Application of Confocal Raman Microscopy for the Analysis of the Distribution of Wood Preservative Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Confocal Raman Microscopy Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, R.S. Weathering of wood. In Handbook of Wood Chemistry and Wood Composites; Rowell, R.M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 139–185. [Google Scholar]
- Evans, P.; Chowdhury, M.J.; Mathews, B.; Schmalzl, K.; Ayer, S.; Kiguchi, M.; Kataoka, Y. Weathering and surface protection of wood. In Handbook of Environmental Degradation of Materials; Kutz, M., Ed.; William Andrew Publishing: Norwich, UK, 2005; pp. 277–297. [Google Scholar]
- Blanchard, V.; Blanchet, P. Color stability for wood products during use: Effects of inorganic nanoparticles. BioResources 2011, 6, 1219–1229. [Google Scholar]
- Isaji, S.; Kojima, Y. Application of copper monoethanolamine solutions as primers for semitransparent exterior wood stains. Eur. J. Wood Prod 2017, 75, 305–314. [Google Scholar]
- Mishra, P.K.; Giagli, K.; Tsalagkas, D.; Mishra, H.; Talegaonkar, S.; Gryc, V.; Wimmer, R. Changing face of wood science in modern era: Contribution of nanotechnology. Recent Pat. Nanotechnol. 2018, 12, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Vardanyan, V.; Galstian, T.; Riedl, B. Effect of addition of cellulose nanocrystals to wood coatings on color changes and surface roughness due to accelerated weathering. J. Coat. Technol. Res. 2015, 12, 247–258. [Google Scholar] [CrossRef]
- Williams, R.S.; Jourdain, C.; Daisey, G.I.; Springate, R.W. Wood properties affecting finish service life. J. Coat. Technol. 2000, 72, 35–42. [Google Scholar] [CrossRef]
- Williams, R.S. Finishing of wood. In Wood Handbook: Wood as an Engineering Material; General Technical Report FPL-GTR-190; Ross, R.J., Ed.; USDA Forest Service: Madison, WI, USA, 2010; pp. 16.1–16.39. [Google Scholar]
- Feist, W.C. Role of pigment concentration in the weathering of semitransparent stains. Forest Prod. J. 1988, 38, 41–44. [Google Scholar]
- Richter, K.; Feist, W.C.; Knaebe, M.T. The effect of surface roughness on the performance of finishes. Part 1. Roughness characterization and stain performance. Forest Prod. J. 1994, 45, 91–97. [Google Scholar]
- Singh, A.P.; Park, B.D.; Nuryawan, A.; Kazayawoko, M. Advances in probing wood-coating interface by microscopy: A review. J. Surf. Eng. Mater. Adv. Technol. 2013, 3, 49–54. [Google Scholar] [CrossRef]
- Schneider, M.H.; Cote, W.A. Studies of wood and coating interactions using fluorescence microscopy and pyrolysis gas-liquid chromatography. J. Paint Technol. 1967, 39, 465–471. [Google Scholar]
- Cote, W.A.; Robison, R.G. A comparative study of wood-wood coating interaction using incident fluorescence and transmitted fluorescence microscopy. J. Paint Technol. 1968, 40, 427–432. [Google Scholar]
- De Meijer, M.; Thurich, K.; Militz, H. Comparative study on penetration characteristics of modern wood coatings. Wood Sci. Technol. 1998, 32, 347–365. [Google Scholar] [CrossRef]
- Rijckaert, V.; Stevens, M.; Van Acker, J.; de Meijer, M.; Militz, H. Quantitative assessment of the penetration of water-borne and solvent-borne wood coatings in Scots pine sapwood. Holz Roh Werkst. 2001, 59, 278–287. [Google Scholar] [CrossRef]
- Schneider, M.H. Coating penetration into wood substance studied with electron microscopy using replica techniques. J. Coat. Technol. 1970, 42, 457–460. [Google Scholar]
- Schneider, M.H. Microscopic distribution of linseed oil after application to wood surface. J. Coat. Technol. 1980, 52, 64–67. [Google Scholar]
- Nussbaum, R.M. Penetration of water-borne alkyd emulsion and solvent-borne alkyds into wood. Holz Roh Werkst. 1994, 52, 389–393. [Google Scholar] [CrossRef]
- Singh, A.P.; Ratz, A.; Dawson, B.S.W. A novel method for high-resolution imaging of coating distribution within a rough-textured plywood surface. J. Coat. Technol. Res. 2007, 4, 207–210. [Google Scholar] [CrossRef]
- Van den Bulcke, J.; Rijckaert, V.; Van Acker, J.; Stevens, M. Quantitative measurement of the penetration of water-borne coatings in wood with confocal lasermicroscopy and image analysis. Holz Roh Werkst. 2003, 61, 303–310. [Google Scholar] [CrossRef]
- Singh, A.P.; Dawson, B.S.W. Confocal microscope―A valuable tool for examining wood-coating interface. J. Coat. Technol. Res. 2004, 1, 235–237. [Google Scholar] [CrossRef]
- Van den Bulcke, J.; Boone, M.; Van Acker, J.; Van Hoorebeke, L. High-Resolution X-ray imaging and analysis of coatings on and in wood. J. Coat. Technol. Res. 2010, 7, 271–277. [Google Scholar] [CrossRef]
- Bessières, J.; Maurin, V.; George, B.; Molina, S.; Masson, E.; Merlin, A. Wood-coating layer studies by X-ray imaging. Wood Sci. Technol. 2013, 47, 853–867. [Google Scholar] [CrossRef]
- Smith, E.; Dent, G. Modern Raman Spectroscopy: A Practical Approach; John Wiley & Sons Ltd.: Chichester, UK, 2005. [Google Scholar]
- Agarwal, U.P. Raman imaging to investigate ultrastructure and composition of plant cell walls: Distribution of lignin and cellulose in black spruce wood (Picea mariana). Planta 2006, 224, 1141–1153. [Google Scholar] [CrossRef]
- Gierlinger, N.; Schwanninger, M. Chemical imaging of poplar wood cell walls by confocal Raman microscopy. Plant Physiol. 2006, 140, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Kanbayashi, T.; Kataoka, Y.; Ishikawa, A.; Matsunaga, M.; Kobayashi, M.; Kiguchi, M. Depth profiling of photodegraded wood surfaces by confocal Raman microscopy. J. Wood Sci. 2018, 64, 169–172. [Google Scholar] [CrossRef]
- Kanbayashi, T.; Kataoka, Y.; Ishikawa, A.; Matsunaga, M.; Kobayashi, M.; Kiguchi, M. Confocal Raman microscopy reveals changes in chemical composition of wood surfaces exposed to artificial weathering. J. Photochem. Photobiol. B Biol. 2018, 187, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Belt, T.; Altgen, M.; Mäkelä, M.; Hänninen, T.; Rautkari, L. Cellular level chemical changes in Scots pine heartwood during incipient brown rot decay. Sci. Rep. 2019, 9, 5188. [Google Scholar] [CrossRef] [PubMed]
- Keplinger, T.; Cabane, E.; Chanana, M.; Hass, P.; Merk, V.; Gierlinger, N.; Burgert, I. A versatile strategy for grafting polymers to wood cell walls. Acta Biomater. 2015, 11, 256–263. [Google Scholar] [CrossRef]
- Ermeydan, M.A. Modification of spruce wood by UV-crosslinked PEG hydrogels inside wood cell walls. React. Funct. Polym. 2018, 131, 100–106. [Google Scholar] [CrossRef]
- Legodi, M.A.; de Waal, D. The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dyes Pigment. 2007, 74, 161–168. [Google Scholar] [CrossRef]
- Zięba-Palus, J.; Michalska, A.; Wesełucha-Birczyńska, A. Characterisation of paint samples by infrared and Raman spectroscopy for criminalistics purposes. J. Mol. Struct. 2011, 993, 134–141. [Google Scholar] [CrossRef]
- Ellis, G.; Claybourn, M.; Richards, S.E. The application of Fourier transform Raman spectroscopy to the study of paint systems. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1990, 46, 227–241. [Google Scholar] [CrossRef]
- Agarwal, U.P. An overview of Raman spectroscopy as applied to lignocellulosic materials. In Advances in Lignocellulosics Characterization; Argyropoulos, D.S., Ed.; TAPPI Press: Atlanta, GA, USA, 1999; pp. 201–225. [Google Scholar]
- Himmelsbach, D.S.; Khahili, S.; Akin, D.E. Near-infrared–Fourier-transform–Raman microspectroscopic imaging of flax stems. Vib. Spectrosc. 1999, 19, 361–367. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Ralph, S.A. FT-Raman spectroscopy of wood: Identifying contributions of lignin and carbohydrate polymers in the spectrum of black spruce (Picea mariana). Appl. Spectrosc. 1997, 51, 1648–1655. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter & Co.: Berlin, Germany, 1989. [Google Scholar]
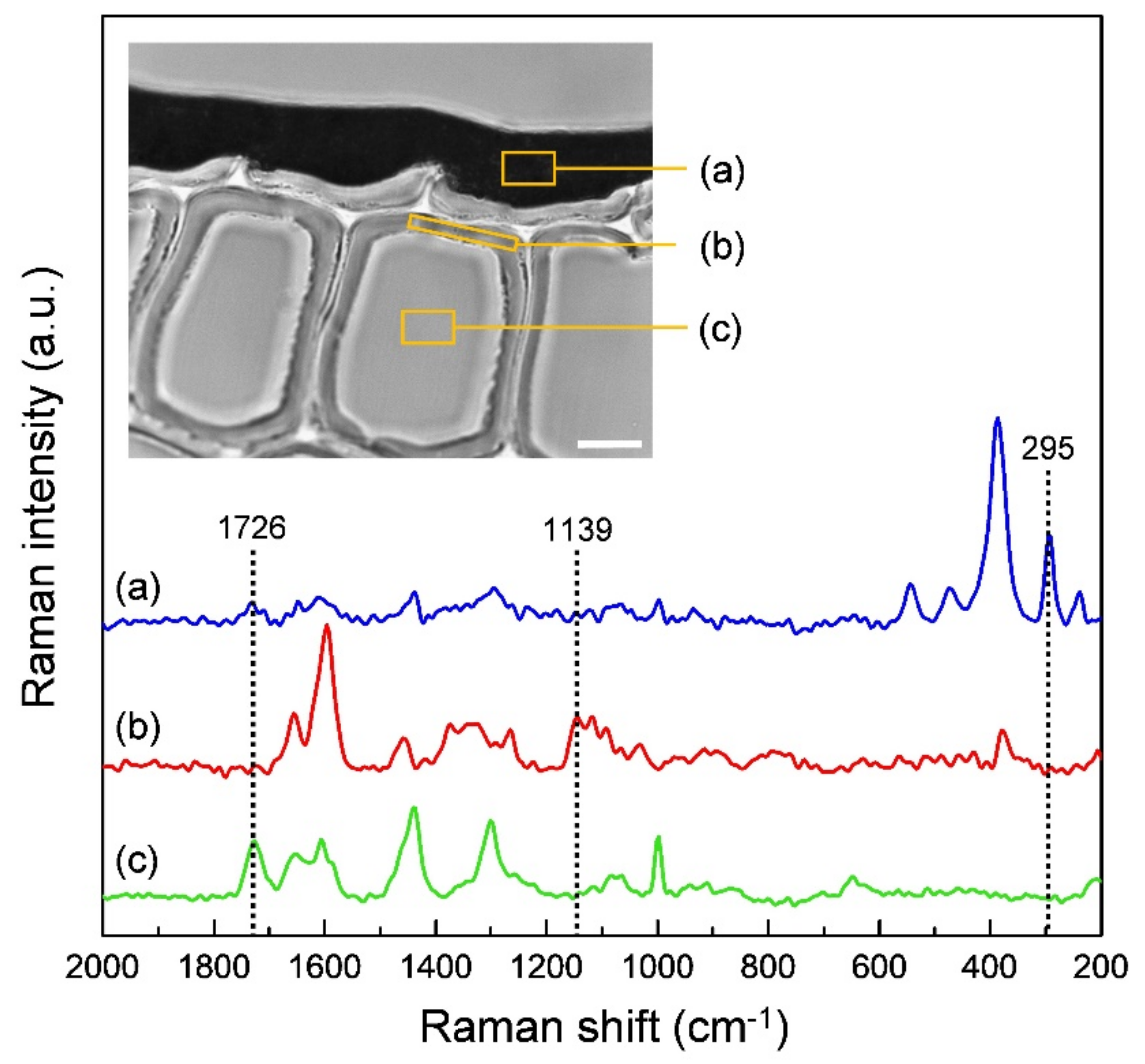
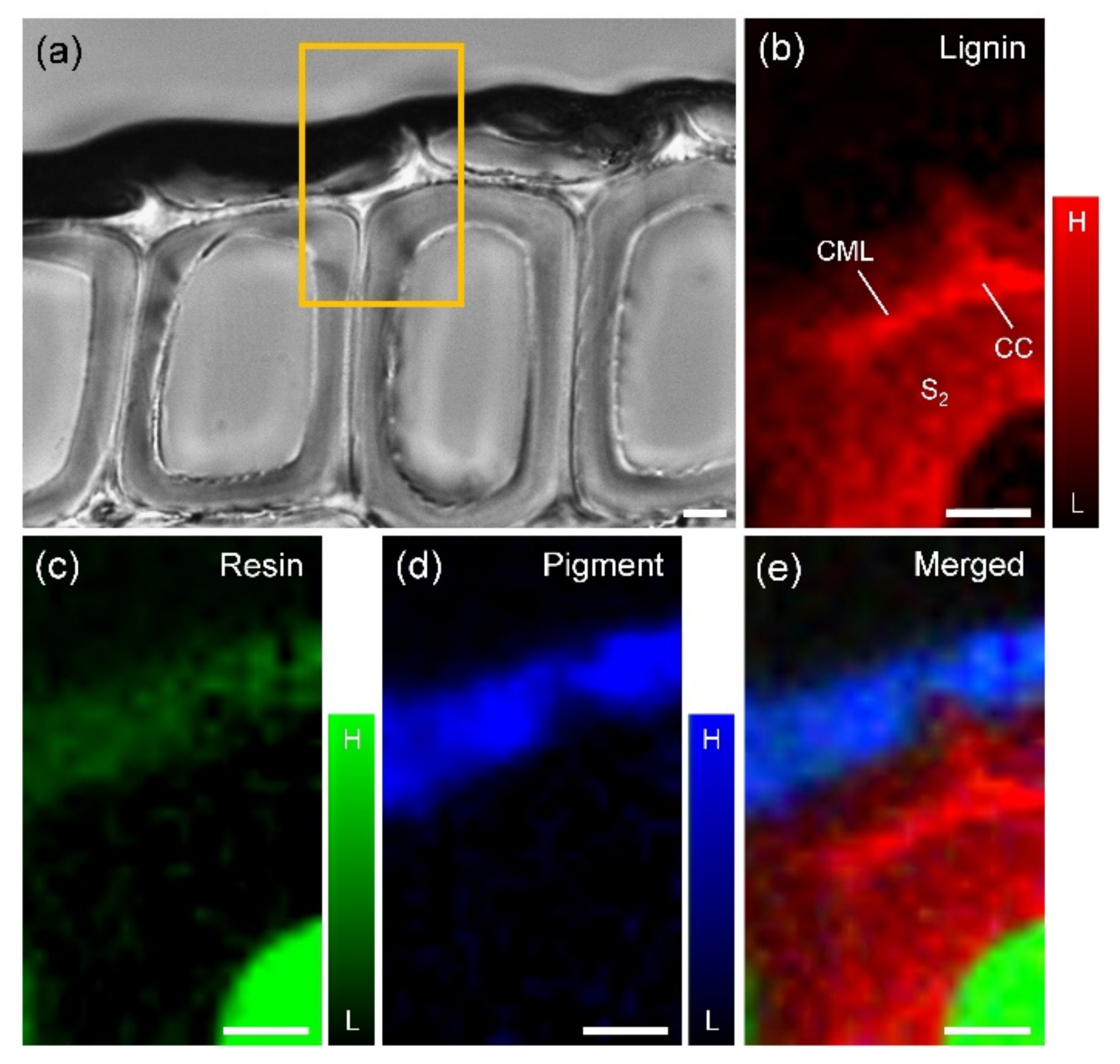
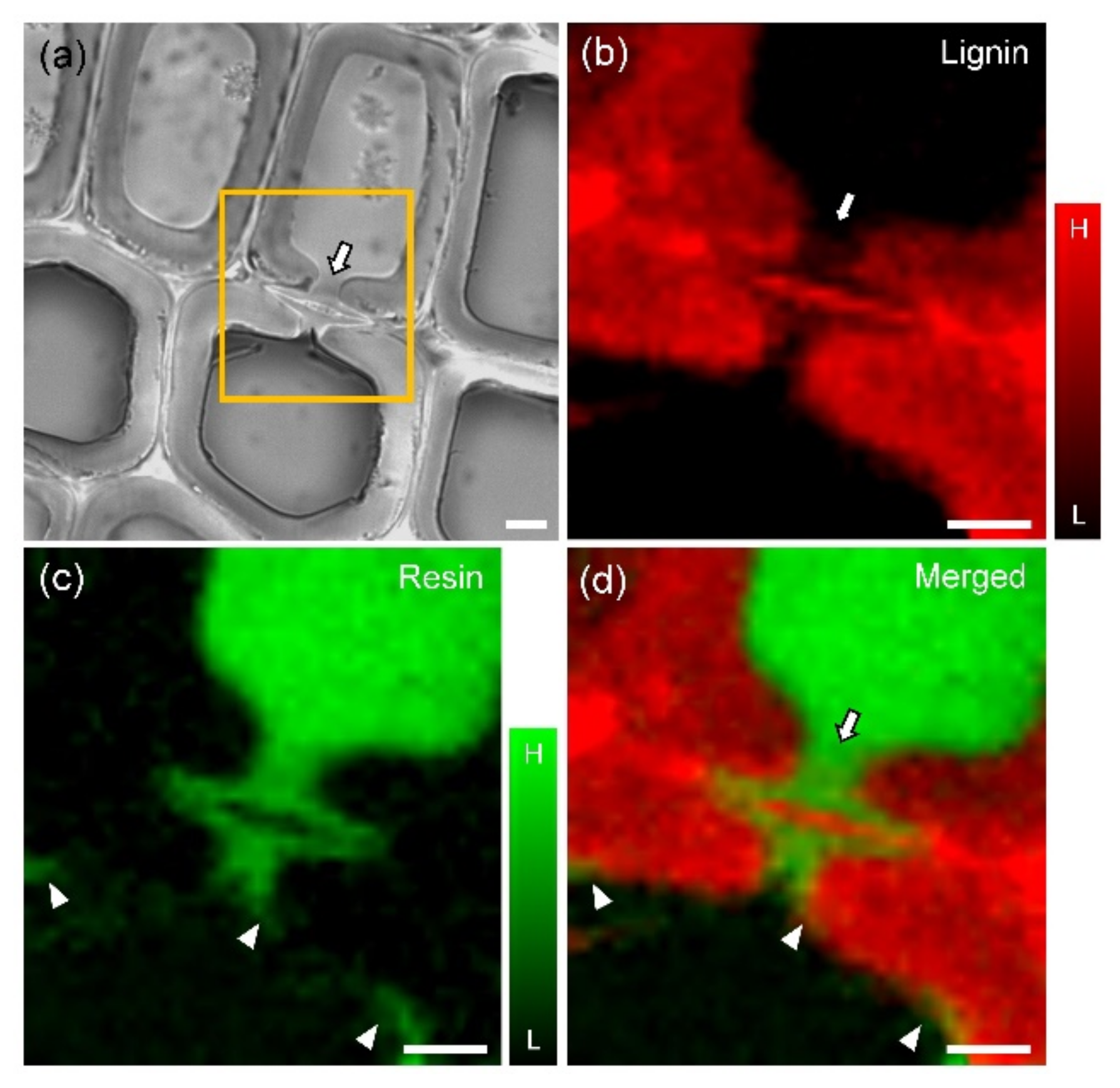
| Raman Band (cm−1) | Component | Band Assignment * |
|---|---|---|
| 1726 | Alkyd resin | C = O stretch |
| 1139 | Lignin | A mode of coniferyl aldehyde |
| 295 | Ferric hydroxide | Fe–OH symmetric bend |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanbayashi, T.; Ishikawa, A.; Matsunaga, M.; Kobayashi, M.; Kataoka, Y. Application of Confocal Raman Microscopy for the Analysis of the Distribution of Wood Preservative Coatings. Coatings 2019, 9, 621. https://doi.org/10.3390/coatings9100621
Kanbayashi T, Ishikawa A, Matsunaga M, Kobayashi M, Kataoka Y. Application of Confocal Raman Microscopy for the Analysis of the Distribution of Wood Preservative Coatings. Coatings. 2019; 9(10):621. https://doi.org/10.3390/coatings9100621
Chicago/Turabian StyleKanbayashi, Toru, Atsuko Ishikawa, Masahiro Matsunaga, Masahiko Kobayashi, and Yutaka Kataoka. 2019. "Application of Confocal Raman Microscopy for the Analysis of the Distribution of Wood Preservative Coatings" Coatings 9, no. 10: 621. https://doi.org/10.3390/coatings9100621
APA StyleKanbayashi, T., Ishikawa, A., Matsunaga, M., Kobayashi, M., & Kataoka, Y. (2019). Application of Confocal Raman Microscopy for the Analysis of the Distribution of Wood Preservative Coatings. Coatings, 9(10), 621. https://doi.org/10.3390/coatings9100621




