Development of Anti-Aging and Anticorrosive Nanoceria Dispersed Alkyd Coating for Decorative and Industrial Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CeO2-Alkyd Based Coating
2.3. Characterization of Alkyd Coatings
2.4. Physical Properties of CeO2-Alkyd Based Coatings
2.5. Mechanical Behaviors of CeO2-Alkyd Based Coatings
2.5.1. Cross-Cut (Adherence) Test
2.5.2. Hardness
2.5.3. Impact Resistance
2.6. Self Cleaning Property of CeO2-Alkyd Based Coatings
2.7. UV Aging of CeO2-Alkyd Based Coating
2.8. Anticorrosive Properties of CeO2-Alkyd Based Coating (Salt Sprey Chamber Test and EIS Tests)
3. Results and Discussion
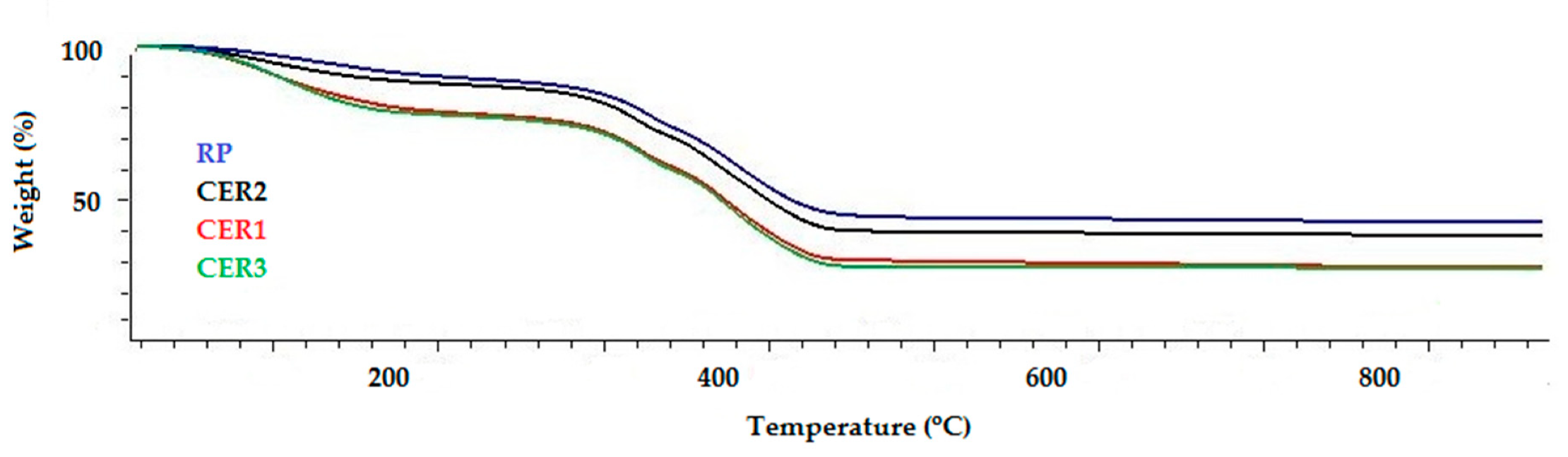
3.1. Characterization of Alkyd Coatings
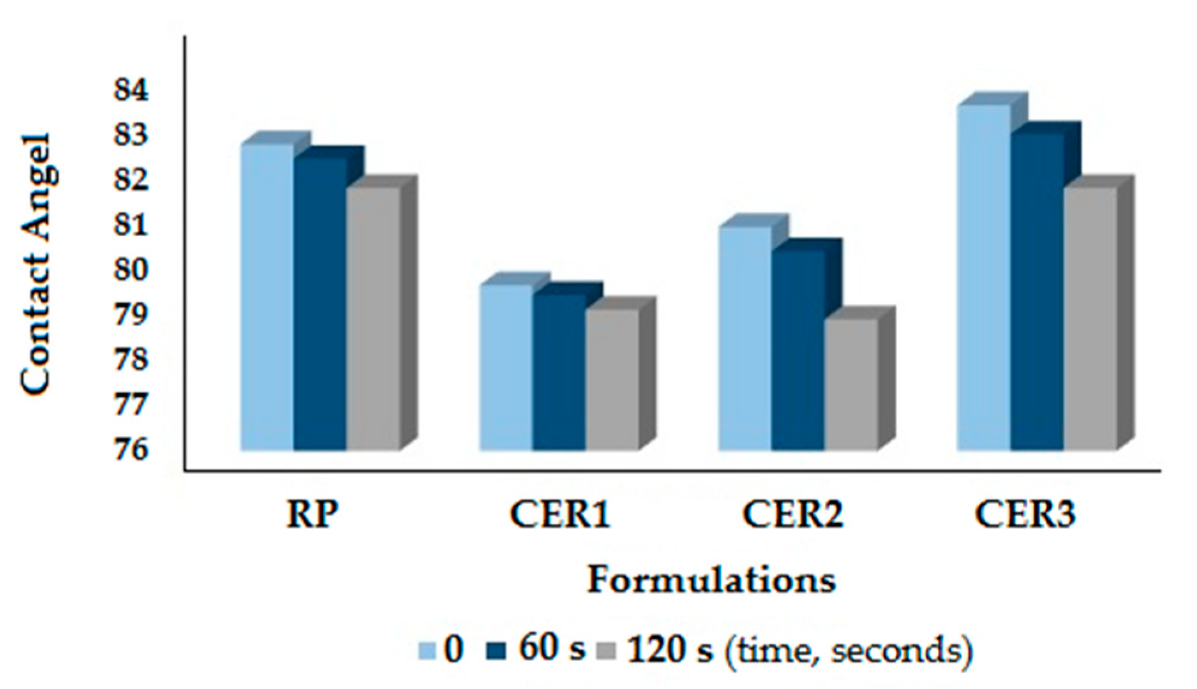
3.2. Physical Properties of CeO2-Alkyd Based Coating
3.3. Mechanical Behaviors of CeO2-Alkyd Based Coating
3.4. Self-Cleaning Behavior
3.5. Effect of UV-Aging
3.6. Anticorrosive Properties of CeO2-Alkyd Based Coating
3.6.1. Salt Spray Test
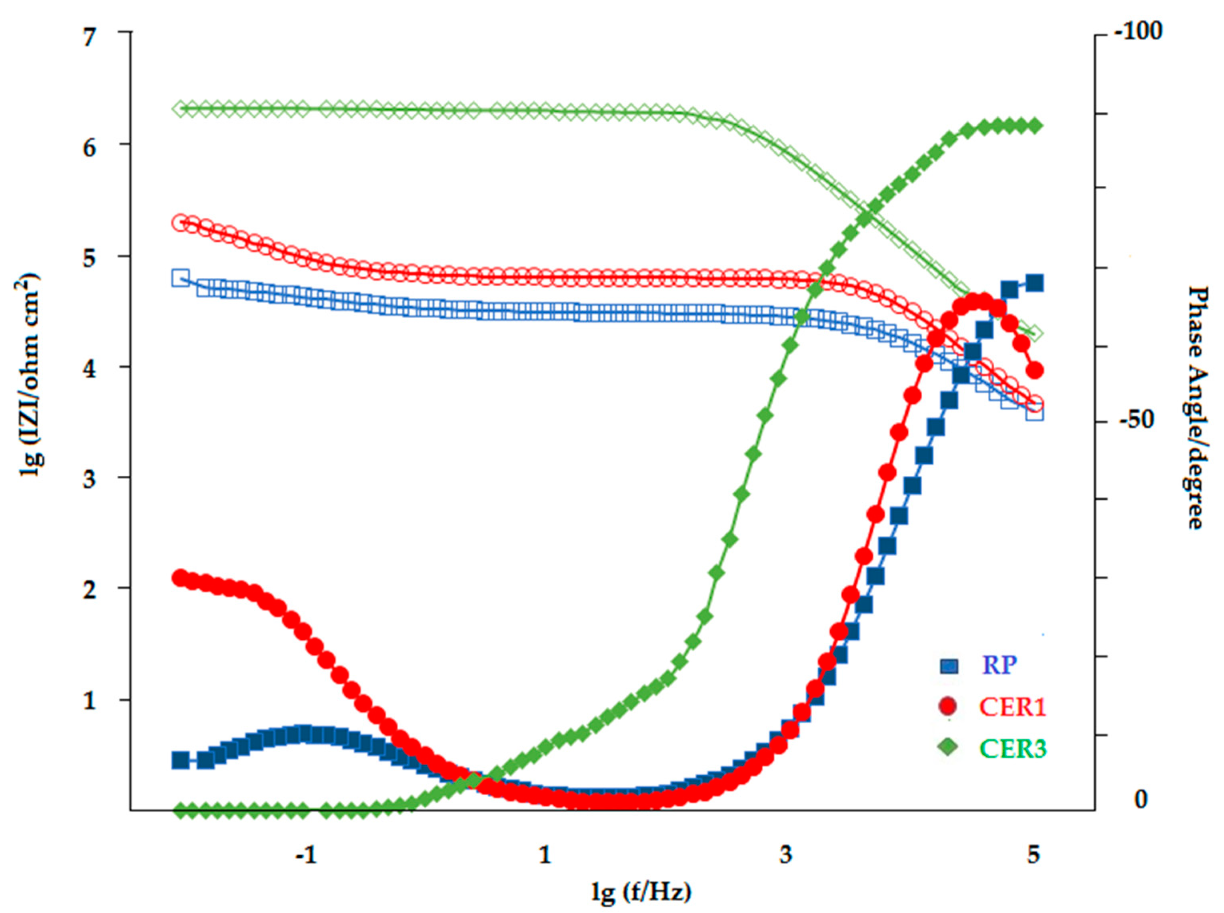
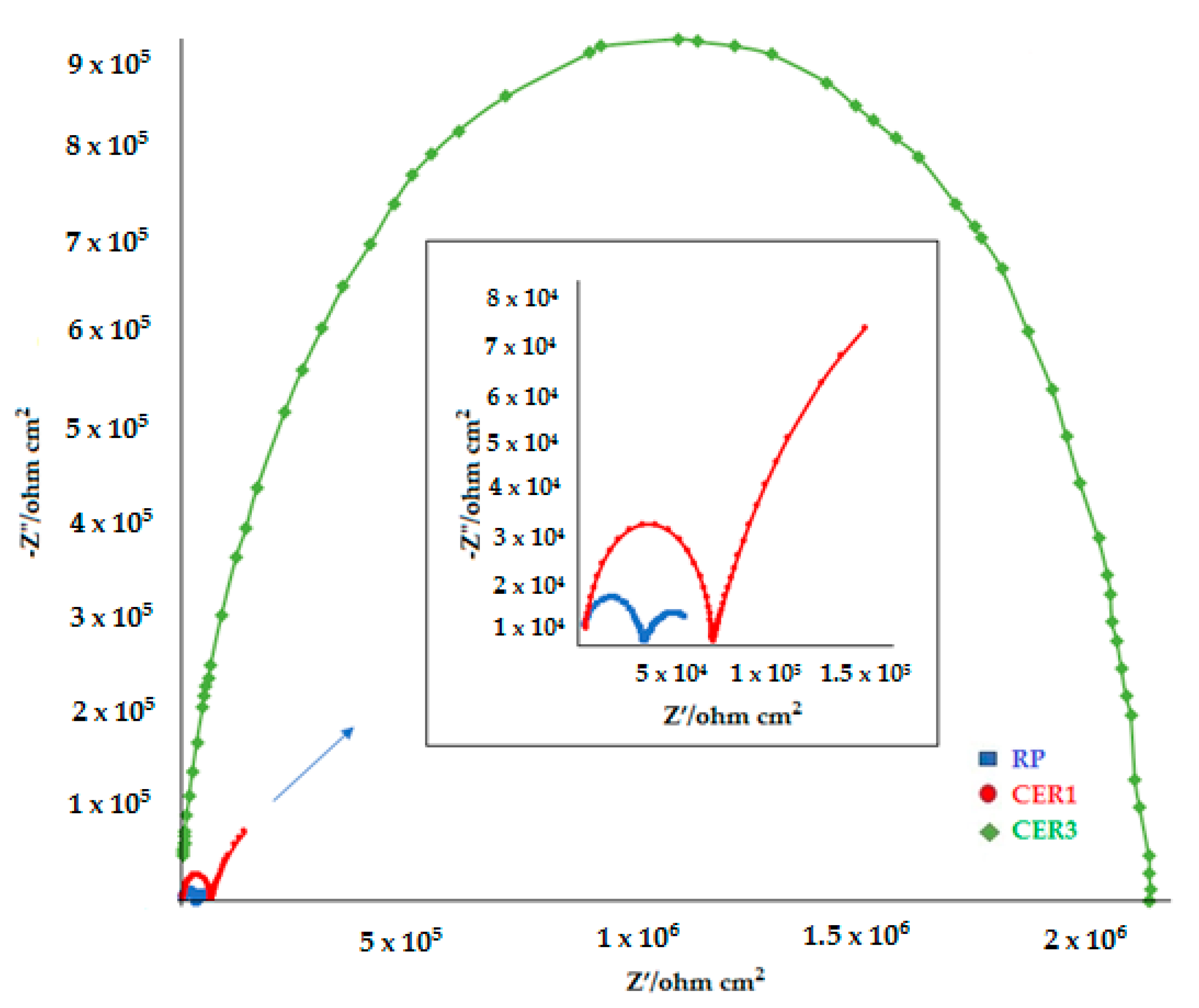
3.6.2. EIS Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- New Research and Developments in Global Paints and Coatings Industry: Market Research Report to 2022. Available online: https://www.reuters.com/brandfeatures/venture-capital/article?id=4426 (accessed on 24 September 2019).
- Ulaeto, S.B.; Rajan, R.; Pancrecious, J.K.; Rajan, T.P.D.; Pai, B.C. Developments in smart anticorrosive coatings with multifunctional characteristics. Prog. Org. Coat. 2017, 111, 294–314. [Google Scholar] [CrossRef]
- Hofland, A. Alkyd resins: From down and out to alive and kicking. Prog. Org. Coat. 2012, 73, 274–282. [Google Scholar] [CrossRef]
- Radoman, T.S.; Dzunuzovic, J.V.; Spasojevic, P.M.; Marinovic Cincovic, M.T.; Jeremic, K.B.; Popovic, I.G.; Dzunuzovic, E.S. Preparation and properties of short oil alkyd resin/TiO2 nanocomposites based on surface modified TiO2 nanoparticles. Polym. Compos. 2018, 39, 1488–1499. [Google Scholar] [CrossRef]
- Dhirde, P.G.; Chada, V.G.R.; Mallik, B.P.; Moitra, N. Alkyd-clay nanocomposites for improved anticorrosion and mechanical performance of coating. Polym. Compos. 2018, 39, 2922–2931. [Google Scholar] [CrossRef]
- Benitha, V.S.; Jeyasubramanian, K.; Hikku, G.S. Investigation of anti-corrosion ability of nano mixed metal oxide pigment dispersed alkyd coating and its optimization for A36 steel. J. Alloy Compd. 2017, 721, 563–576. [Google Scholar] [CrossRef]
- Hikku, G.S.; Jeyasubramanien, K.; Kumar, S.V. Nanoporous MgO as self-cleaning and anti-bacterial pigment for alkyd based coating. J. Ind. Eng. Chem. 2017, 52, 168–178. [Google Scholar] [CrossRef]
- Castano, C.E.; O’Keefe, M.J.; Fahrenholtz, W.G. Cerium-based oxide coatings. Curr. Opin. Solid State Mater. Sci. 2015, 19, 69–76. [Google Scholar] [CrossRef]
- Xu, C.; Qu, X.G. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Ecco, L.G.; Fedel, M.; Ahniyaz, A.; Deflorian, F. Influence of polyaniline and cerium oxide nanoparticles on the corrosion protection properties of alkyd coating. Prog. Org. Coat. 2014, 77, 2031–2038. [Google Scholar] [CrossRef]
- Ecco, L.G.; Fedel, M.; Deflorian, F.; Becker, J.; Iversen, B.B.; Mamakhel, A. Waterborne acrylic paint system based on nanoceria for corrosion protection of steel. Prog. Org. Coat. 2016, 96, 19–25. [Google Scholar] [CrossRef]
- Kumar, A.M.; Rahman, M.M.; Gasem, Z.M. A promising nanocomposite from CNTs and nanoceria: Nanostructured fillers in polyurethane coatings for surface protection. RSC Adv. 2015, 5, 63537–63544. [Google Scholar] [CrossRef]
- Xu, W.; Song, J.; Sun, J.; Lu, Y.; Yu, Z. Rapid fabrication of large-area, corrosion-resistant superhydrophobic Mg alloy surfaces. ACS Appl. Mater. Interfaces 2011, 3, 4404–4414. [Google Scholar] [CrossRef] [PubMed]
- Ecco, L.G.; Rossi, S.; Fedel, M.; Deflorian, F. Color variation of electrophoretic styrene-acrylic paints under field and accelerated ultraviolet exposure. Mater. Des. 2017, 116, 554–564. [Google Scholar] [CrossRef]
- Nelson, G.L. Adhesion in Paint and Coating Testing Manual, 14th ed.; Koleske, J.V., Ed.; ASTM: Philadelphia, PA, USA, 1995. [Google Scholar]
- ASTM D3359-17 Standard Test Methods for Rating Adhesion by Tape Test; ASTM International: West Conshohocken, Montgomery County, PA, USA, 2017.
- Çağlar, D.İ.; Emre, B.; Oktay, B.; Kahraman, M.V. Preparation and evaluation of linseed oil based alkyd paints. Prog. Org. Coat. 2014, 77, 81–86. [Google Scholar] [CrossRef]
- ISO 4892-3 Plastics; Methods of Exposure to Laboratory Light Sources Part 3: Fluorescent UV Lamps; ISO: Geneva, Switzerland, 2016.
- ASTM B117 Standard Practice for Operating Salt Spray (Fog) Apparatus; ASTM International: West Conshohocken, PA, USA, 2018.
- ISO 4628-1 Paints and Varnishes; Evaluation of Degradation of Coatings, Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance Part 1: General Introduction and Designation System; ISO: Geneva, Switzerland, 2016.
- ASTM D1210-05 Standard Test Method for Fineness of Dispersion of Pigment-Vehicle Systems by Hegman-Type Gage; ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D562-10 Standard Test Method for Consistency of Paints Measuring Krebs Unit (KU) Viscosity Using a Stormer-Type Viscometer; ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D1475-13 Standard Test Method for Density of Liquid Coatings, Inks, and Related Products; ASTM International: West Conshohocken, PA, USA, 2013.
- ISO 6504-3 Paints and Varnishes; Determination of Hiding Power Part 3: Determination of Contrast Ratio of Light-Coloured Paints at a Fixed Spreading Rate; ISO: Geneva, Switzerland, 2006.
- ASTM D523-14 Standard Test Method for Specular Gloss; ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D5895-13 Standard Test Methods for Evaluating Drying or Curing During Film Formation of Organic Coatings Using Mechanical Recorders; ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM E1347-06 Standard Test Method for Color and Color-Difference Measurement by Tristimulus Colorimetry; ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM D2244-16 Standard Practice for Calculation of Color Tolerances and Color Differences from Instrumentally Measured Color Coordinates; ASTM International: West Conshohocken, PA, USA, 2016.
- DIN EN ISO 1522 Paints and Varnishes—Pendulum Damping Test; ISO: Geneva, Switzerland, 2007.
- ASTM D 2794-93 Standard Test Method for Resistance of Organic Coatings to the Effects of Rapid Deformation (Impact); ASTM International: West Conshohocken, PA, USA, 2019.
- ISO 4628-2 Paints and Varnishes; Evaluation of Degradation of Coatings, Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance Part 2: Assessment of Degree of Blistering; ISO: Geneva, Switzerland, 2016.
- ISO 4628-3 Paints and Varnishes; Evaluation of Degradation of Coatings, Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance Part 3: Assessment of Degree of Rusting; ISO: Geneva, Switzerland, 2016.
- ISO 4628-4 Paints and Varnishes; Evaluation of Degradation of Coatings, Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance Part 4: Assessment of Degree of Cracking; ISO: Geneva, Switzerland, 2016.
- ISO 4628-8 Paints and Varnishes; Evaluation of Degradation of Coatings, Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance Part 8: Assessment of Degree of Delamination and Corrosion around a Scribe or other Artificial Defect; ISO: Geneva, Switzerland, 2012.
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M. Enhancement of barrier and corrosion protection performance of anepoxy coating through wet transfer of amino functionalized grapheneoxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Marti, M.; Fabregat, G.; Azambuja, D.S.; Aleman, C.; Armelin, E. Evaluation of an environmentally friendly anticorrosive pigment for alkyd primer. Prog. Org. Coat. 2012, 73, 321–329. [Google Scholar] [CrossRef]
- Amirudin, A.; Thierry, D. Application of electrochemical impedance spectroscopy to study the degradation of polymer-coated metals. Prog. Org. Coat. 1995, 26, 1–28. [Google Scholar] [CrossRef]
- Kumar, A.M.; Khan, A.; Suleiman, R.; Qamar, M.; Saravanan, S.; Dafalla, H. Bifunctional CuO/TiO2 nanocomposite as nanofiller for improved corrosion resistance and antibacterial protection. Prog. Org. Coat. 2018, 114, 9–18. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Z.; Yu, L. Hydrophobic polyaniline/modified SiO2 coatings for anticorrosion protection. Synth. Met. 2017, 233, 94–100. [Google Scholar] [CrossRef]
- Benedetti, A.V.; Sumodjo, P.T.A.; Nobe, K.; Cabot, P.L.; Proud, W.G. Electrochemical studies of Copper, Copper-Aluminium and Copper-Aluminium-Silver alloys: Impedance results in 0.5 M NaCl. Electrochim. Acta 1995, 40, 2657–2668. [Google Scholar] [CrossRef]


| Ingredients | RP (wt %) | CER1 (wt %) | CER2 (wt %) | CER3 (wt %) |
|---|---|---|---|---|
| Alkyd | 61.6 | 61.6 | 61.6 | 61.6 |
| Dispersion agent | 0.8 | 0.8 | 0.8 | 0.8 |
| Rheology agent | 0.12 | 0.12 | 0.12 | 0.12 |
| Solvent | 7.78 | 7.78 | 7.78 | 9.78 |
| TiO2 | 24 | 24 | 24 | 24 |
| Wetting agent | 0.2 | 0.2 | 0.2 | 0.2 |
| Inner coat drier | 0.5 | 0.5 | 0.5 | 0.5 |
| Middle coat drier | 0.5 | 0.5 | 0.5 | 0.5 |
| Anti-skinning agent | 0.5 | 0.5 | 0.5 | 0.5 |
| Plasticizer | 1 | 1 | 1 | 1 |
| Commercial anticorrosive agent | 3 | − | − | − |
| CeO2-5 micron | − | 3 | − | − |
| CeO2-25 nm | − | − | 3 | 1 |
| 100 | 100 | 100 | 100 |
| Characteristics | Unit | Method | RP | CER1 | CER2 | CER3 |
|---|---|---|---|---|---|---|
| Grinding value | µm | ASTM D1210 | 15 ± 1 | 10 ± 1 | 15 ± 1 | 12 ± 1 |
| Viscosity (25 °C) | KU | ASTM D562 | 116 ± 2 | 128 ± 2 | 132 ± 1 | 134 ± 2 |
| Density (25 °C) | g/mL | ASTM D1475 | 1.22 ± 0.02 | 1.27 ± 0.03 | 1.25 ± 0.01 | 1.24 ± 0.01 |
| Hiding power | % | ISO 6504-3 | 99.2 ± 0.1 | 99.3 ± 0.1 | 99.3 ± 0.1 | 99.5 ± 0.1 |
| Gloss | 20° | ASTM D523 | 84.0 ± 0.2 | 90.3 ± 0.3 | 95.9 ± 0.4 | 89.5 ± 0.3 |
| 60° | 93.8 ± 0.3 | 96.8 ± 0.2 | 98.9 ± 0.5 | 96.9 ± 0.4 | ||
| 80° | 97.4 ± 0.5 | 100.8 ± 0.6 | 98.7 ± 0.4 | 99.6 ± 0.4 | ||
| Drying time | hour | ASTM D5895/A | 10 | 9 | 9 | 9 |
| Color | L | ASTM E1347 ASTM D2244 | 96.93 | 97.12 | 97.01 | 97.09 |
| a | −0.92 | −0.98 | −1.36 | −1.1 | ||
| b | 1.40 | 1.88 | 3.22 | 2.29 | ||
| Yellowness index | YI | 1.44 | 2.17 | 3.74 | 2.79 | |
| Whiteness index | WI | 86.48 | 84.24 | 79.87 | 81.89 |
| Test Methods | RP | CER1 | CER2 | CER3 |
|---|---|---|---|---|
| Adherence/ASTM 3359 | 4B | 3B | 5B | 5B |
| Hardness (Cycle)/DIN EN ISO 1522 | 24 | 39 | 45 | 45 |
| Impact Resistance (weight, kg)/ASTM D2794 | 30 | 50 | 50 | 50 |
| Color Difference | RP | CER1 | CER2 | CER3 |
|---|---|---|---|---|
| ΔE | 5.47 ± 0.08 | 3.88 ± 0.10 | 0.80 ± 0.19 | 2.51 ± 0.17 |
| Color Differences | RP | CER1 | CER2 | CER3 |
|---|---|---|---|---|
| ΔE | 9.25 ± 0.2 | 3.40 ± 0.11 | 2.66 ± 0.12 | 2.93 ± 0.05 |
| YI before UV | 1.44 | 2.17 | 3.74 | 2.79 |
| YI after UV | 10.44 | 8.72 | 5.54 | 7.01 |
| Test Parameter | Reference Test Method | Blank Coating | RP | CER1 | CER2 | CER3 |
|---|---|---|---|---|---|---|
| Blistering (size) | ISO 4628-2 | 5 | 3 | 2 | 1 | 1 |
| Blistering (density) | ISO 4628-2 | 5 | 3 | 2 | 1 | 1 |
| Degree of rusting | ISO 4628-3 | Ri5 | Ri4 | Ri4 | Ri2 | Ri3 |
| Cracking (size) | ISO 4628-4 | 4 | 2 | 2 | 0 | 1 |
| Cracking rate (quantity) | ISO 4628-4 | 4 | 3 | 2 | 1 | 1 |
| Delamination (mm) | ISO 4628-8 | 21 | 13.5 | 9.5 | 6 | 6.5 |
| Degree of delamination | ISO 4628-8 | severe | moderate | slight | very slight | very slight |
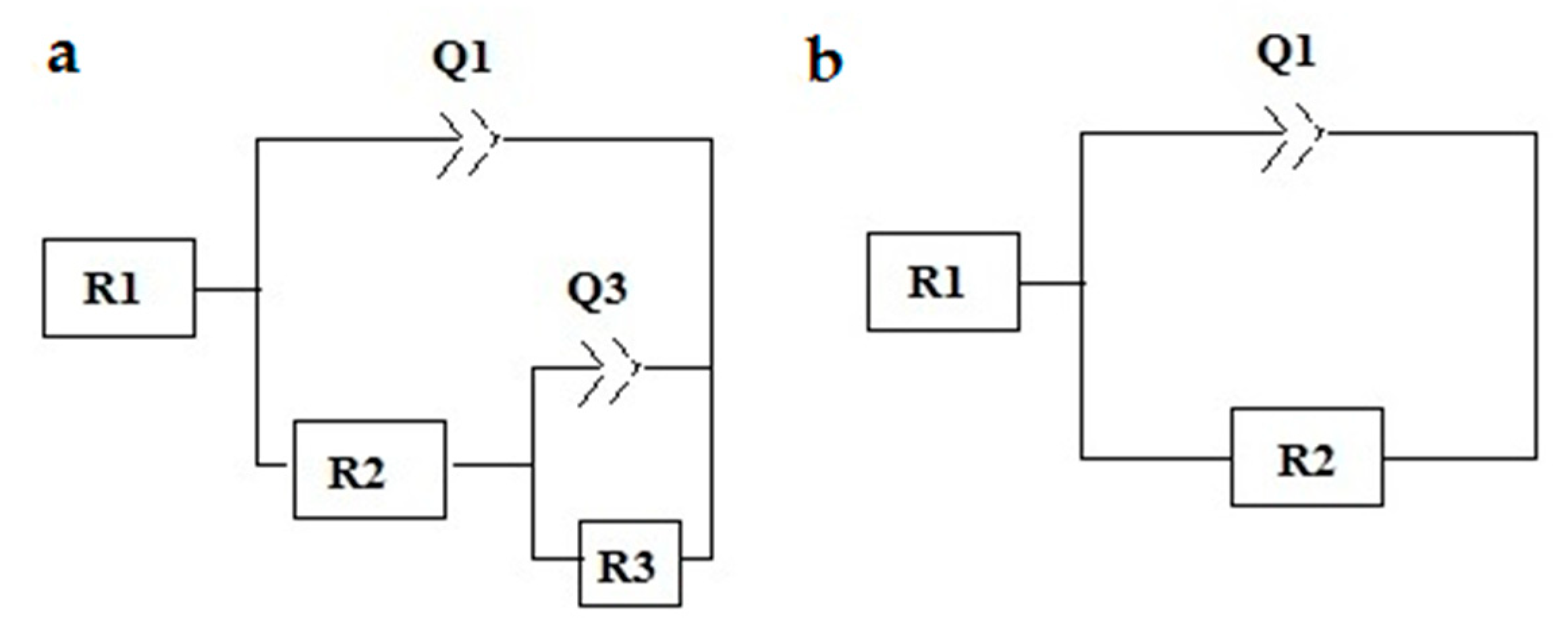
| Sample | Q1 (Q2), F cm−2 | n1 | R2, Ω cm2 | Q3, F cm−2 | n2 | R3, Ω cm2 | |Z| at 0.01 Hz (Ω cm2, log10) |
|---|---|---|---|---|---|---|---|
| RP | 6.34 × 10−9 | 0.79 | 31,100 | 6.30 × 10−5 | 0.62 | 28,102 | 4.8 |
| CER1 | 7.6 × 10−10 | 0.95 | 61,299 | 2.84 × 10−5 | 0.71 | 295,380 | 5.3 |
| CER3 | 3.77 × 10−10 | 0.91 | 2,142,000 | − | − | − | 6.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kızılkonca, E.; Erim, F.B. Development of Anti-Aging and Anticorrosive Nanoceria Dispersed Alkyd Coating for Decorative and Industrial Purposes. Coatings 2019, 9, 610. https://doi.org/10.3390/coatings9100610
Kızılkonca E, Erim FB. Development of Anti-Aging and Anticorrosive Nanoceria Dispersed Alkyd Coating for Decorative and Industrial Purposes. Coatings. 2019; 9(10):610. https://doi.org/10.3390/coatings9100610
Chicago/Turabian StyleKızılkonca, Ezgi, and F. Bedia Erim. 2019. "Development of Anti-Aging and Anticorrosive Nanoceria Dispersed Alkyd Coating for Decorative and Industrial Purposes" Coatings 9, no. 10: 610. https://doi.org/10.3390/coatings9100610
APA StyleKızılkonca, E., & Erim, F. B. (2019). Development of Anti-Aging and Anticorrosive Nanoceria Dispersed Alkyd Coating for Decorative and Industrial Purposes. Coatings, 9(10), 610. https://doi.org/10.3390/coatings9100610






