect of Thioacetamide Concentration on the Preparation of Single-Phase SnS and SnS2 Thin Films for Optoelectronic Applications" Coatings 9, no. 10: 632. https://doi.org/10.3390/coatings9100632
APA StyleGedi, S., Minnam Reddy, V. R., Alhammadi, S., Moon, D., Seo, Y., Kotte, T. R. R., Park, C., & Kim, W. K. (2019). Effect of Thioacetamide Concentration on the Preparation of Single-Phase SnS and SnS2 Thin Films for Optoelectronic Applications. Coatings, 9(10), 632. https://doi.org/10.3390/coatings9100632