Study on a Novel Recyclable Anticorrosion Gel Coating Based on Ethyl Cellulose and Thermoplastic Polyurethane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gel and Coating
2.3. Material Characterization and Property Measurement
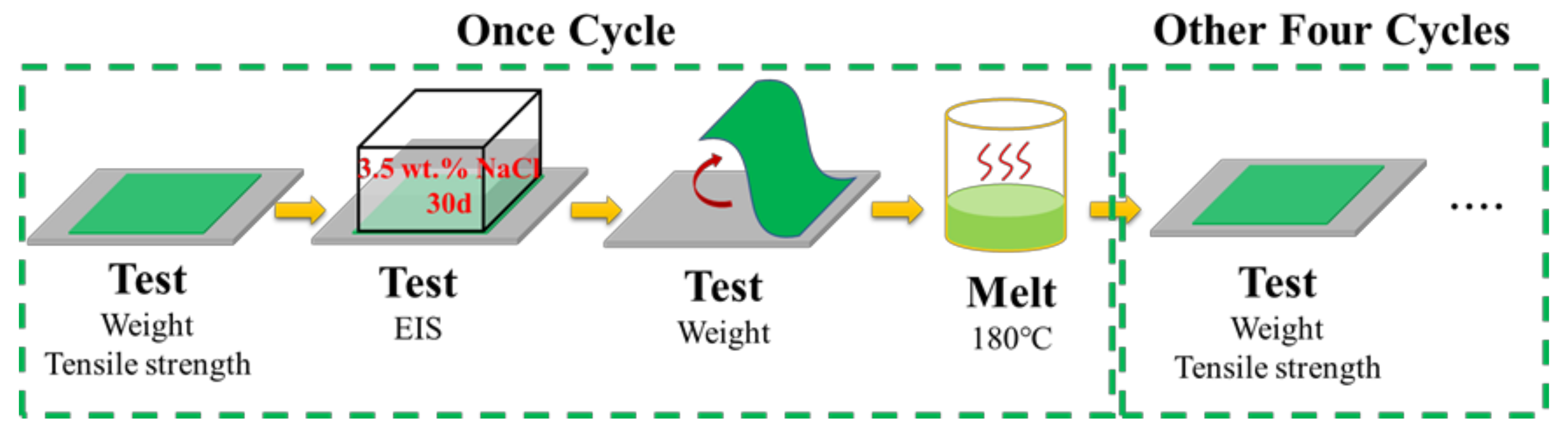
2.4. Cyclic Spraying and Performance Test of Coating
3. Results and Discussion
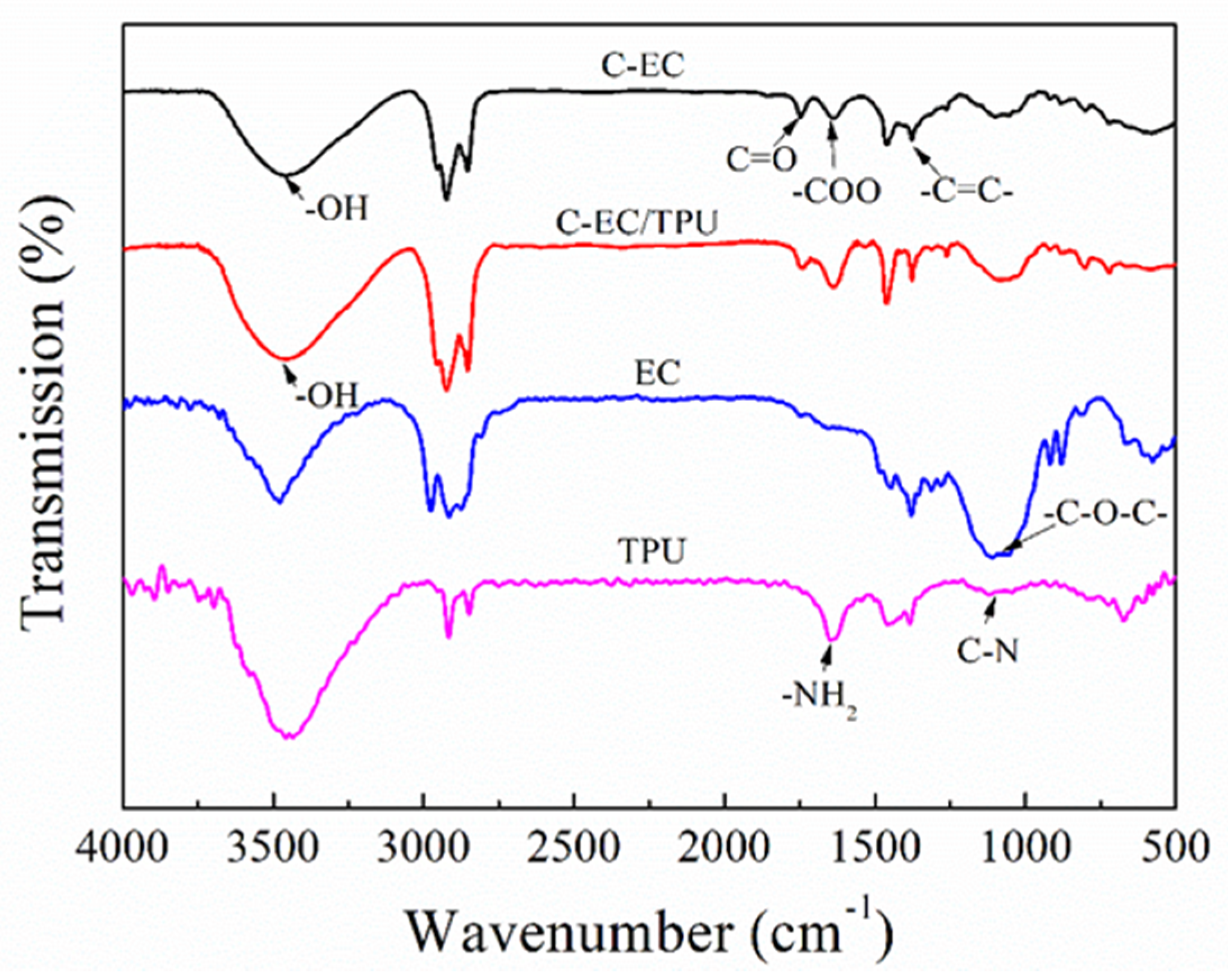
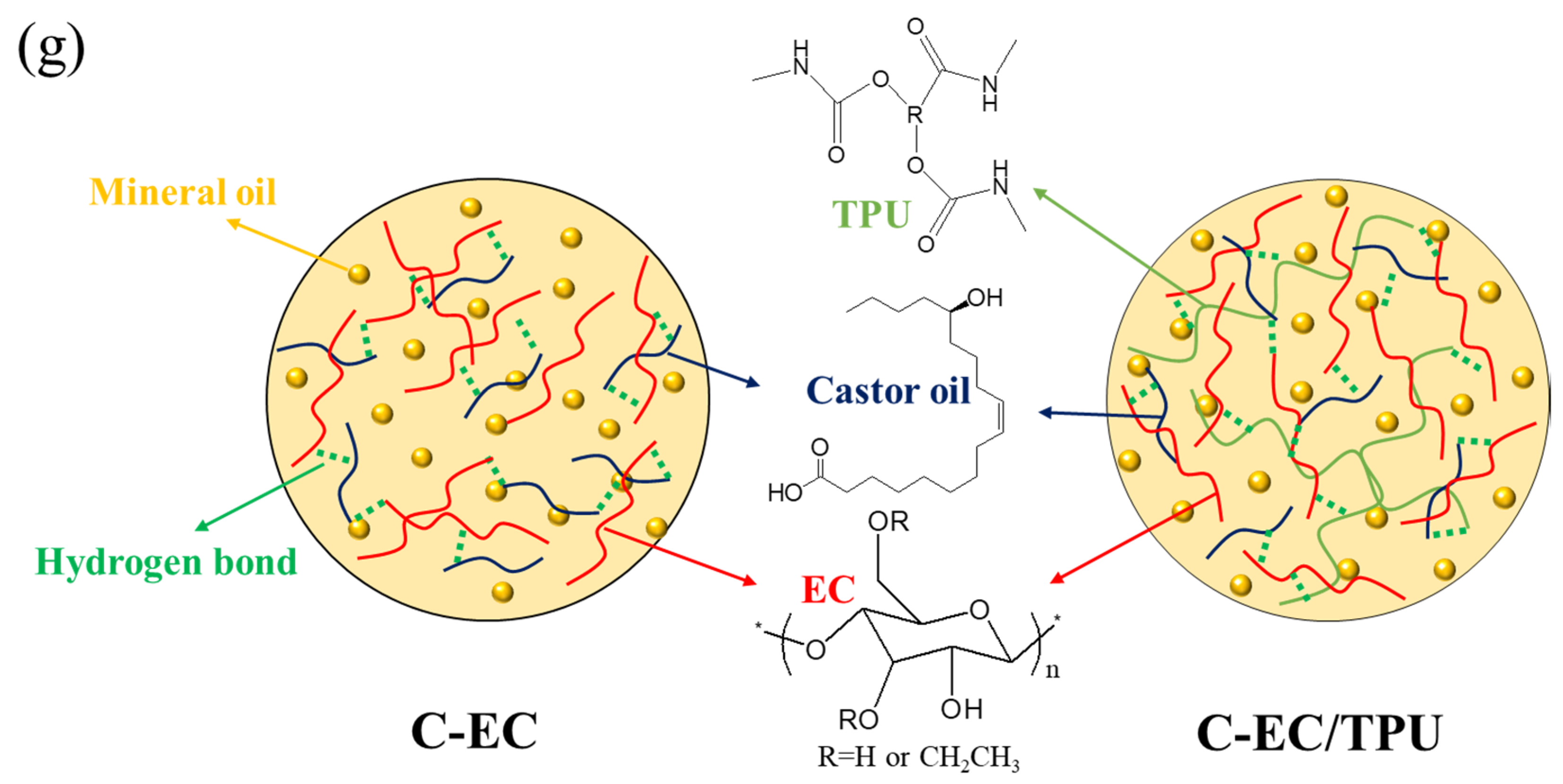
3.1. FT-IR Spectra
3.2. SEM Observation
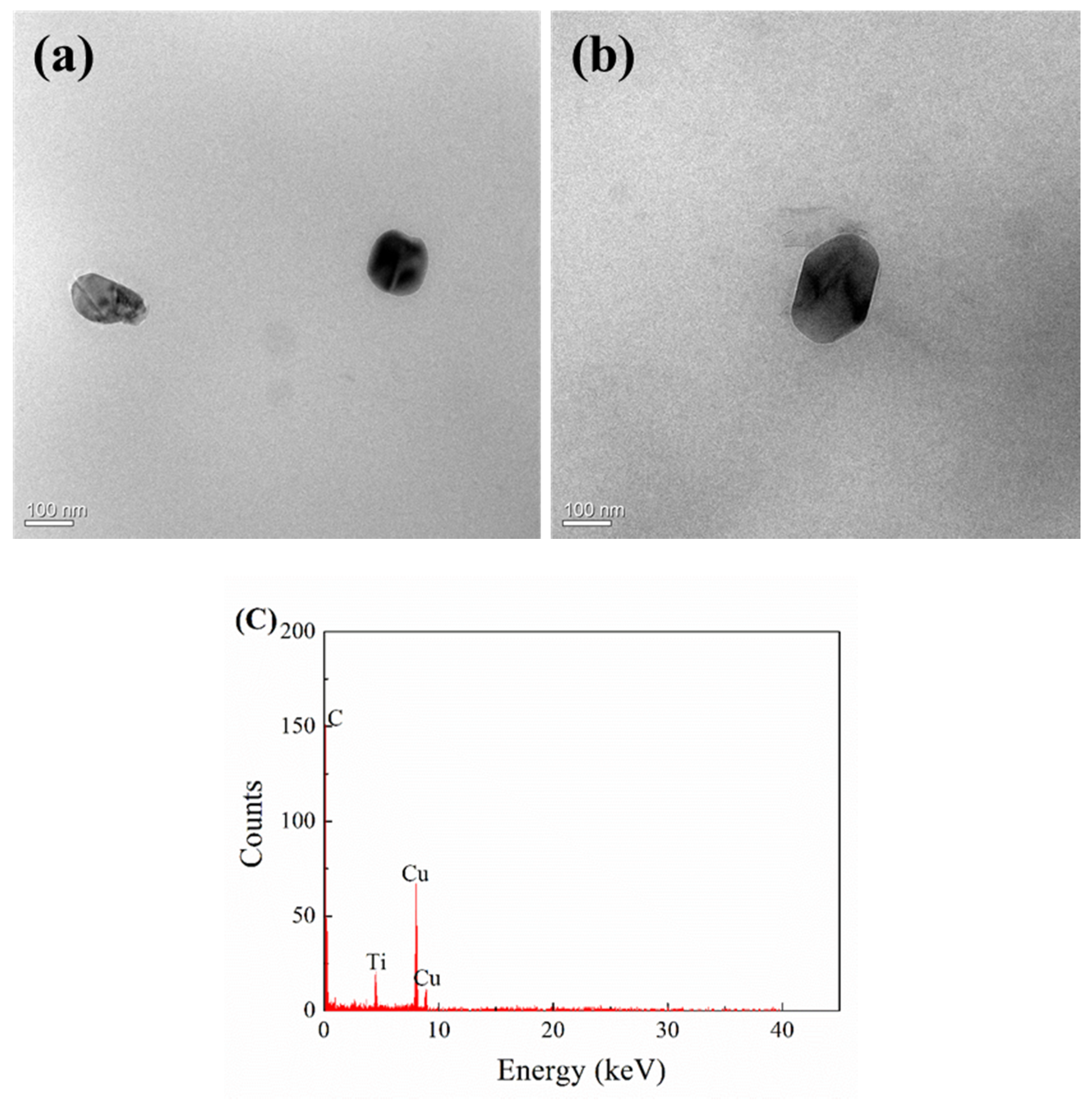
3.3. TEM Observation
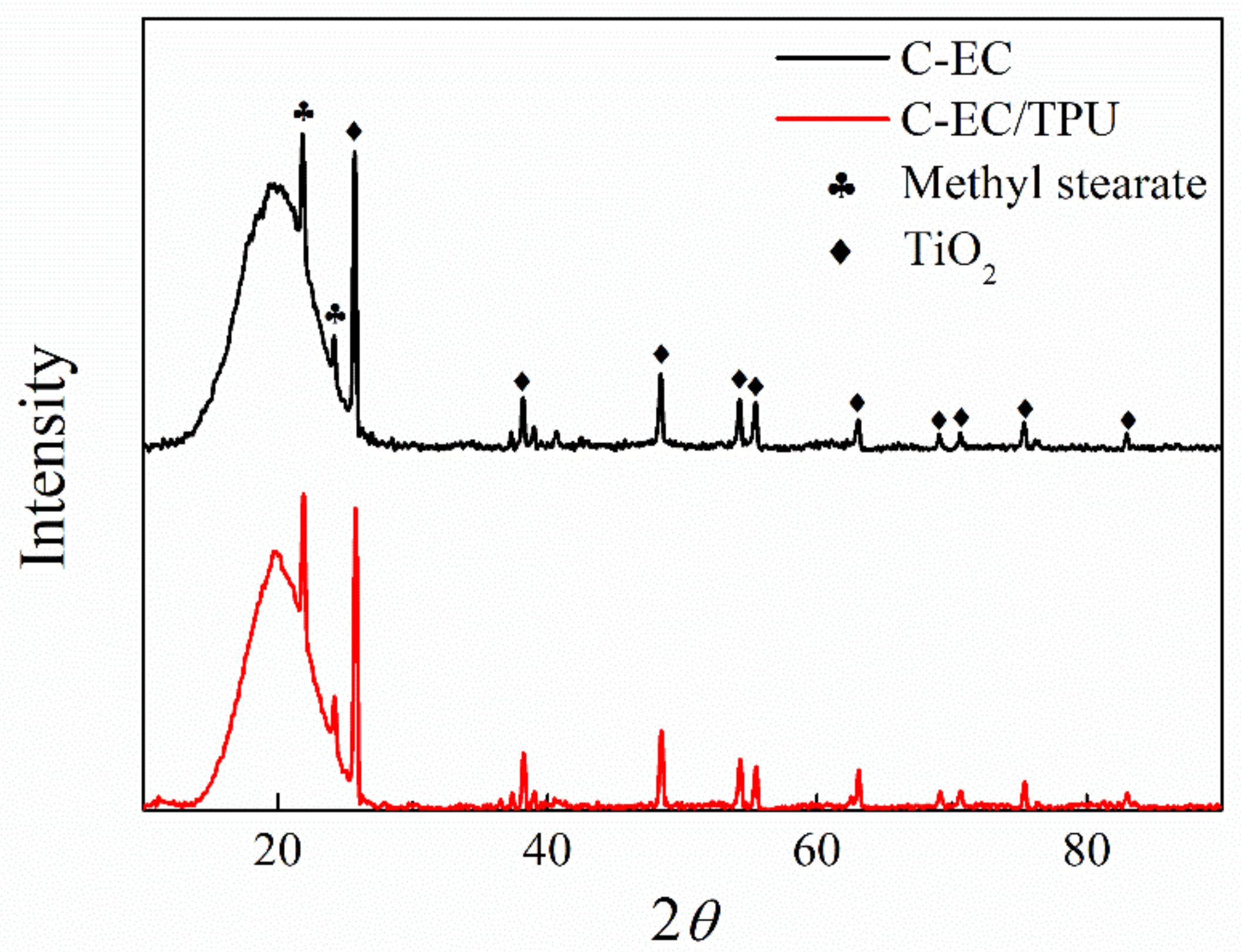
3.4. XRD Analysis
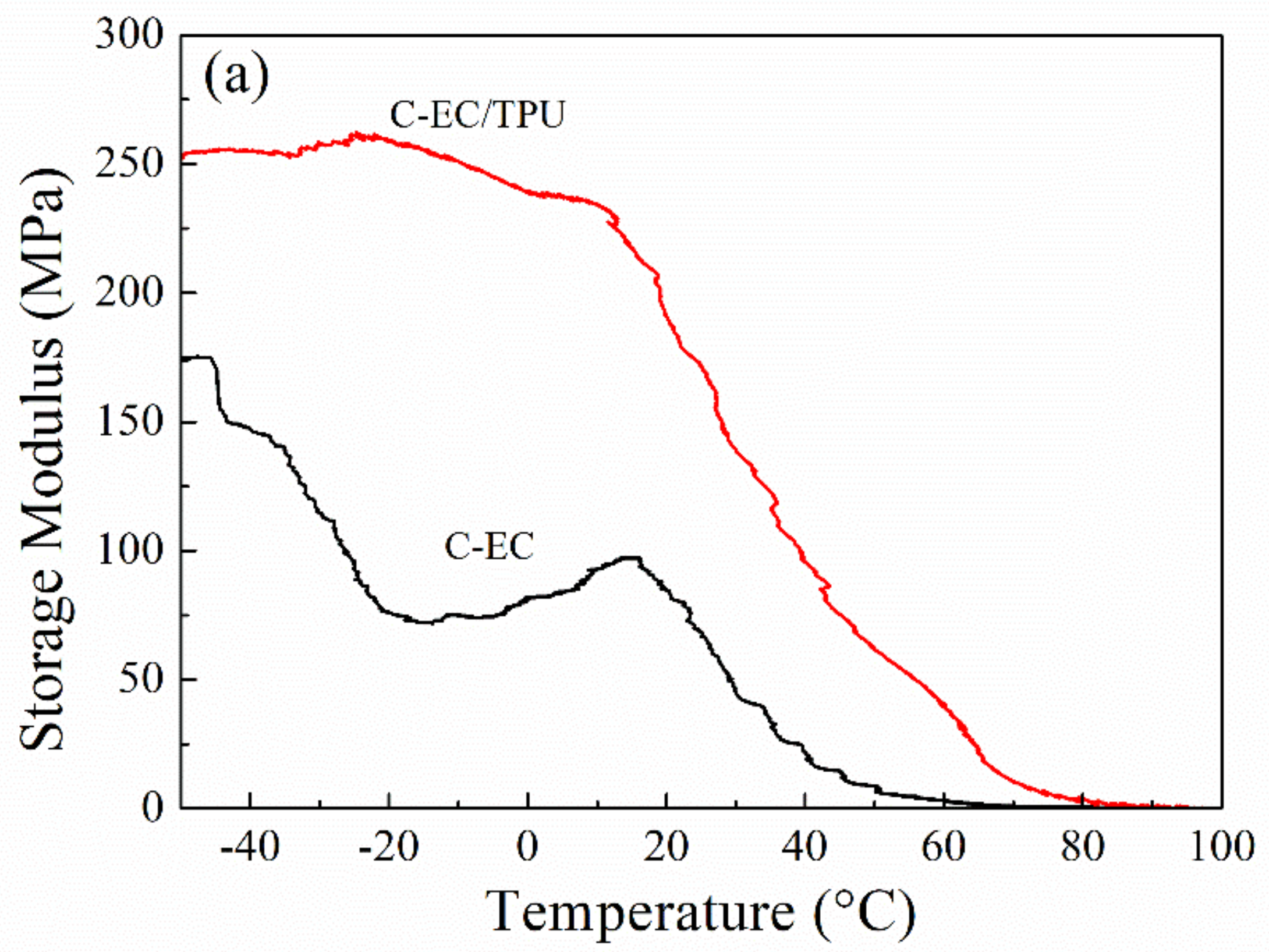
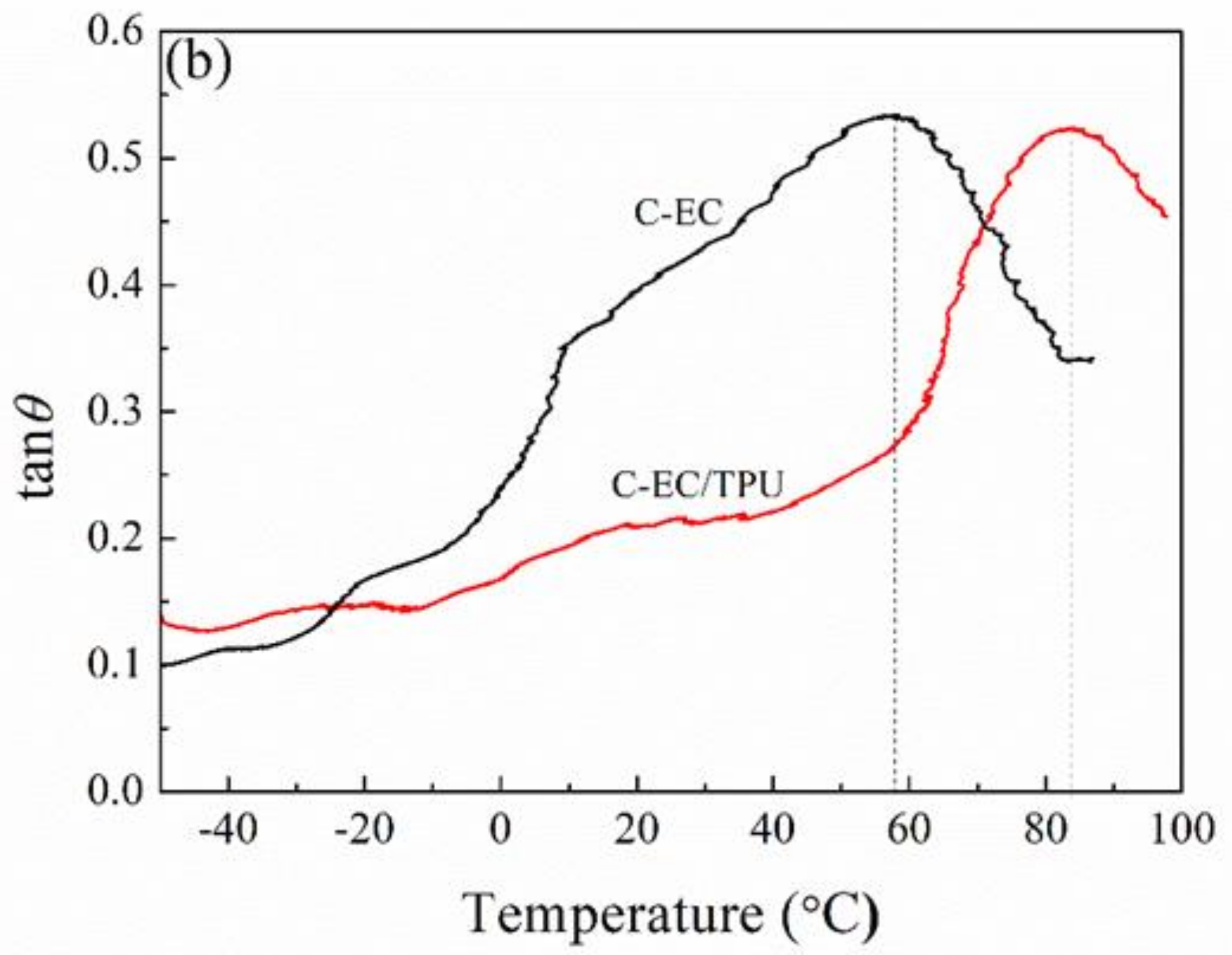
3.5. DMA Analysis
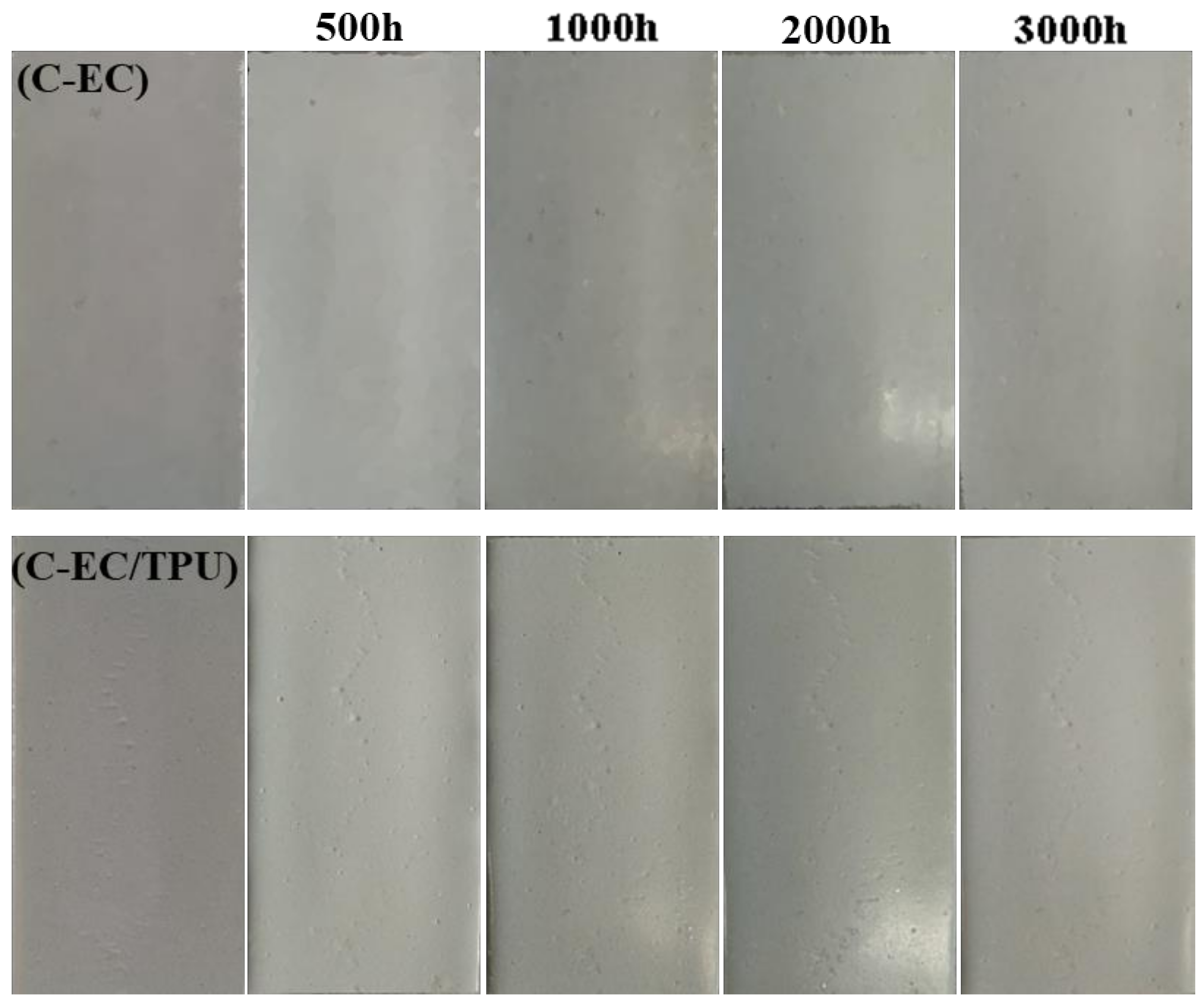
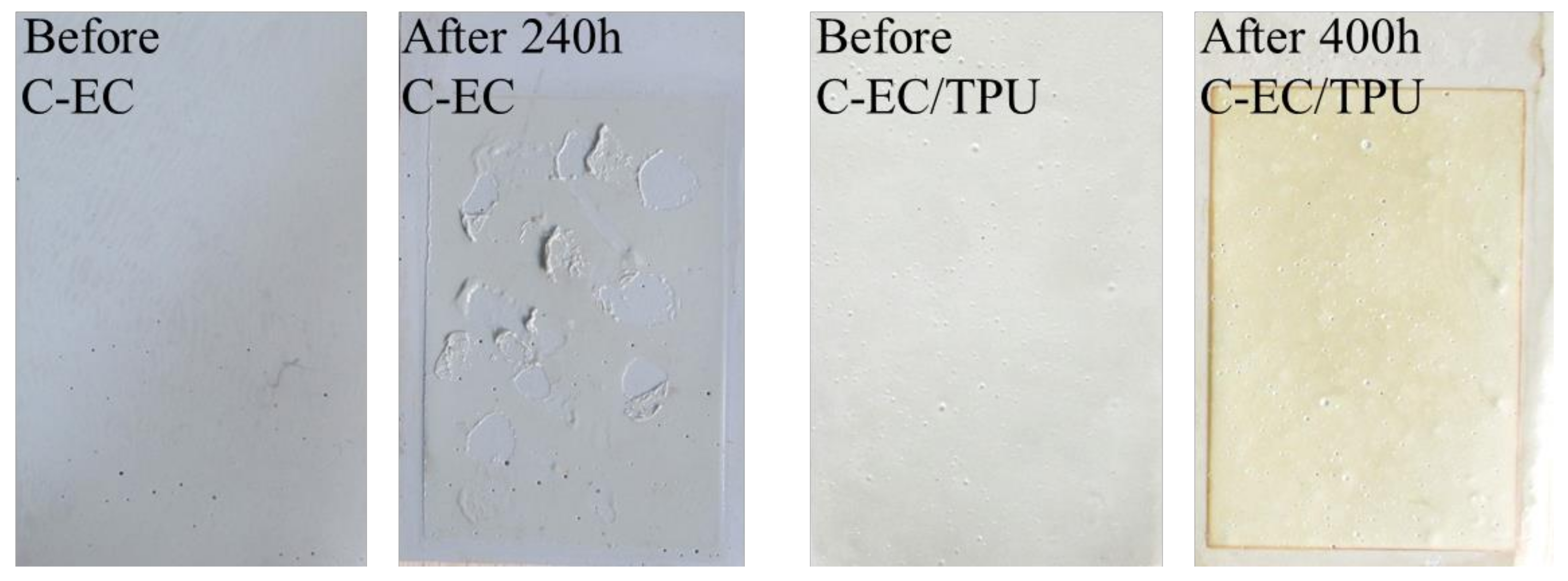
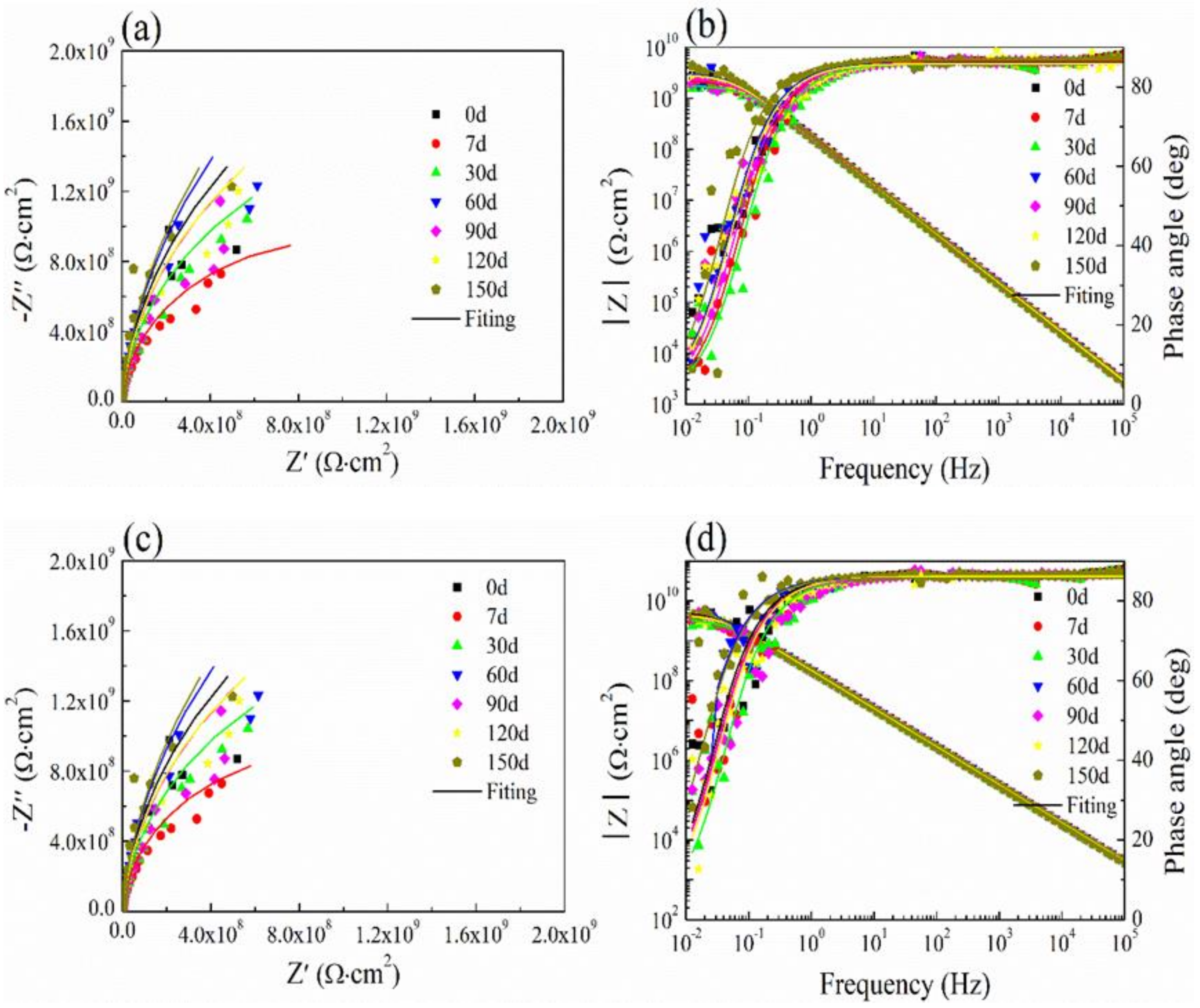
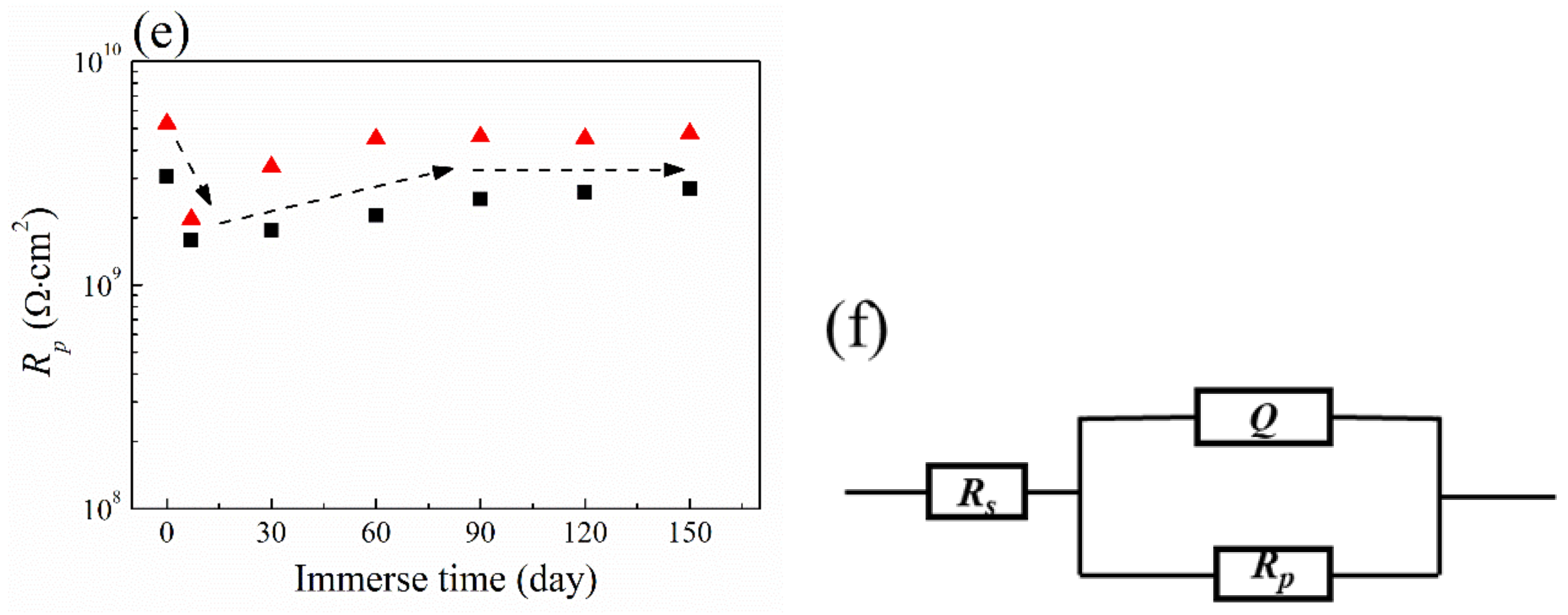
3.6. Salt Spray Test and Ultraviolet Aging Test
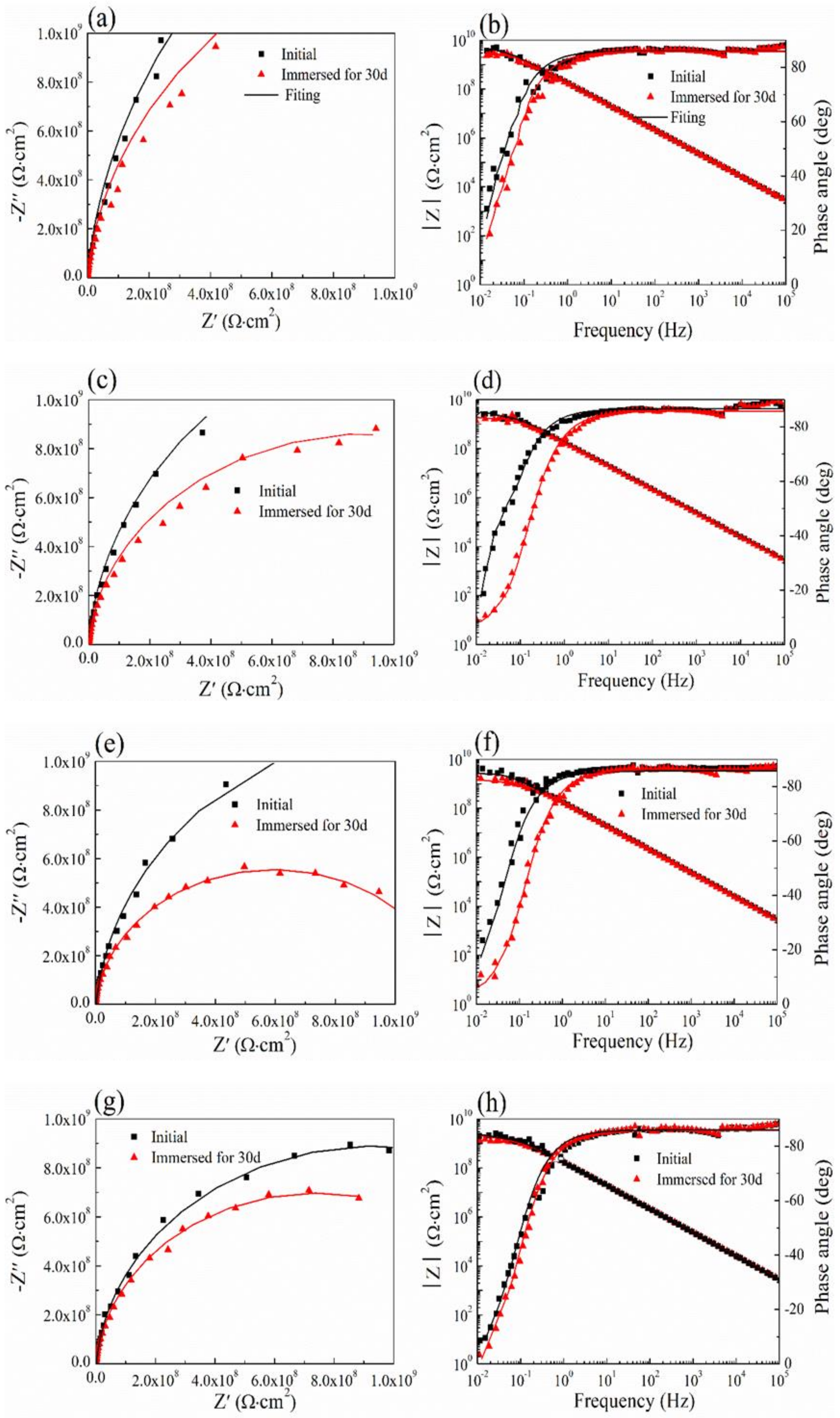
3.7. Cyclic Performance of Coating
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- King, A.D.; Kannan, B.; Scully, J.R. Environmental degradation of a Mg-rich primer in selected field and laboratory environments: Part 1-without a topcoat. Corrosion 2014, 70, 512–535. [Google Scholar] [CrossRef]
- Wood, R.J.K. Marine wear and tribocorrosion. Wear 2017, 376–377, 893–910. [Google Scholar] [CrossRef]
- Dong, S.; Zhao, B.; Lin, C.; Du, R.; Hu, R.; Zhang, G.X. Corrosion behavior of epoxy/zinc duplex coated rebar embedded in concrete in ocean environment. Constr. Build. Mater. 2012, 28, 72–78. [Google Scholar] [CrossRef]
- Liu, B.; Fang, Z.G.; Wang, H.B.; Wang, T. Effect of cross linking degree and adhesion force on the anti-corrosion performance of epoxy coatings under simulated deep sea environment. Prog. Org. Coat. 2013, 76, 1814–1818. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, T.; Ji, C.; Cheng, F.; Cui, W.; Chen, Y. Optimizing heavy-duty anticorrosive performances of coating films formed by acrylate-vinylidene chloride copolymer latexes through twice-painting technique. Chin. J. Polym. Sci. 2015, 33, 14–22. [Google Scholar] [CrossRef]
- Wang, N.; Gao, H.; Zhang, J.; Kang, P. Effect of graphene oxide/ZSM-5 hybrid on corrosion resistance of waterborne epoxy coating. Coatings 2018, 8, 179. [Google Scholar] [CrossRef]
- Dastpak, A.; Yliniemi, K.; De Oliveira Monteiro, M.; Höhn, S.; Virtanen, S.; Lundström, M.; Wilson, B. From waste to valuable resource: Lignin as a sustainable anti-corrosion coating. Coatings 2018, 8, 454. [Google Scholar] [CrossRef]
- Fu, C.; Qin, H.W.; Ben, H.J.; Han, J.; Cui, W.Z.; Cheng, F.; Chen, Y. Acrylate-vinylidene chloride copolymers derived from corresponding water-borne latexes: Influence of acrylate units on their potential as heavy-duty anticorrosive coating materials. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, Z.; Wang, L.; Du, X. Investigation on anti-corrosion property of nano-TiO2 fluoro-carbon coatings. Proc. SPIE 2009, 7493, 74933D. [Google Scholar]
- Fu, C.; Zhang, T.X.; Cheng, F.; Cui, W.Z.; Chen, Y. Double-layer coating films prepared from water-borne latexes of acrylate-vinylidene chloride copolymers: Investigating their heavy-duty anticorrosive properties. Ind. Eng. Chem. Res. 2014, 53, 4534–4543. [Google Scholar] [CrossRef]
- Kismet, Y.; Wagner, M.H. Enhancing the potential of employing thermosetting powder recyclates as filler in LLDPE by structural modifications. J. Polym. Eng. 2017, 37, 287–296. [Google Scholar] [CrossRef]
- Kismet, Y. Change of mechanical properties of powder recyclate reinforced polyolefin based on gamma radiation. Polymers 2017, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, Y.; Kamisho, T.; Sakata, S.; Sawada, T.; Watanuki, Y.; Nishio, R.; Ueda, T. Mixed powder coating film using thermoplastic polyester and its alkaline resistance. J. Coat. Technol. Res. 2013, 10, 503–514. [Google Scholar] [CrossRef]
- Tohidi, S.D.; Rocha, A.M.; Dencheva, N.V.; Pouzada, A.S.; Denchev, Z. Comparative structural and mechanical studies on polyamide 6 knitted-reinforced single polymer composites prepared by different reactive processing techniques. Polym. Compos. 2019, 40, E886–E897. [Google Scholar] [CrossRef]
- Takeshita, Y.; Kamisho, T.; Sakata, S.; Sawada, T.; Watanuki, Y.; Nishio, R.; Ueda, T. Dual layer structural thermoplastic polyester powder coating film and its weathering resistance. J. Appl. Polym. Sci. 2013, 128, 1732–1739. [Google Scholar] [CrossRef]
- Koning, C.E.; Sablong, R.J.; Hosseini Nejad, E.; Duchateau, R.; Buijsen, P. Novel coating resins based on polycarbonates and poly(ester-co-carbonate)s made by catalytic chain growth polymerization of epoxides with CO2 and with anhydride/CO2. Prog. Org. Coat. 2013, 76, 1704–1711. [Google Scholar] [CrossRef]
- Gioia, C.; Vannini, M.; Celli, A.; Colonna, M.; Minesso, A. Chemical recycling of post-consumer compact discs towards novel polymers for powder coating applications. RSC Adv. 2016, 6, 31462–31469. [Google Scholar] [CrossRef]
- Sauer, D.; Cerea, M.; Dinunzio, J.; McGinity, J. Dry powder coating of pharmaceuticals: A review. Int. J. Pharm. 2013, 457, 488–502. [Google Scholar] [CrossRef]
- Adhikari, S.; Selvaraj, S.; Kim, D.H. Progress in powder coating technology using atomic layer deposition. Adv. Mater. Interfaces 2018, 5, 1–20. [Google Scholar] [CrossRef]
- Yuan, W.; Yuan, J.; Zhang, F.; Xie, X. Syntheses, characterization, and in vitro degradation of ethyl cellulose-graft-poly(ε-caprolactone)-block-poly(L-lactide) copolymers by sequential ring-opening polymerization. Biomacromolecules 2007, 8, 1101–1108. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, L.; Zhang, C.; Chen, K.; Feng, F.; Zhang, H. Comparison of ethyl cellulose–gelatin composite films fabricated by electrospinning versus solvent casting. J. Appl. Polym. Sci. 2018, 135, 1–10. [Google Scholar] [CrossRef]
- Masood, A.; Shoukat, Z.; Yousaf, Z.; Sana, M.; Faisal Iqbal, M.; Rehman, A.R.; Sultana, I.; Razaq, A. High capacity natural fiber coated conductive and electroactive composite papers electrode for energy storage applications. J. Appl. Polym. Sci. 2019, 136, 1–6. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Davidovich-Pinhas, M.; Zetzl, A.K.; Barbut, S.; Marangoni, A.G. Influence of solvent quality on the mechanical strength of ethylcellulose oleogels. Carbohydr. Polym. 2016, 135, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Lakdawala, D.H.; Shaikh, A.A.; Desai, A.R.; Choksi, H.H.; Vaidya, R.J.; Ranch, K.M.; Koli, A.R.; Vyas, B.A.; Shah, D.O. In vitro and in vivo evaluation of novel implantation technology in hydrogel contact lenses for controlled drug delivery. J. Control. Release 2016, 226, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Li, H.; Ou, Z.; Yang, G. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release. Eur. J. Pharm. Sci. 2018, 115, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kempin, W.; Franz, C.; Koster, L.C.; Schneider, F.; Bogdahn, M.; Weitschies, W.; Seidlitz, A. Assessment of different polymers and drug loads for fused deposition modeling of drug loaded implants. Eur. J. Pharm. Biopharm. 2017, 115, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lu, C.; Wang, C.; Wang, J.; Fan, Y.; Chu, F. Sustainable thermoplastic elastomers derived from cellulose, fatty acid and furfural via ATRP and click chemistry. Carbohydr. Polym. 2017, 176, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Abbaspoor, S.; Ashrafi, A.; Abolfarsi, R. Development of self-healing coatings based on ethyl cellulose micro/nano-capsules. Surf. Eng. 2019, 35, 273–280. [Google Scholar] [CrossRef]
- Pei, A.; Malho, J.M.; Ruokolainen, J.; Zhou, Q.; Berglund, L.A. Strong nanocomposite reinforcement effects in polyurethane elastomer with low volume fraction of cellulose nanocrystals. Macromolecules 2011, 44, 4422–4427. [Google Scholar] [CrossRef]
- Mehta, B.; Kathalewar, M.; Sabnis, A. Benzyl ester of dehydrated castor oil fatty acid as plasticizer for poly(vinyl chloride). Polym. Int. 2014, 63, 1456–1464. [Google Scholar] [CrossRef]
- El-Wahab, H.A.; El-Fattah, M.A.; Ghazy, M.B.M. Synthesis and characterization of new modified anti-corrosive polyesteramide resins incorporated pyromellitimide ring for surface coating. Prog. Org. Coat. 2011, 72, 353–359. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mishra, R.S.; Narayan, R.; Raju, K.V.S.N. Effect of nano ZnO on the phase mixing of polyurethane hybrid dispersions. Prog. Org. Coat. 2010, 67, 405–413. [Google Scholar] [CrossRef]
- Mishra, S.K.; Pathak, K. Formulation and evaluation of oil entrapped gastroretentive floating gel beads of loratadine. Acta Pharm. 2008, 58, 187–197. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Abd-Elrahman, M.I.; Ahmed, M.O.; Abdel-Aleem, J.A. Optical properties of cellulose derivatives blend film carrying a chalcogenide material. Mater. Sci. Semicond. Process. 2013, 16, 1052–1056. [Google Scholar] [CrossRef]
- Selim, M.S.; El-safty, S.A.; El-sockary, M.A.; Hashem, A.I.; Abo, O.M.; El-saeed, A.M.; Fatthallah, N.A. Smart photo-induced silicone/TiO2 nanocomposites with dominant [110] exposed surfaces for self-cleaning foul-release coatings of ship hulls. JMADE 2016, 101, 218–225. [Google Scholar]
- Mohanan, A.; Bouzidi, L.; Narine, S.S. Mitigating crystallization of saturated FAMEs in biodiesel 6: The binary phase behavior of 1, 2-dioleoyl-3-stearoyl sn-glycerol—Methyl stearate. Energy 2016, 100, 273–284. [Google Scholar] [CrossRef]
- Ogunniyi, D.S. Castor oil: A vital industrial raw material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef]
- Huda, M.S.; Drzal, L.T.; Mohanty, A.K.; Misra, M. Effect of fiber surface-treatments on the properties of laminated biocomposites from poly(lactic acid) (PLA) and kenaf fibers. Compos. Sci. Technol. 2008, 68, 424–432. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Biocomposites from wheat straw nanofibers: Morphology, thermal and mechanical properties. Compos. Sci. Technol. 2008, 68, 557–565. [Google Scholar] [CrossRef]
- Das, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Investigation into the Influence of UV Aging on green polyurethane/nanosilica composite coatings based on transesterified castor oil and palm oil isocyanate. J. Inorg. Organomet. Polym. Mater. 2017, 27, 641–657. [Google Scholar] [CrossRef]
- Zhang, H.; Dun, Y.; Tang, Y.; Zuo, Y.; Zhao, X. Correlation between natural exposure and artificial ageing test for typical marine coating systems. J. Appl. Polym. Sci. 2016, 43893, 1–9. [Google Scholar] [CrossRef]
- Liu, J.; Liu, T.; Guo, Z.; Guo, N.; Lei, Y.; Chang, X.; Yin, Y. Promoting barrier performance and cathodic protection of zinc-rich epoxy primer via single-layer graphene. Polymers 2018, 10, 591. [Google Scholar] [CrossRef]
- Nawaz, M.; Yusuf, N.; Habib, S.; Shakoor, R.A.; Ubaid, F.; Ahmad, Z.; Kahraman, R.; Mansour, S.; Gao, W. Development and properties of polymeric nanocomposite coatings. Polymers 2019, 11, 852. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, Z.D.; Liu, Q.; Dyer, P. Dissolved gas analysis of thermal faults in transformer liquids simulated using immersed heating method. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1749–1757. [Google Scholar] [CrossRef]


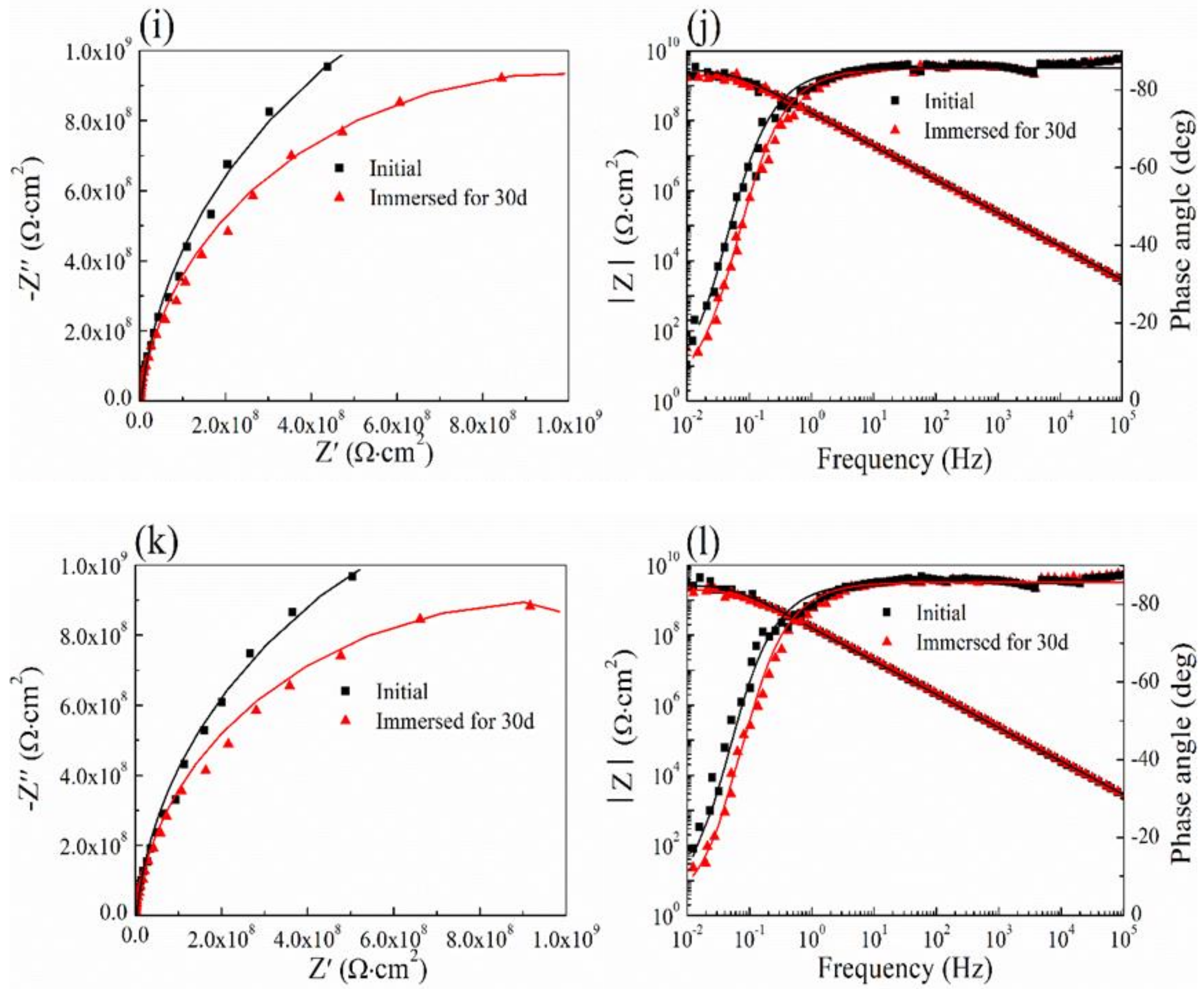
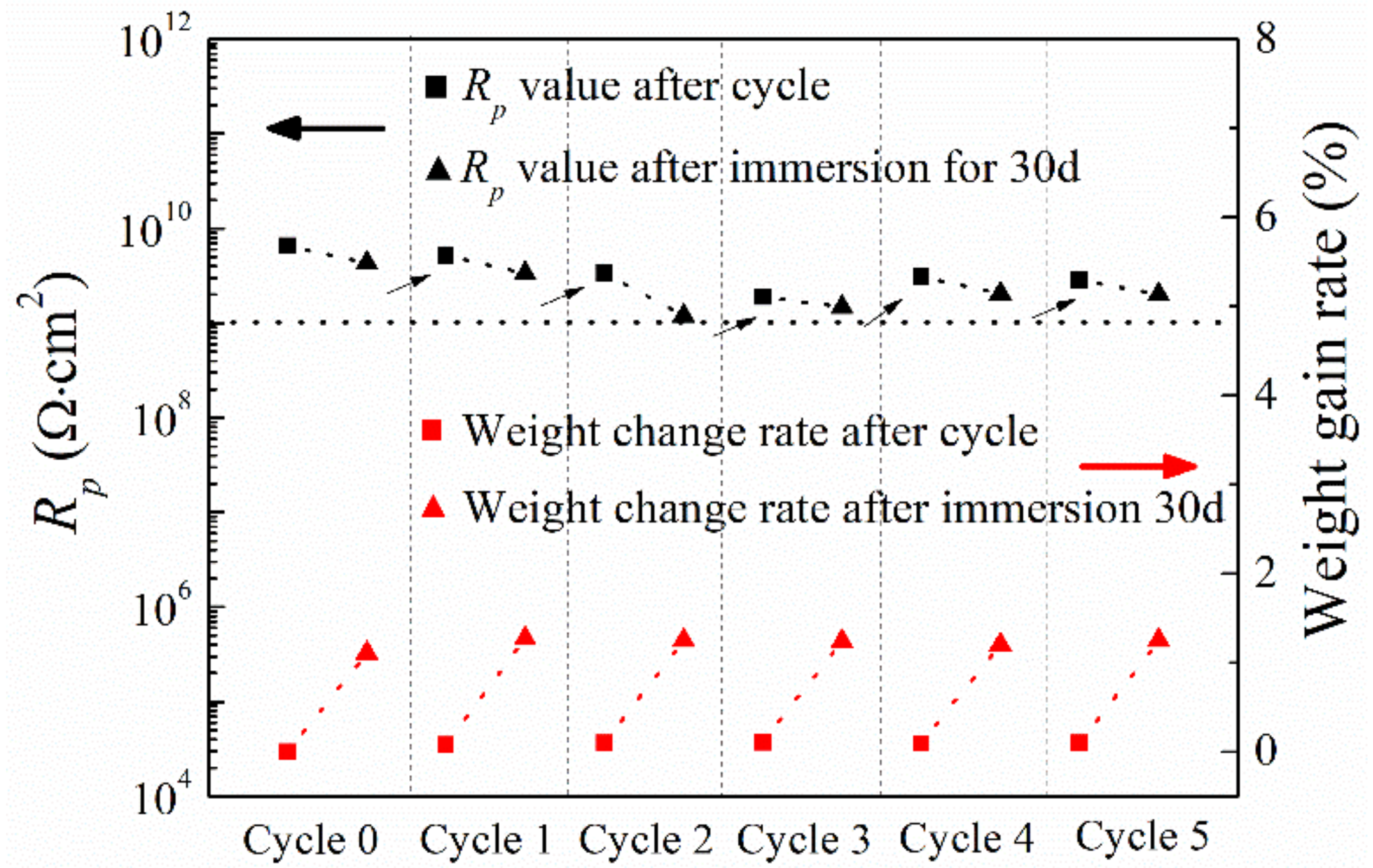
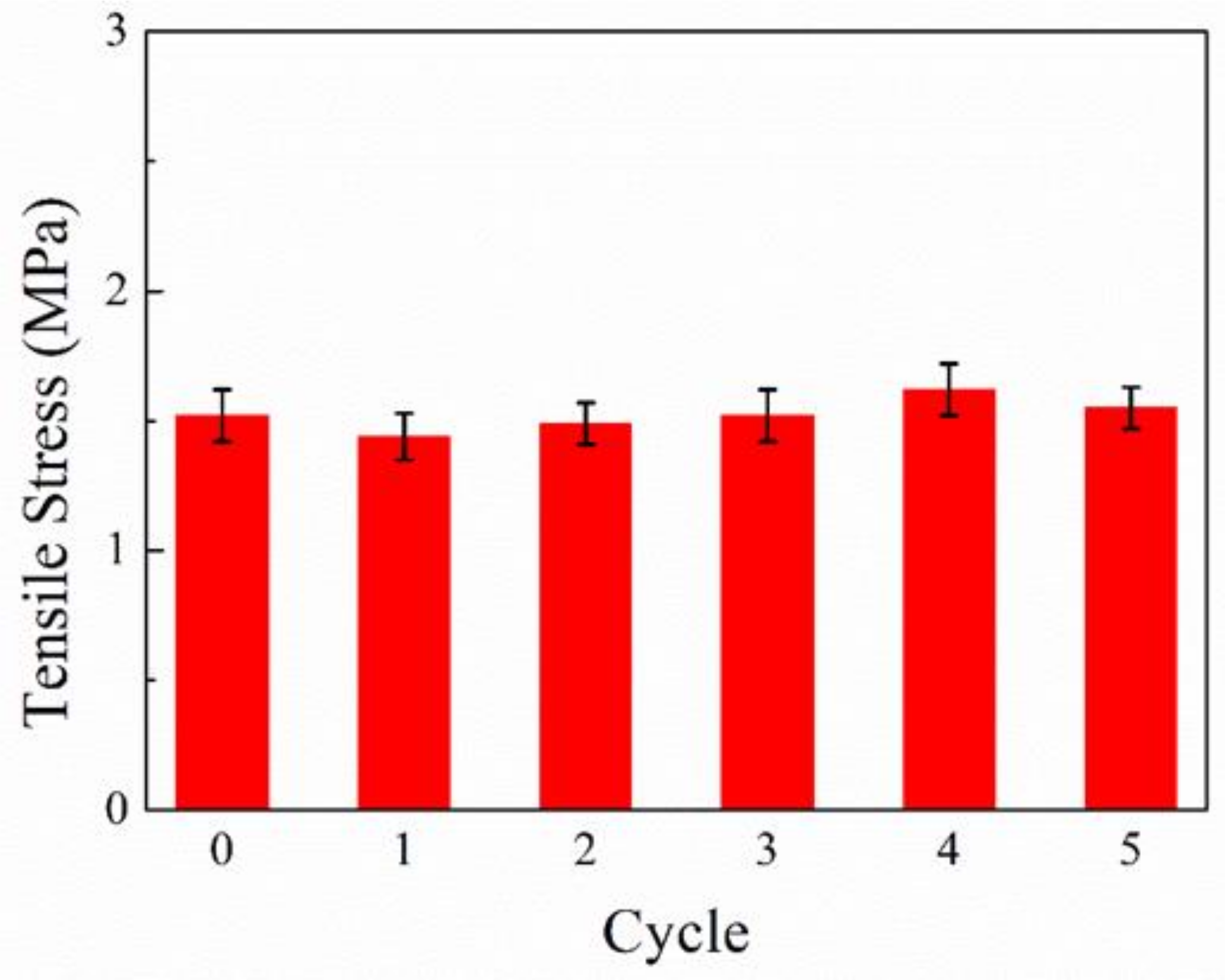
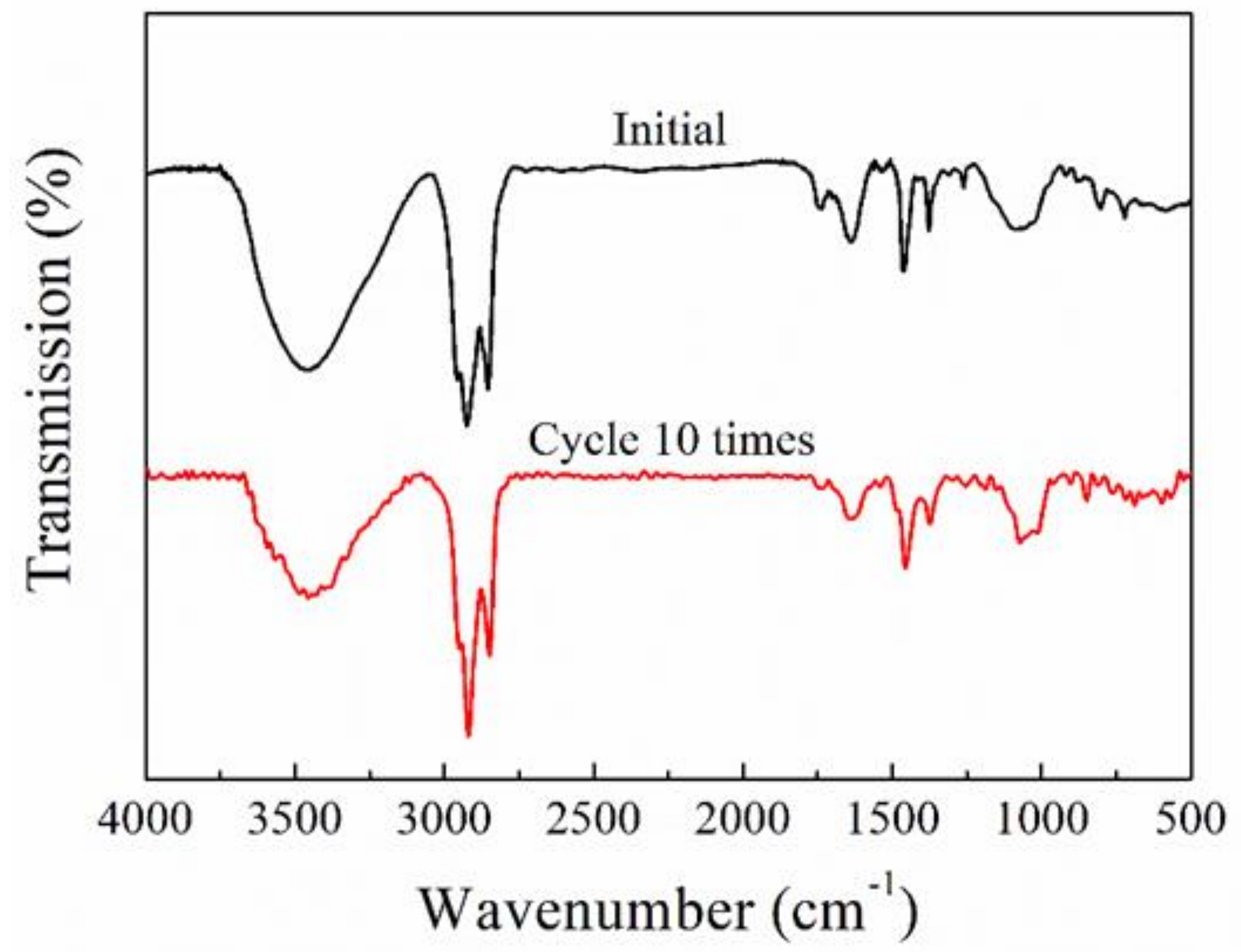
| Test Conditions | Rs (Ω⋅cm2) | Q (F⋅cm−2) | Rp (Ω⋅cm2) | n | |
|---|---|---|---|---|---|
| Before the cycle | Before immersion | 5.420 × 10−2 | 1.3 × 10−9 | 6.454 × 109 | 0.9523 |
| After immersion | 4.01 × 10−3 | 1.063 × 10−9 | 4.331 × 109 | 0.9495 | |
| Cycle once | Before immersion | 3.805 × 10−4 | 1.033 × 10−9 | 5.193 × 109 | 0.9517 |
| After immersion | 1.023 × 10−2 | 9.553 × 10−10 | 3.357 × 109 | 0.9610 | |
| Cycle 2 times | Before immersion | 2.45 × 10−3 | 1.035 × 10−9 | 3.402 × 109 | 0.9533 |
| After immersion | 7.67 × 10−3 | 9.929 × 10−10 | 1.191 × 109 | 0.9590 | |
| Cycle 3 times | Before immersion | 1.487 × 10−2 | 1.023 × 10−9 | 1.898 × 109 | 0.9553 |
| After immersion | 2.42 × 10−3 | 1.021 × 10−9 | 1.491 × 109 | 0.9538 | |
| Cycle 4 times | Before immersion | 8.07 × 10−3 | 1.049 × 10−9 | 3.102 × 109 | 0.9519 |
| After immersion | 1.196 × 10−2 | 1.07 × 10−9 | 2.055 × 109 | 0.9506 | |
| Cycle 5 times | Before immersion | 1.953 × 10−4 | 1.073 × 10−9 | 2.870 × 109 | 0.9512 |
| After immersion | 4.17 × 10−3 | 1.029 × 10−9 | 2.015 × 109 | 0.9525 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Tang, J.; Han, H.; Zhang, S.; Wang, H.; Wang, Y.; Li, T.; Lin, B. Study on a Novel Recyclable Anticorrosion Gel Coating Based on Ethyl Cellulose and Thermoplastic Polyurethane. Coatings 2019, 9, 618. https://doi.org/10.3390/coatings9100618
Zhang H, Tang J, Han H, Zhang S, Wang H, Wang Y, Li T, Lin B. Study on a Novel Recyclable Anticorrosion Gel Coating Based on Ethyl Cellulose and Thermoplastic Polyurethane. Coatings. 2019; 9(10):618. https://doi.org/10.3390/coatings9100618
Chicago/Turabian StyleZhang, Hailong, Junlei Tang, Hongchang Han, Shengwei Zhang, Hu Wang, Yingying Wang, Tian Li, and Bing Lin. 2019. "Study on a Novel Recyclable Anticorrosion Gel Coating Based on Ethyl Cellulose and Thermoplastic Polyurethane" Coatings 9, no. 10: 618. https://doi.org/10.3390/coatings9100618
APA StyleZhang, H., Tang, J., Han, H., Zhang, S., Wang, H., Wang, Y., Li, T., & Lin, B. (2019). Study on a Novel Recyclable Anticorrosion Gel Coating Based on Ethyl Cellulose and Thermoplastic Polyurethane. Coatings, 9(10), 618. https://doi.org/10.3390/coatings9100618






