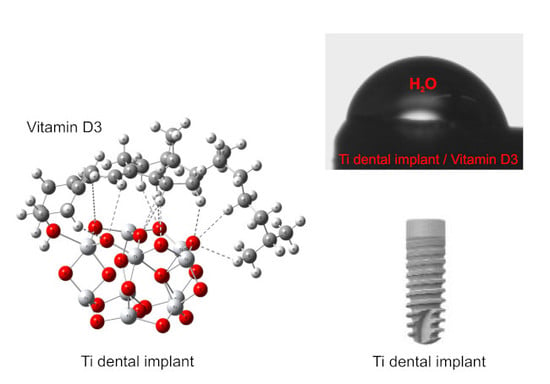
Bioactive Coating on Titanium Dental Implants for Improved Anticorrosion Protection: A Combined Experimental and Theoretical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
2.2. Formation of Bioactive Coating
2.3. Characterisation Methods
2.4. Computational Details
3. Results and Discussion
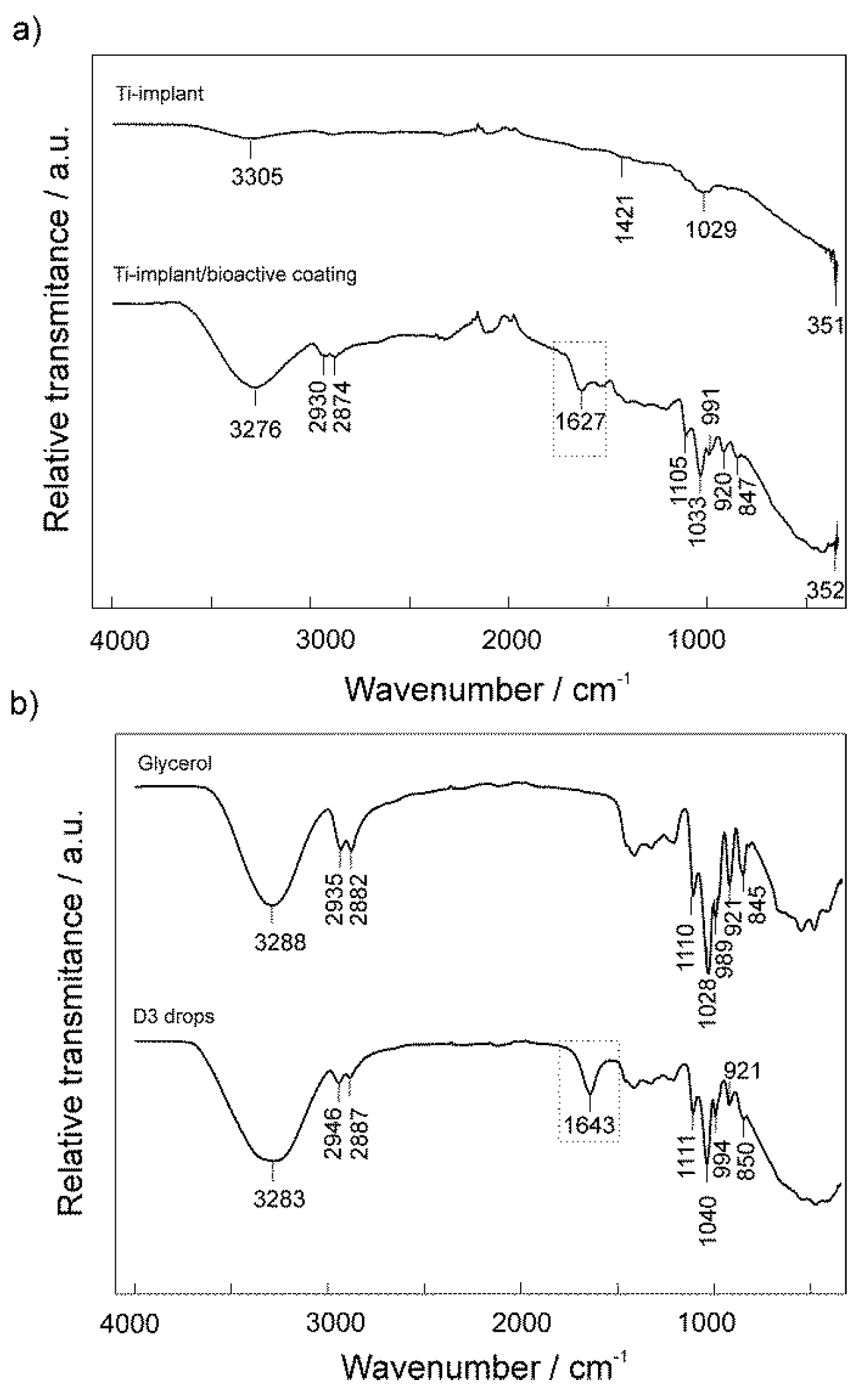
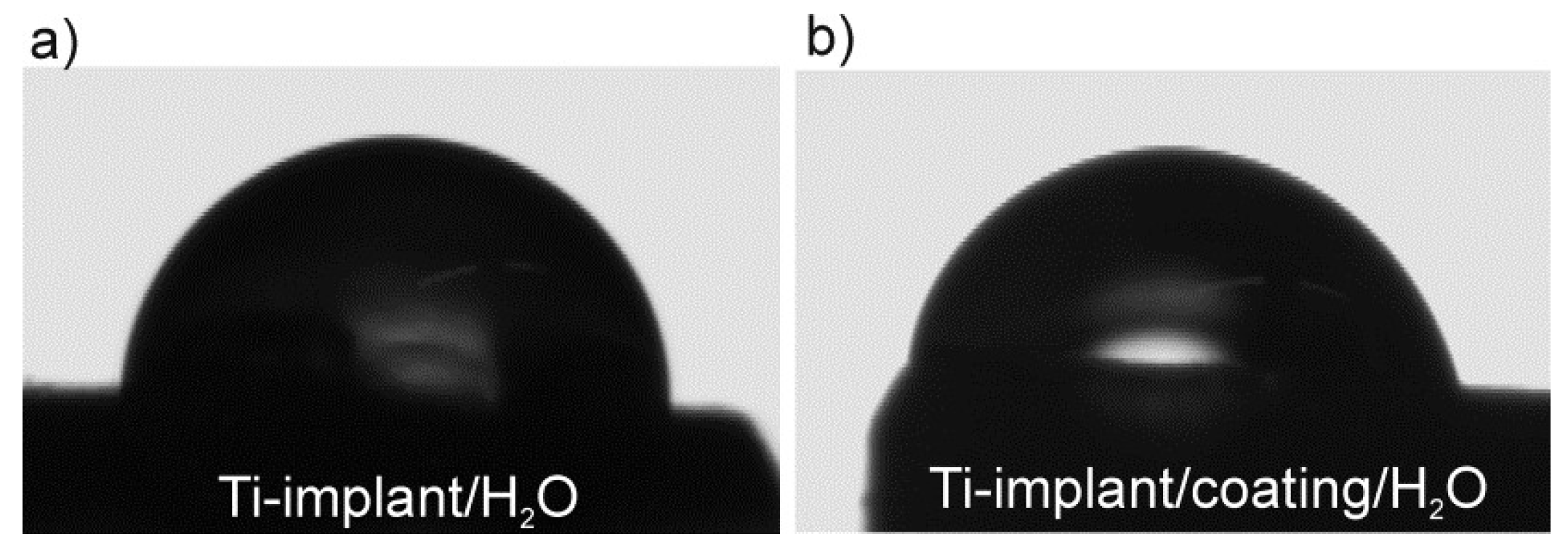
3.1. Surface Characterization of the Unmodified and Modified Ti-Implant Samples
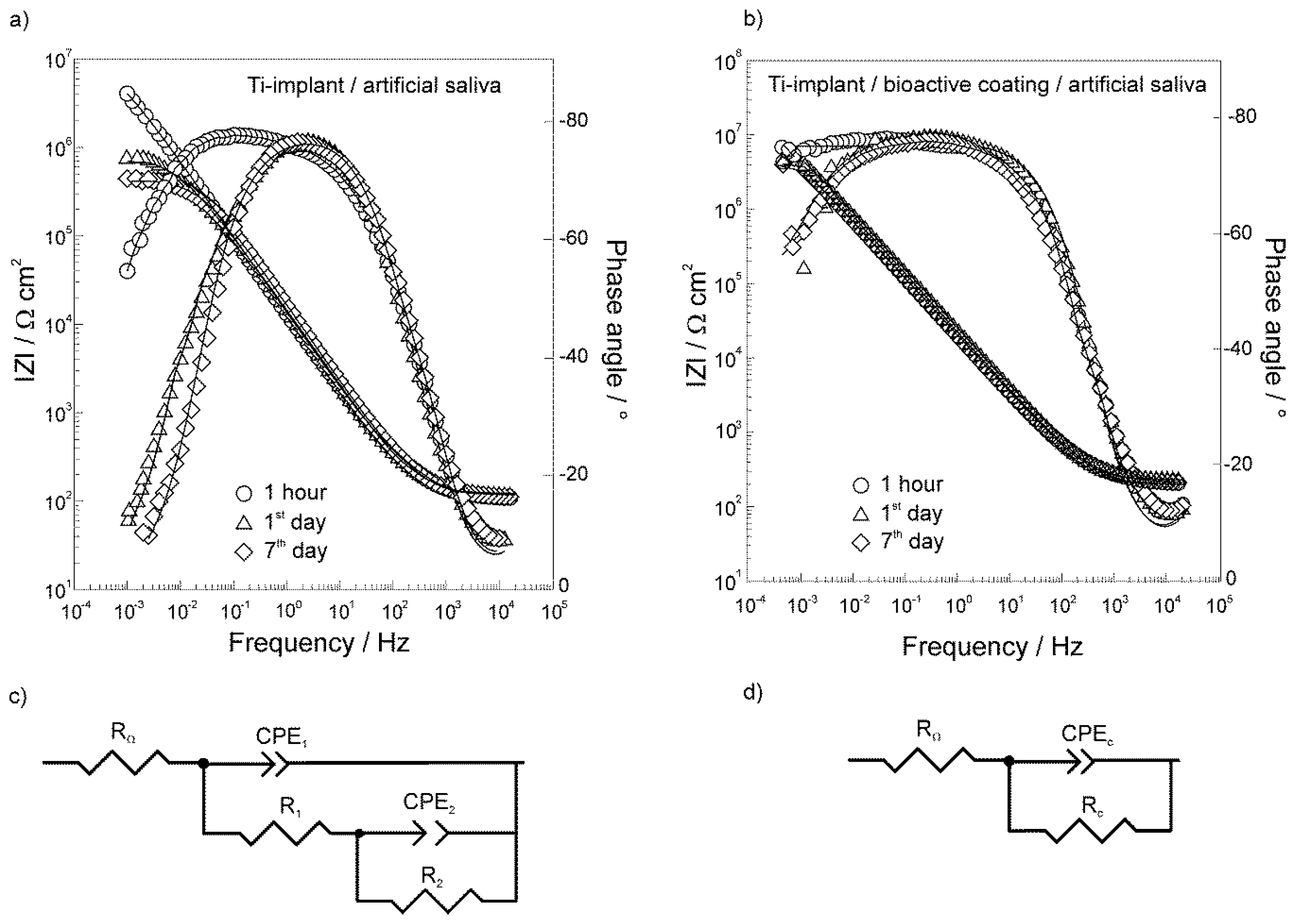
3.2. Electrochemical Characterization of the Unmodified and Modified Ti-Implant Samples
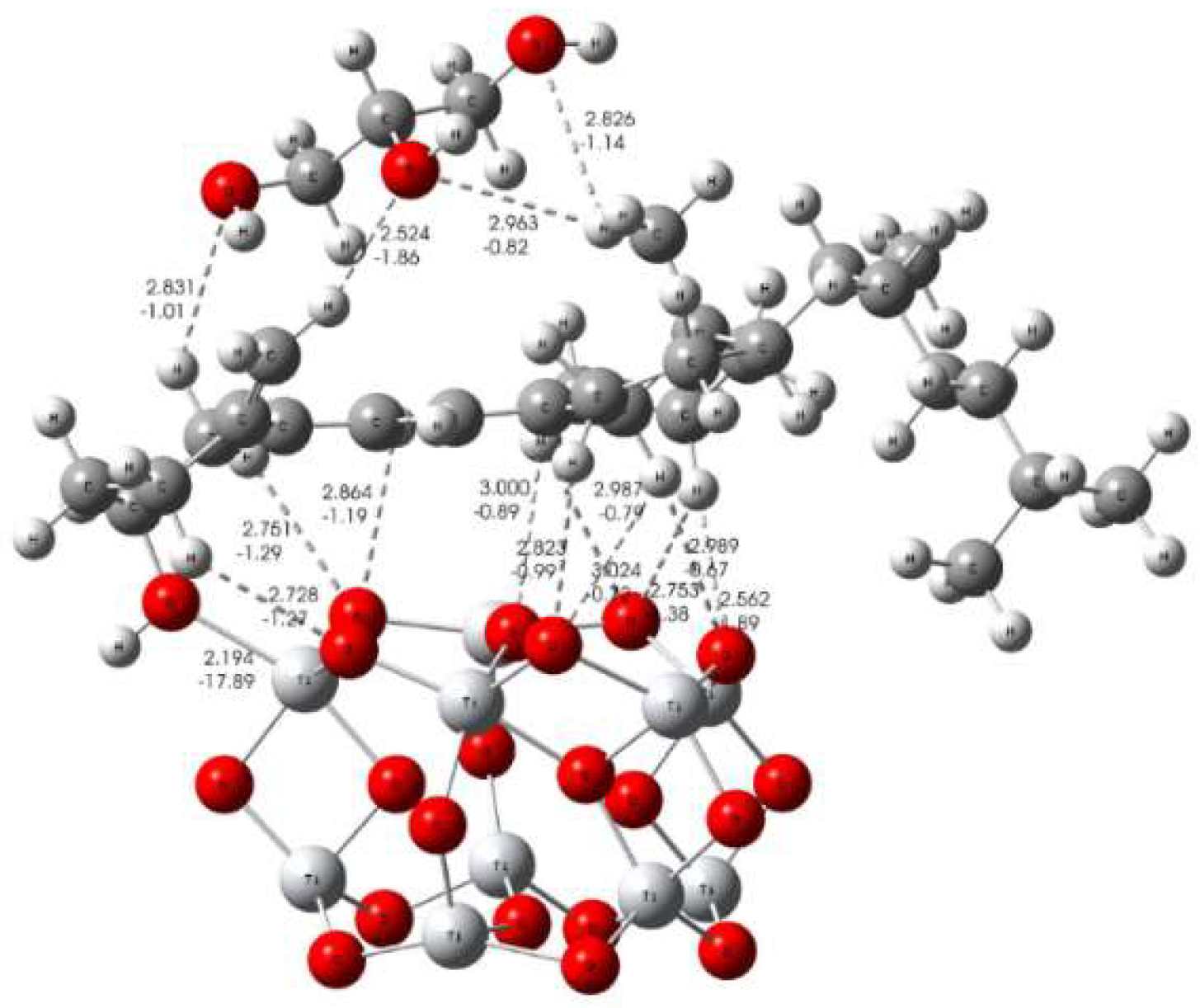
3.3. Formation Mechanism of Bioactive Implant Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.C. Metal Corrosion in the Human Body: The Ultimate Bio-Corrosion Scenario. Electrochem. Soc. Interface 2008, 17, 31. [Google Scholar]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Afroz, S.; Dalanon, J.; Goto, N.; Hosoki, M.; Matsuka, Y. Metal allergy patient treated by titanium implant denture: A case report with at least 4-year follow-up. Clin. Case Rep. 2018, 6, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Payne, A.G.T.; De Silva, R.K.; Duncan, W.J. Titanium allergy: Could it affect dental implant integration? Clin. Oral Implant. Res. 2011, 22, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.; Chopra, R.; Sachdev, V. Allergic Reactions to Dental Materials—A Systematic Review. J. Clin. Diagn. Res. 2015, 9, ZE04. [Google Scholar] [CrossRef] [PubMed]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin. Oral Implant. Res. 2008, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]
- Al Mugeiren, O.M.; Baseer, M.A. Dental Implant Bioactive Surface Modifiers: An Update. J. Int. Soc. Prev. Community Dent. 2019, 9, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Xie, D.B.; Wang, F.H. Corrosion behavior of TiN and TiN/Ti composite films on Ti6Al4V alloy in Hanks solution. J. Appl. Electrochem. 2009, 39, 447–453. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, Z.; Li, Y.; Zhang, X.; De Bruijn, J.D.; De Groot, K. Osteoinduction by calcium phosphate biomaterials. J. Mater. Sci. Mater. Electron. 1998, 9, 723–726. [Google Scholar] [CrossRef]
- Sousa, L.L.; Ricci, V.P.; Prado, D.G.; Apolinario, R.C.; Vercik, L.C.; Rigo, E.C.; Fernandes, M.C.; Mariano, N.A. Titanium Coating with Hydroxyapatite and Chitosan Doped with Silver Nitrate. Mater. Res. 2017, 20, 863–868. [Google Scholar] [CrossRef][Green Version]
- Łukaszewska-Kuska, M.; Krawczyk, P.; Martyla, A.; Hedzelek, W.; Dorocka-Bobkowska, B. Hydroxyapatite coating on titanium endosseous implants for improved osseointegration: Physical and chemical considerations. Adv. Clin. Exp. Med. 2018, 27, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.H.; Ben-Nissan, B.; Matinlinna, J.P.; Conway, R.C. Current perspectives: Calcium phosphates nanocoatings and nanocomposite coatings in dentistry. J. Dent. Res. 2013, 92, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.; Gharibi, B.; McLister, R.; Meenan, B.J.; Deb, S. Self-assembled monolayers of alendronate on Ti6Al4V alloy surfaces enhance osteogenesis in mesenchymal stem cells. Sci. Rep. 2016, 6, 30548. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Neoh, K.G.; Shi, Z.; Kang, E.-T.; Wang, W. An In Vitro Assessment of Fibroblast and Osteoblast Response to Alendronate-Modified Titanium and the Potential for Decreasing Fibrous Encapsulation. Tissue Eng. Part A 2013, 19, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Trybek, G.; Aniko-Wlodarczyk, M.; Kwiatek, J.; Preuss, O.; Brodkiewicz, A.; Sinicyn, A.; Grzywacz, A. The effect of vitamin D3 on the osteointegration of dental implant. Balt. J. Health Phys. Act. 2018, 10, 25–33. [Google Scholar] [CrossRef]
- Satué, M.; Monjo, M.; Ronold, H.J.; Lyngstadaas, S.P.; Ramis, J.M. Titanium implants coated with UV-irradiated vitamin D precursor and vitamin E: In vivo performance and coating stability. Clin. Oral Implant. Res. 2016, 28, 424–431. [Google Scholar] [CrossRef]
- ISO 5832-2:2018 Implants for surgery - Metallic materials—Part 2: Unalloyed titanium; ISO: Geneva, Switzerland, 2018.
- Available online: https://www.upmet.com/products/titanium/cp-grade-2 (accessed on 25 June 2019).
- Gouzman, I.; Dubey, M.; Carolus, M.D.; Schwartz, J.; Bernasek, S.L. Monolayer vs. multilayer self-assembled alkylphosphonate films: X-ray photoelectron spectroscopy studies. Surf. Sci. 2006, 600, 773–781. [Google Scholar] [CrossRef]
- Petrović, Ž.; Katić, J.; Metikoš-Huković, M.; Dadafarin, H.; Omanovic, S. Modification of a Nitinol Surface by Phosphonate Self-Assembled Monolayers. J. Electrochem. Soc. 2011, 158, F159–F165. [Google Scholar] [CrossRef]
- Mellado-Valero, A.; Muñoz, A.I.; Pina, V.G.; Sola-Ruiz, M.F. Electrochemical Behaviour and Galvanic Effects of Titanium Implants Coupled to Metallic Suprastructures in Artificial Saliva. Materials 2018, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, A. A Nonlinear Least Squares Fit procedure for analysis of immittance data of electrochemical systems. Solid State Ionics 1986, 20, 31–44. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Allard, M.M.; Merlos, S.N.; Springer, B.N.; Cooper, J.; Zhang, G.; Boskovic, D.S.; Kwon, S.R.; Nick, K.E.; Perry, C.C. Role of TiO2 Anatase Surface Morphology on Organophosphorus Interfacial Chemistry. J. Phys. Chem. C 2018, 122, 29237–29248. [Google Scholar] [CrossRef]
- Qu, Z.-W.; Kroes, G.-J. Theoretical Study of Stable, Defect-Free (TiO2)n Nanoparticles with n = 10–16. J. Phys. Chem. C 2007, 111, 16808–16817. [Google Scholar] [CrossRef]
- Zhao, Y.; Thrular, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. Density Functional Theory for Reaction Energies: Test of Meta and Hybrid Meta Functionals, Range-Separated Functionals, and Other High-Performance Functionals. J. Chem. Theory Comput. 2011, 7, 669–676. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Keith, T.A. AIMAll (Version 17.01.25). Available online: http://aim.tkgristmill.com/versionhistory.html (accessed on 12 February 2019).
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, NY, USA, 1994. [Google Scholar]
- Rupp, F.; Scheideler, L.; Rehbein, D.; Axmann, D.; Geis-Gerstorfer, J. Roughness induced dynamic changes of wettability of acid etched titanium implant modifications. Biomaterials 2004, 25, 1429–1438. [Google Scholar] [CrossRef]
- Webster, T.J.; Ejiofor, J.U. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomatererials 2004, 25, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.C.; Pereira Ricci, V.; de Sousa, L.L.; Marianoa, N.A.; Campos, M.G.N. Improvement of Titanium Corrosion Resistance by Coating with Poly-Caprolactone and Poly-Caprolactone/Titanium Dioxide: Potential Application in Heart Valves. Mater. Res. 2017, 20, 126–133. [Google Scholar] [CrossRef][Green Version]
- Baig, M.I.; Ingole, P.G.; Choi, W.K.; Lee, H.K. Synthesis and characterization of polyamide/polyester thin-film nanocomposite membranes achieved by functionalized TiO2 nanoparticles for water vapor separation. J. Mater. Chem. A 2016, 4, 5592–5604. [Google Scholar]
- Othayoth, R.; Mathi, P.; Bheemanapally, K.; Kakarla, L.; Botlagunta, M. Characterization of vitamin–cisplatin-loaded chitosan nano-particles for chemoprevention and cancer fatigue. J. Microencapsul. 2015, 32, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; Wiley: Hoboken, NJ, USA, 2008; pp. 233–265. [Google Scholar]
- Brug, G.; Eeden, A.V.D.; Sluyters-Rehbach, M.; Sluyters, J. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Jorcin, J.-B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef]
- Pan, J.; Thierry, D.; Leygraf, C. Electrochemical impedance spectroscopy study of the passive oxide film on titanium for implant application. Electrochimica Acta 1996, 41, 1143–1153. [Google Scholar] [CrossRef]
- Kosec, T.; Legat, A.; Kovač, J.; Klobčar, D. Influence of Laser Colour Marking on the Corrosion Properties of Low Alloyed Ti. Coatings 2019, 9, 375. [Google Scholar] [CrossRef]
- Aziz-Kerrzo, M.; Conroy, K.G.; Fenelon, A.M.; Farrell, S.T.; Breslin, C. Electrochemical studies on the stability and corrosion resistance of titanium-based implant materials. Biomaterials 2001, 22, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- De Assis, S.L.; Wolynec, S.; Costa, I. Corrosion characterization of titanium alloys by electrochemical techniques. Electrochim. Acta 2006, 51, 1815–1819. [Google Scholar] [CrossRef]
- Qu, Q.; Wang, L.; Chen, Y.; Li, L.; He, Y.; Ding, Z. Corrosion Behavior of Titanium in Artificial Saliva by Lactic Acid. Materials 2014, 7, 5528–5542. [Google Scholar] [CrossRef] [PubMed]
- Guo, X. Corrosion and Electrochemical Impedance Properties of Ti Alloys as Orthopaedic Trauma Implant Materials. Int. J. Electrochem. Sci. 2017, 12, 9007–9016. [Google Scholar] [CrossRef]
- Mishnaevsky, L.; Levashov, E.; Valiev, R.Z.; Segurado, J.; Sabirov, I.; Enikeev, N.; Prokoshkin, S.; Solov’Yov, A.V.; Korotitskiy, A.; Gutmanas, E.; et al. Nanostructured titanium-based materials for medical implants: Modeling and development. Mater. Sci. Eng. R Rep. 2014, 81, 1–19. [Google Scholar] [CrossRef]
- Scully, J.R. Polarization Resistance Method for Determination of Instantaneous Corrosion Rates. Corrosion 2000, 56, 199–218. [Google Scholar] [CrossRef]
- Tamilselvi, S.; Murugaraj, R.; Rajendran, N. Electrochemical impedance spectroscopic studies of titanium and its alloys in saline medium. Mater. Corros. 2007, 58, 113–120. [Google Scholar] [CrossRef]
- Evans, S.D.; Urankar, E.; Ulman, A.; Ferris, N. Self-assembled monolayers of alkanethiols containing a polar aromatic group: Effects of the dipole position on molecular packing, orientation, and surface wetting properties. J. Am. Chem. Soc. 1991, 113, 4121–4131. [Google Scholar] [CrossRef]
- Boubour, E.; Lennox, R.B. Insulating Properties of Self-Assembled Monolayers Monitored by Impedance Spectroscopy. Langmuir 2000, 16, 4222–4228. [Google Scholar] [CrossRef]


| Element | N | C | O | Fe | H | Ti | Other |
|---|---|---|---|---|---|---|---|
| wt % | 0.03 | 0.10 | 0.25 | 0.30 | 0.0155 | Balance | 0.4 |
| Exposure Time | RΩ/Ω cm2 | 106 × Q1/Ω−1 cm−2 sn | n1 | C1/μF cm−2 | R1/Ω cm2 | 106 × Q2/Ω−1 cm−2 sn | n2 | C2/μF cm−2 | R2/MΩ cm2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 h | 111 | 9.98 | 0.853 | 3.02 | 760 | 5.16 | 0.850 | 1.38 | 9.90 |
| 1 day | 123 | 2.88 | 0.997 | 2.88 | 174 | 12.1 | 0.788 | 2.10 | 0.79 |
| 7 days | 123 | 3.26 | 0.978 | 2.71 | 307 | 9.21 | 0.810 | 1.88 | 0.44 |
| Exposure Time | RΩ/Ω cm2 | 106 × Qc/Ω−1 cm−2 sn | n | Cc/μF cm−2 | Rc/MΩ cm2 | η/% |
|---|---|---|---|---|---|---|
| 1 h | 157 | 7.97 | 0.851 | 2.52 | 51.7 | 80.9 |
| 1 day | 157 | 6.95 | 0.862 | 2.54 | 17.4 | 95.5 |
| 7 days | 157 | 8.14 | 0.846 | 2.47 | 17.6 | 97.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katić, J.; Šarić, A.; Despotović, I.; Matijaković, N.; Petković, M.; Petrović, Ž. Bioactive Coating on Titanium Dental Implants for Improved Anticorrosion Protection: A Combined Experimental and Theoretical Study. Coatings 2019, 9, 612. https://doi.org/10.3390/coatings9100612
Katić J, Šarić A, Despotović I, Matijaković N, Petković M, Petrović Ž. Bioactive Coating on Titanium Dental Implants for Improved Anticorrosion Protection: A Combined Experimental and Theoretical Study. Coatings. 2019; 9(10):612. https://doi.org/10.3390/coatings9100612
Chicago/Turabian StyleKatić, Jozefina, Ankica Šarić, Ines Despotović, Nives Matijaković, Marin Petković, and Željka Petrović. 2019. "Bioactive Coating on Titanium Dental Implants for Improved Anticorrosion Protection: A Combined Experimental and Theoretical Study" Coatings 9, no. 10: 612. https://doi.org/10.3390/coatings9100612
APA StyleKatić, J., Šarić, A., Despotović, I., Matijaković, N., Petković, M., & Petrović, Ž. (2019). Bioactive Coating on Titanium Dental Implants for Improved Anticorrosion Protection: A Combined Experimental and Theoretical Study. Coatings, 9(10), 612. https://doi.org/10.3390/coatings9100612