Optimization of the Oxidation Behavior and Mechanical Properties by Designing the TiB2/ZrO2 Multilayers
Abstract
1. Introduction
2. Experimental Details
3. Results and Discussion
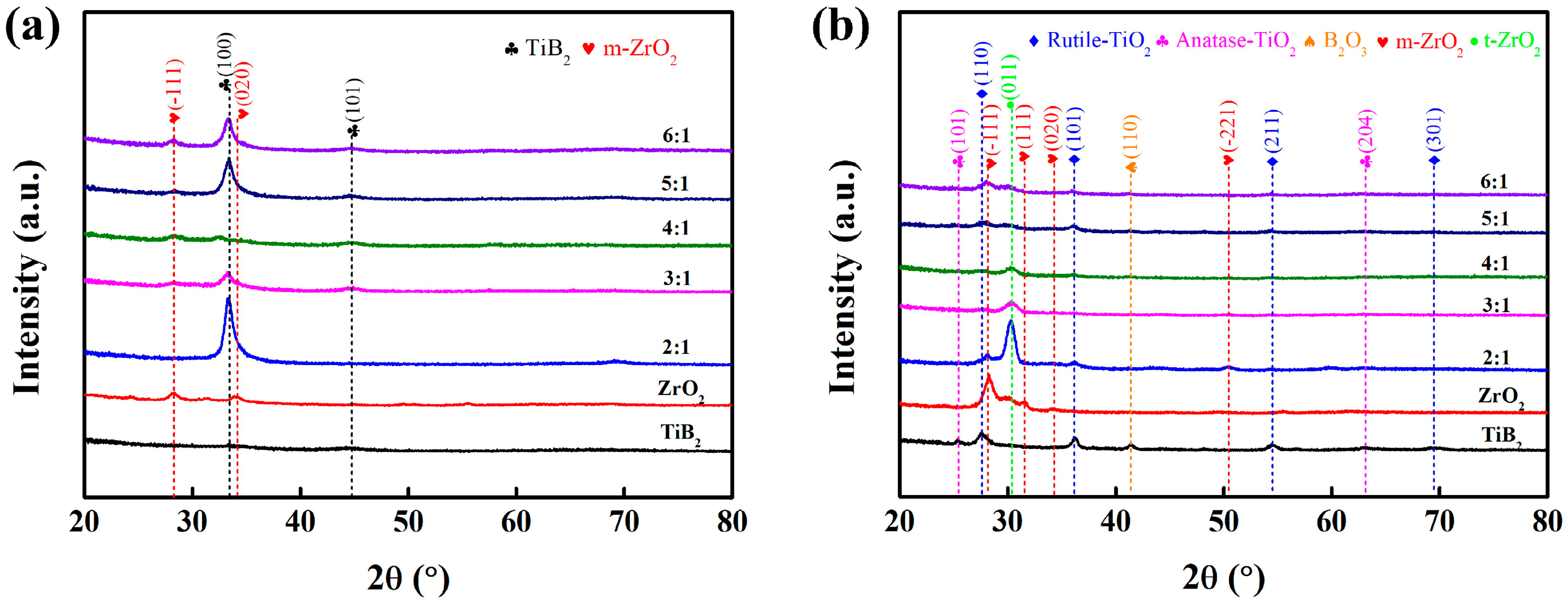
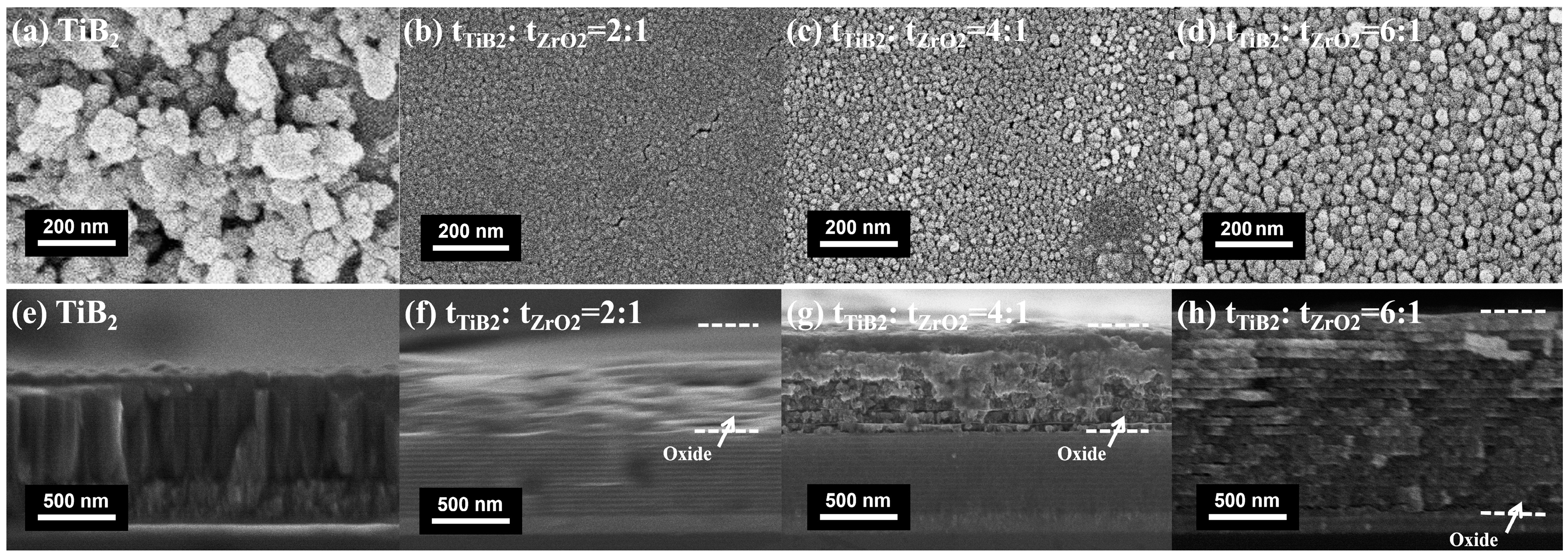
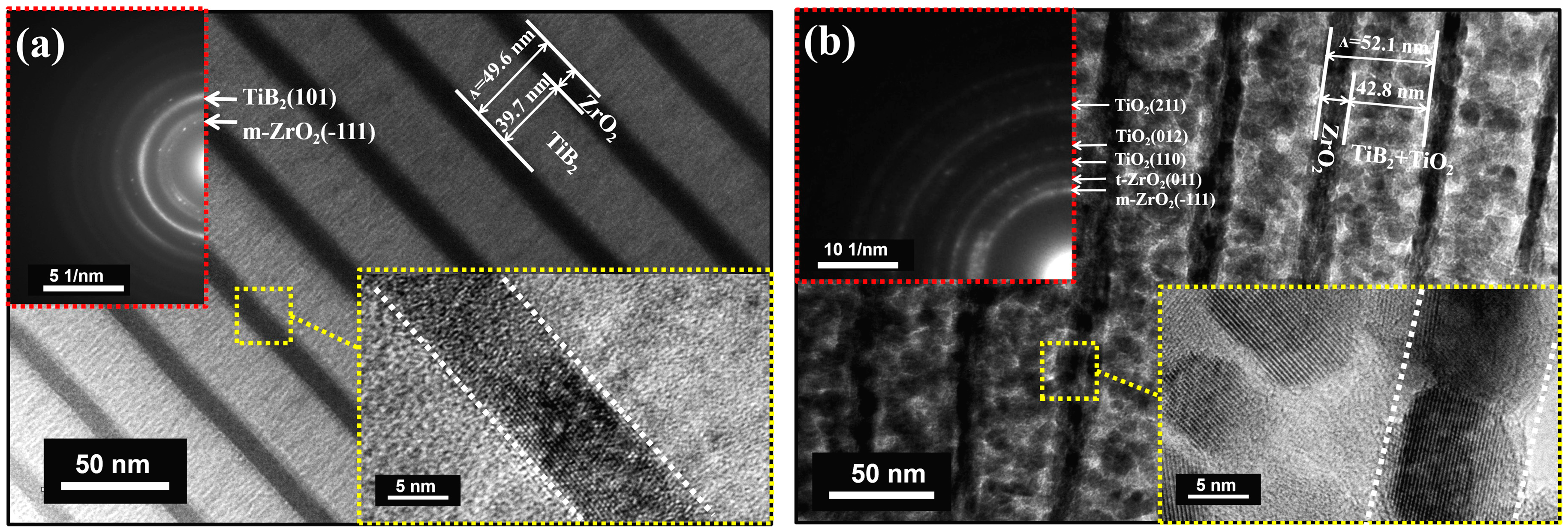
3.1. Microstructure and Crystallographic Characterization before and after Annealing in Air
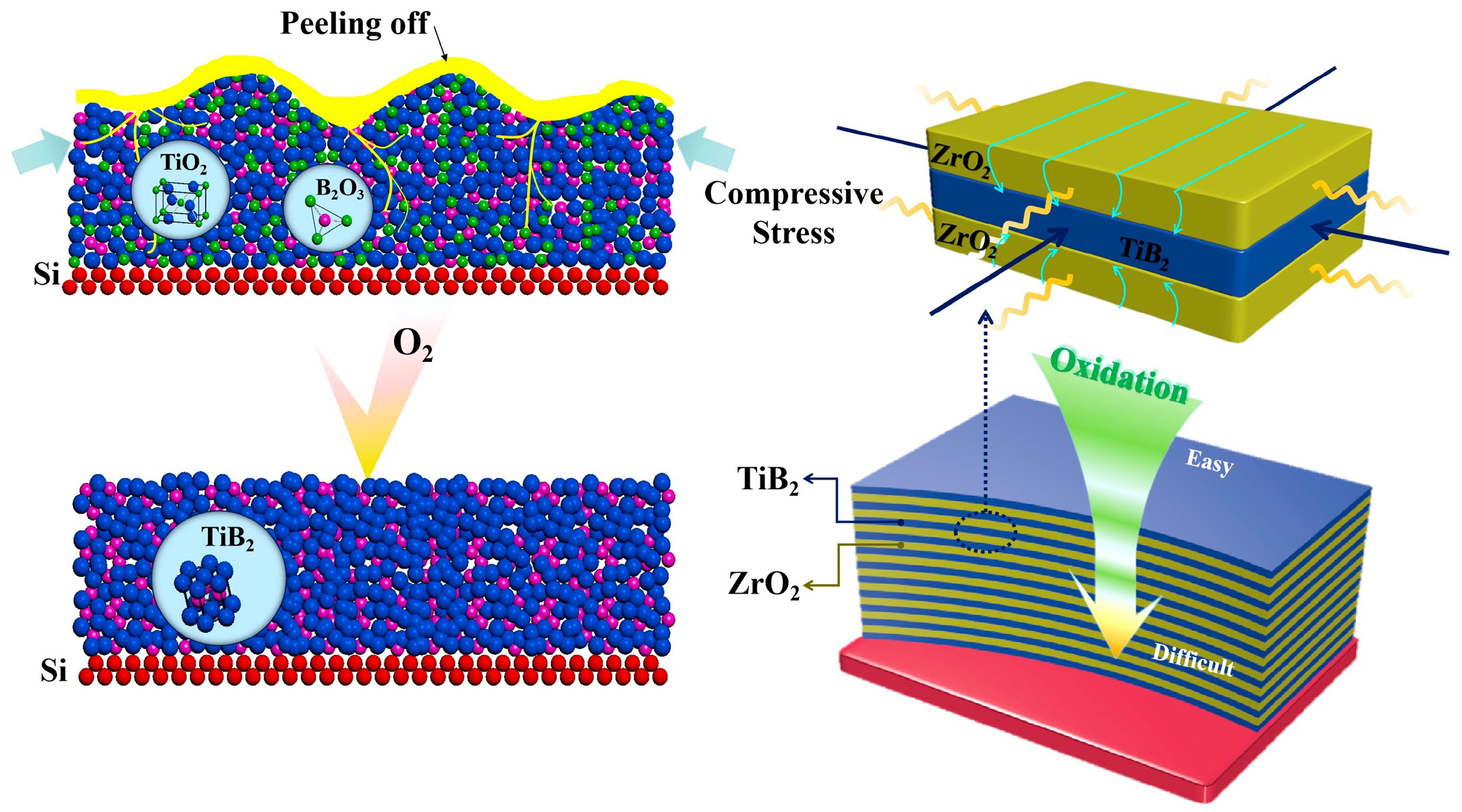
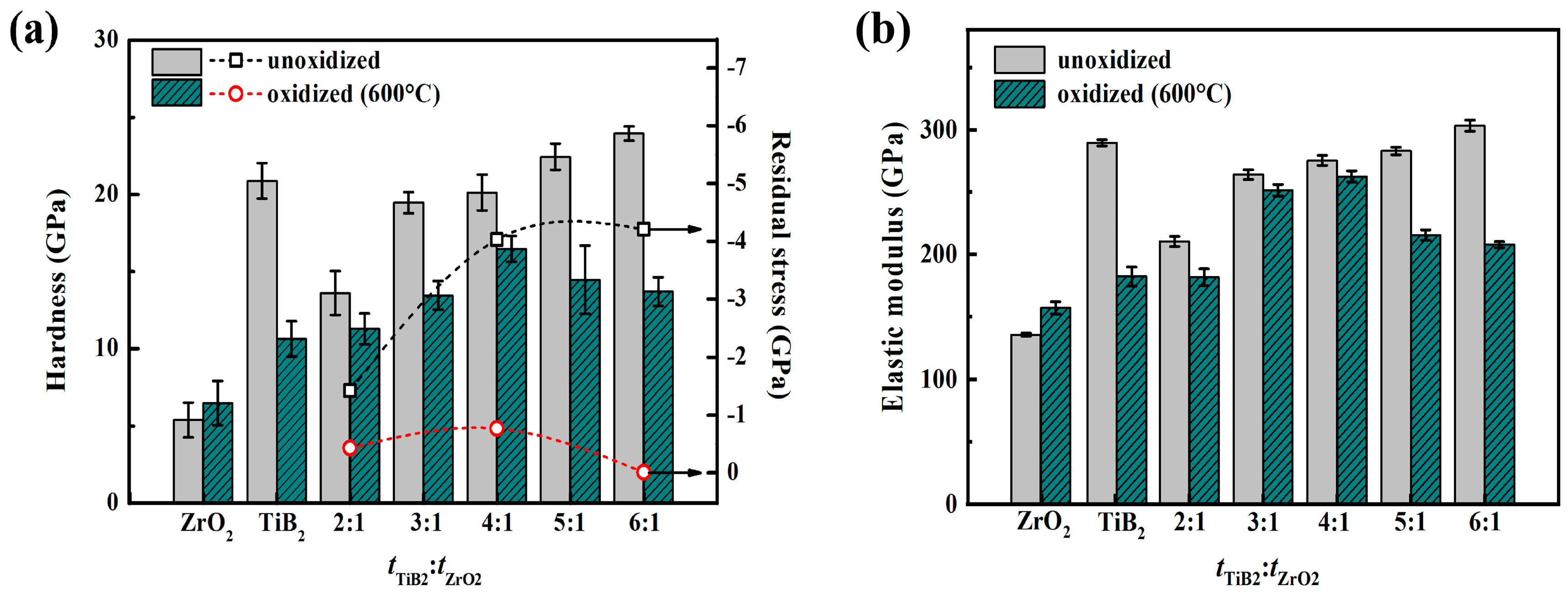
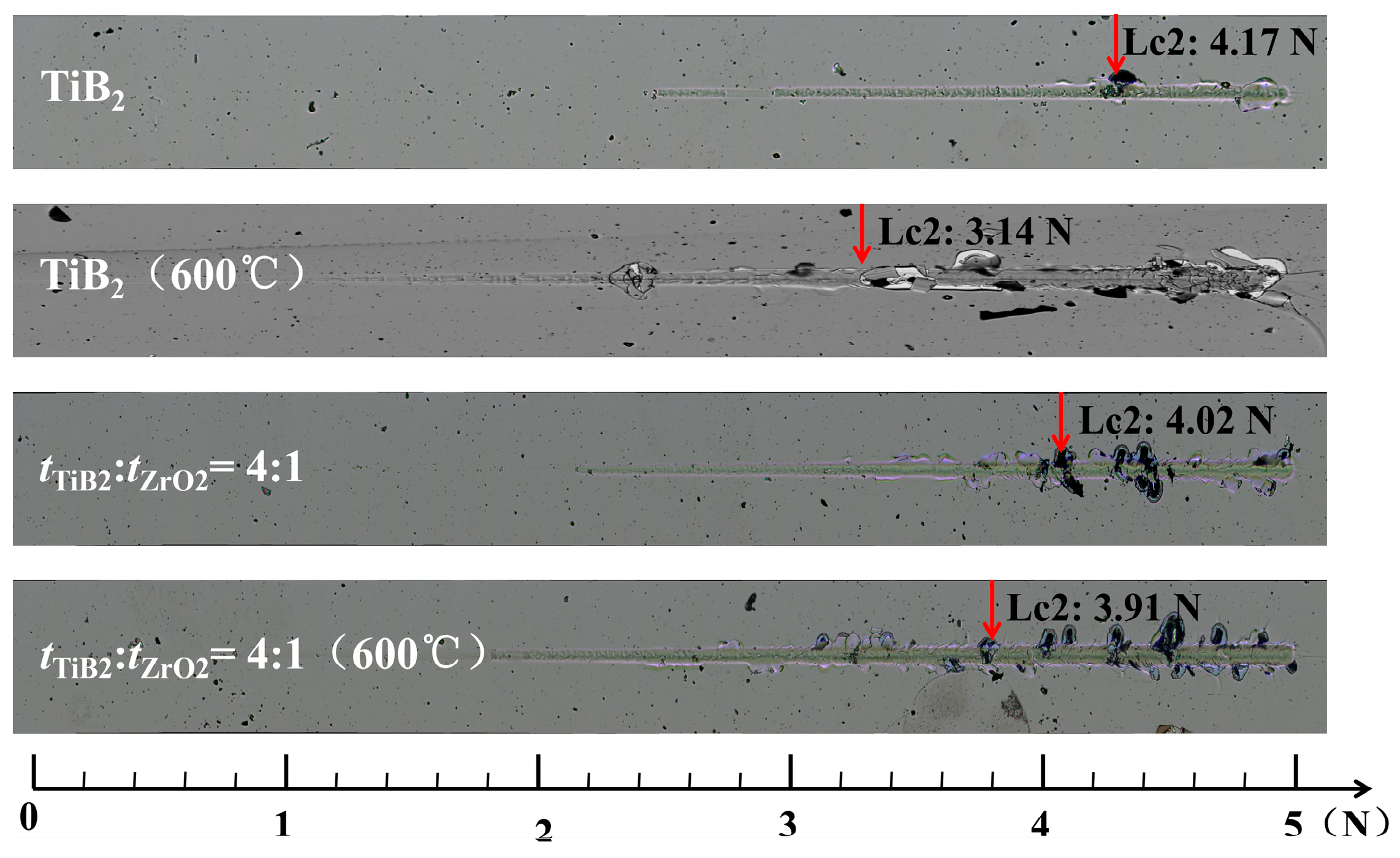
3.2. Mechanism of Improved Oxidation Resistance and Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Monclus, M.A.; Callisti, M.; Polcar, T.; Yang, L.W.; Llorca, J.; Molina-Aldareguia, J.M. Selective oxidation-induced strengthening of Zr/Nb nanoscale multilayers. Acta Mater. 2017, 122, 1–10. [Google Scholar] [CrossRef]
- Cho, S.; Jo, I.; Lee, Y.-H.; Yoo, Y.W.; Byon, E.; Lee, S.-K.; Lee, S.-B. Highly improved oxidation resistance of TiC-SKD11 composite by SiC/TiB2 based hybrid coating. Appl. Surf. Sci. 2018, 448, 407–415. [Google Scholar] [CrossRef]
- Shimada, S.; Takahashi, M.; Kiyono, H.; Tsujino, J. Coatings and microstructures of monolithic TiB2 films and double layer and composite TiCN/TiB2 films from alkoxide solutions by thermal plasma CVD. Thin Solid Films 2008, 516, 6616–6621. [Google Scholar] [CrossRef]
- Ma, A.; Jiang, M. The Study on oxidative dynamic of TiB2 ceramic. Ceramics 2006, 7, 19–21. (In Chinese) [Google Scholar] [CrossRef]
- Mokhayer, M.M.; Kakroudi, M.G.; Milani, S.S.; Ghiasi, H.; Vafa, N.P. Investigation of AlN addition on the microstructure and mechanical properties of TiB2 ceramics. Ceram. Int. 2019, 45, 16577–16583. [Google Scholar] [CrossRef]
- Zhirkov, I.; Petruhins, A.; Naslund, L.-A.; Kolozsvári, S.; Polcik, P.; Rosen, J. Vacuum arc plasma generation and thin film deposition from a TiB2 cathode. Appl. Phys. Lett. 2015, 107, 184103. [Google Scholar] [CrossRef]
- Li, H.; Sun, Y.; Zhang, J. Effect of ZrO2 particle on the performance of micro-arc oxidation coatings on Ti6Al4V. Appl. Surf. Sci. 2015, 342, 183–190. [Google Scholar] [CrossRef]
- Moon, J.; Choi, H.; Kim, H.; Lee, C. The effects of heat treatment on the phase transformation behavior of plasma-sprayed stabilized ZrO2 coatings. Surf. Coat. Technol. 2002, 155, 1–10. [Google Scholar] [CrossRef]
- Mahnicka-Goremikina, L.; Svinka, R.; Svinka, V. Influence of ZrO2 and WO3 doping additives on the thermal properties of porous mullite ceramics. Ceram. Int. 2018, 44, 16873–16879. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Liang, R.; Zhao, R.; Xiong, B.; Liu, H.; Tian, H.; Yang, Y.; Ren, T.-L. Switching dynamics of ferroelectric HfO2–ZrO2 with various ZrO2 contents. Appl. Phys. Lett. 2019, 114, 142902. [Google Scholar] [CrossRef]
- Ramana, C.V.; Vemuri, R.S.; Fernandez, I.; Campbell, A.L. Size-effects on the optical properties of zirconium oxide thin films. Appl. Phys. Lett. 2009, 95, 231905. [Google Scholar] [CrossRef]
- Yao, J.; He, Y.; Wang, D.; Lin, J. High-temperature oxidation resistance of (Al2O3–Y2O3)/(Y2O3-stabilized ZrO2) laminated coating on 8Nb–TiAl alloy prepared by a novel spray pyrolysis. Corrosion Sci. 2014, 80, 19–27. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, J.; Chen, J.; Zhao, J.; Yu, Y.; Zhou, H. Improvement of the oxidation and wear resistance of pure Ti by laser cladding at elevated temperature. Surf. Coat. Technol. 2010, 205, 2142–2151. [Google Scholar] [CrossRef]
- Ryabchikov, A.I.; Kashkarov, E.B.; Pushilina, N.S.; Syrtanov, M.S.; Shevelev, A.E.; Korneva, O.S.; Sutygina, A.N.; Lider, A.M. High-intensity low energy titanium ion implantation into zirconium alloy. Appl. Surf. Sci. 2018, 439, 106–112. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, P.; Zhao, H.; Wang, L.; Xie, A. Effect of different alloyed layers on the high temperature oxidation behavior of newly developed Ti2AlNb-based alloys. Appl. Surf. Sci. 2011, 257, 1835–1839. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Li, G. Coherent growth and superhardness effect in VC/Si3N4 nanomultilayers. Surf. Coat. Technol. 2011, 205, 3881–3884. [Google Scholar] [CrossRef]
- Fu, T.; Zhang, Z.; Peng, X.; Weng, S.; Miao, Y.; Zhao, Y.; Fu, S.; Hu, N. Effects of modulation periods on mechanical properties of V/VN nano-multilayers. Ceram. Int. 2019, 45, 10295–10303. [Google Scholar] [CrossRef]
- Yin, D.; Peng, X.; Qin, Y.; Wang, Z. Template effect in TiN/AlN multilayered coatings from first principles. Ceram. Int. 2015, 41, 10095–10101. [Google Scholar] [CrossRef]
- Shah, S.A.; Khan, A.F.; Khan, A.; Rahim, N.A.; Mehmood, M. Multilayer Si/Ge thin films with quantum confinement effects for photovoltaic applications. Appl. Surf. Sci. 2014, 296, 185–188. [Google Scholar] [CrossRef]
- Yang, F.J.; Wang, H.; Wang, H.B.; Cao, X.; Li, Q.; Zhou, M.J. Effect of interfacial diffusion on microstructure and properties of FePt/B4C multifunctional multilayer composite films. Appl. Surf. Sci. 2008, 254, 2516–2520. [Google Scholar] [CrossRef]
- Pan, Y.; Lei, D.; Na, L.; Yu, J.; Li, C.; Li, D. Investigating the influence of epitaxial modulation on the evolution of superhardness of the VN/TiB2 multilayers. Appl. Surf. Sci. 2016, 390, 406–411. [Google Scholar] [CrossRef]
- Junhua, X.; Geyang, L.; Mingyuan, G. The microstructure and mechanical properties of TaN/TiN and TaWN/TiN superlattice films. Thin Solid Films 2000, 370, 45–49. [Google Scholar] [CrossRef]
- Saladukhin, I.A.; Abadias, G.; Uglov, V.V.; Zlotski, S.V.; Michel, A.; Janse van Vuuren, A. Thermal stability and oxidation resistance of ZrSiN nanocomposite and ZrN/SiNx multilayered coatings: A comparative study. Surf. Coat. Technol. 2017, 332, 428–439. [Google Scholar] [CrossRef]
- Yao, X.; Li, H.; Zhang, Y.; Ren, J.; Yao, D.; Tao, J. A SiC/ZrB2–SiC/SiC oxidation resistance multilayer coating for carbon/carbon composites. Corrosion Sci. 2012, 57, 148–153. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, Y.; Fei, M.; Song, Z.; Li, Y. Oxidation resistance of TiAlN/ZrN multilayer coatings. Vacuum 2016, 127, 22–29. [Google Scholar] [CrossRef]
- Janssen, G.C.A.M.; Abdalla, M.M.; van Keulen, F.; Pujada, B.R.; van Venrooy, B. Celebrating the 100th anniversary of the Stoney equation for film stress: developments from polycrystalline steel strips to single crystal silicon wafers. Thin Solid Films 2009, 517, 1858–1867. [Google Scholar] [CrossRef]
- Legoues, F.K.; Mooney, P.M.; Tersoff, J. Measurement of the activation barrier to nucleation of dislocations in thin films. Phys. Rev. Lett. 1993, 71, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sheng, X.L.; Zhang, T.F.; Liu, M.; Xin, X.L. Raman spectrum and friction behaviors of carbon-doped TiB2 films prepared by magnetron sputtering. J. Mater. Eng. 2012, 2, 30–32. [Google Scholar] [CrossRef]
- Bondarev, A.V.; Kiryukhantsev-Korneev, P.V.; Levashov, E.A.; Shtansky, D.V. Tribological behavior and self-healing functionality of TiNbCN–Ag coatings in wide temperature range. Appl. Surf. Sci. 2017, 396, 110–120. [Google Scholar] [CrossRef]
- Maniu, D.; Iliescu, T.; Ardelean, I.; Cinta-Pinzaru, S.; Tarcea, N.; Kiefer, W. Raman study on B2O3–CaO glasses. J. Mol. Struct. 2003, 651–653, 485–488. [Google Scholar] [CrossRef]
- Song, Y.; Zheng, X.; Yu, G.; Zhao, J.; Jiang, L.; Liu, Y.; Yang, B.; Yang, Y. The characteristics of laser-driven shock wave investigated by time-resolved Raman spectroscopy. J. Raman Spectrosc. 2011, 42, 345–348. [Google Scholar] [CrossRef]
- Tampieri, A.; Bellosi, A. Oxidation of monolithic TiB2 and of Al2O3−TiB2composite. J. Mater. Sci. 1993, 28, 649–653. [Google Scholar] [CrossRef]
- Tao, G.; He, J.; Pang, X.; Volinsky, A.A.; Su, Y.; Qiao, L. High temperature brittle film adhesion measured from annealing-induced circular blisters. Acta Mater. 2017, 138, 1–9. [Google Scholar] [CrossRef]
- Liang, B.; Chen, H. Overview of the application and development of ZrO2 coatings (films). Bull. Chin. Ceram. Soc. 2003. (In Chinese) [Google Scholar] [CrossRef]
- Dang, C.; Li, J.; Wang, Y.; Chen, J. Structure, mechanical and tribological properties of self-toughening TiSiN/Ag multilayer coatings on Ti6Al4V prepared by arc ion plating. Appl. Surf. Sci. 2016, 386, 224–233. [Google Scholar] [CrossRef]
- Xiao, B.; Li, H.; Mei, H.; Dai, W.; Zuo, F.; Wu, Z.; Wang, Q. A study of oxidation behavior of AlTiN- and AlCrN-based multilayer coatings. Surf. Coat. Technol. 2018, 333, 229–237. [Google Scholar] [CrossRef]
- Xu, Y.X.; Riedl, H.; Holec, D.; Li, C.; Yong, D.; Mayrhofer, P.H. Thermal stability and oxidation resistance of sputtered Ti−Al−Cr−N hard coatings. Surf. Coat. Technol. 2017, 324, 48–56. [Google Scholar] [CrossRef]
- Dong, K.F.; Li, H.H.; Peng, Y.G.; Ju, G.; Chow, G.M.; Chen, J.S. L10FePt−ZrO2 (001) nanostructured films with high aspect ratio columnar grains. Appl. Phys. Lett. 2014, 104, 192404. [Google Scholar] [CrossRef]
- Sarkar, B.K.; Mukherjee, M.K.; Natarajan, A. A modification of the rule of mixture in estimating strengths of a composite. Mater. Sci. Eng. Technol. 1982, 13, 269–273. [Google Scholar] [CrossRef]
- Bouaouina, B.; Besnard, A.; Abaidia, S.E.; Haid, F. Residual stress, mechanical and microstructure properties of multilayer Mo2N/CrN coating produced by R.F Magnetron discharge. Appl. Surf. Sci. 2017, 395, 117–121. [Google Scholar] [CrossRef]

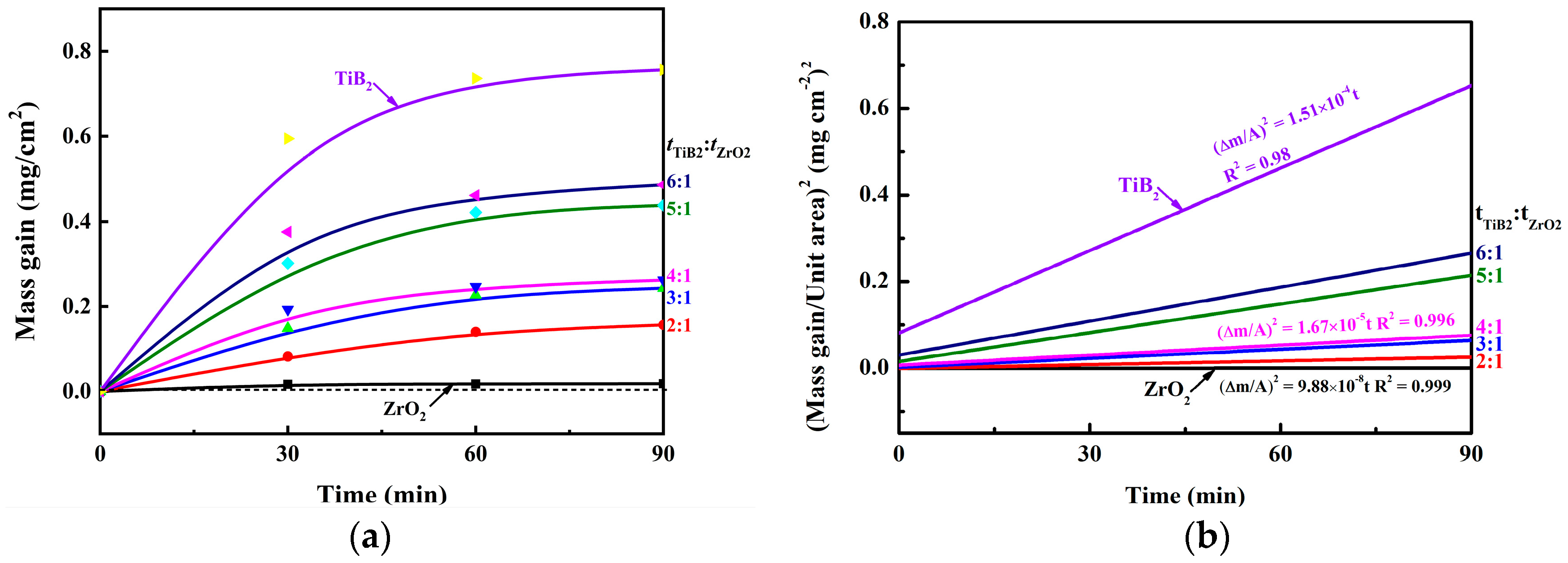
| Coating Style | Absolute Temperature (K) | K (mg2 cm−4 t−1) | R2 (Correlation Coefficient) | Ea (KJ mol−1) |
|---|---|---|---|---|
| ZrO2 | 873.15 | 9.88 × 10−8 | 0.99 | 140.87 |
| tTiB₂:tZrO₂ =2:1 | 873.15 | 4.58 × 10−6 | 0.99 | 107.34 |
| tTiB₂:tZrO₂ =3:1 | 873.15 | 1.25 × 10−5 | 0.99 | 98.60 |
| tTiB₂:tZrO₂ =4:1 | 873.15 | 1.67 × 10−5 | 0.99 | 96.07 |
| tTiB₂:tZrO₂ =5:1 | 873.15 | 4.52 × 10−5 | 0.99 | 87.34 |
| tTiB₂:tZrO₂ =6:1 | 873.15 | 6.05 × 10−5 | 0.98 | 84.83 |
| TiB2 | 873.15 | 1.51 × 10−5 | 0.98 | 76.84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, D.; Xu, Y.; Dong, L.; Wu, J.; Zhao, M.; Li, D. Optimization of the Oxidation Behavior and Mechanical Properties by Designing the TiB2/ZrO2 Multilayers. Coatings 2019, 9, 600. https://doi.org/10.3390/coatings9100600
Mao D, Xu Y, Dong L, Wu J, Zhao M, Li D. Optimization of the Oxidation Behavior and Mechanical Properties by Designing the TiB2/ZrO2 Multilayers. Coatings. 2019; 9(10):600. https://doi.org/10.3390/coatings9100600
Chicago/Turabian StyleMao, Dong, Yang Xu, Lei Dong, Jie Wu, Mengli Zhao, and Dejun Li. 2019. "Optimization of the Oxidation Behavior and Mechanical Properties by Designing the TiB2/ZrO2 Multilayers" Coatings 9, no. 10: 600. https://doi.org/10.3390/coatings9100600
APA StyleMao, D., Xu, Y., Dong, L., Wu, J., Zhao, M., & Li, D. (2019). Optimization of the Oxidation Behavior and Mechanical Properties by Designing the TiB2/ZrO2 Multilayers. Coatings, 9(10), 600. https://doi.org/10.3390/coatings9100600





