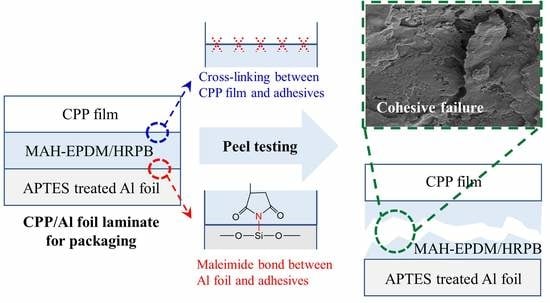
High-Performance Adhesives Based on Maleic Anhydride-g-EPDM Rubbers and Polybutene for Laminating Cast Polypropylene Film and Aluminum Foil
Abstract
:1. Introduction
2. Experiment
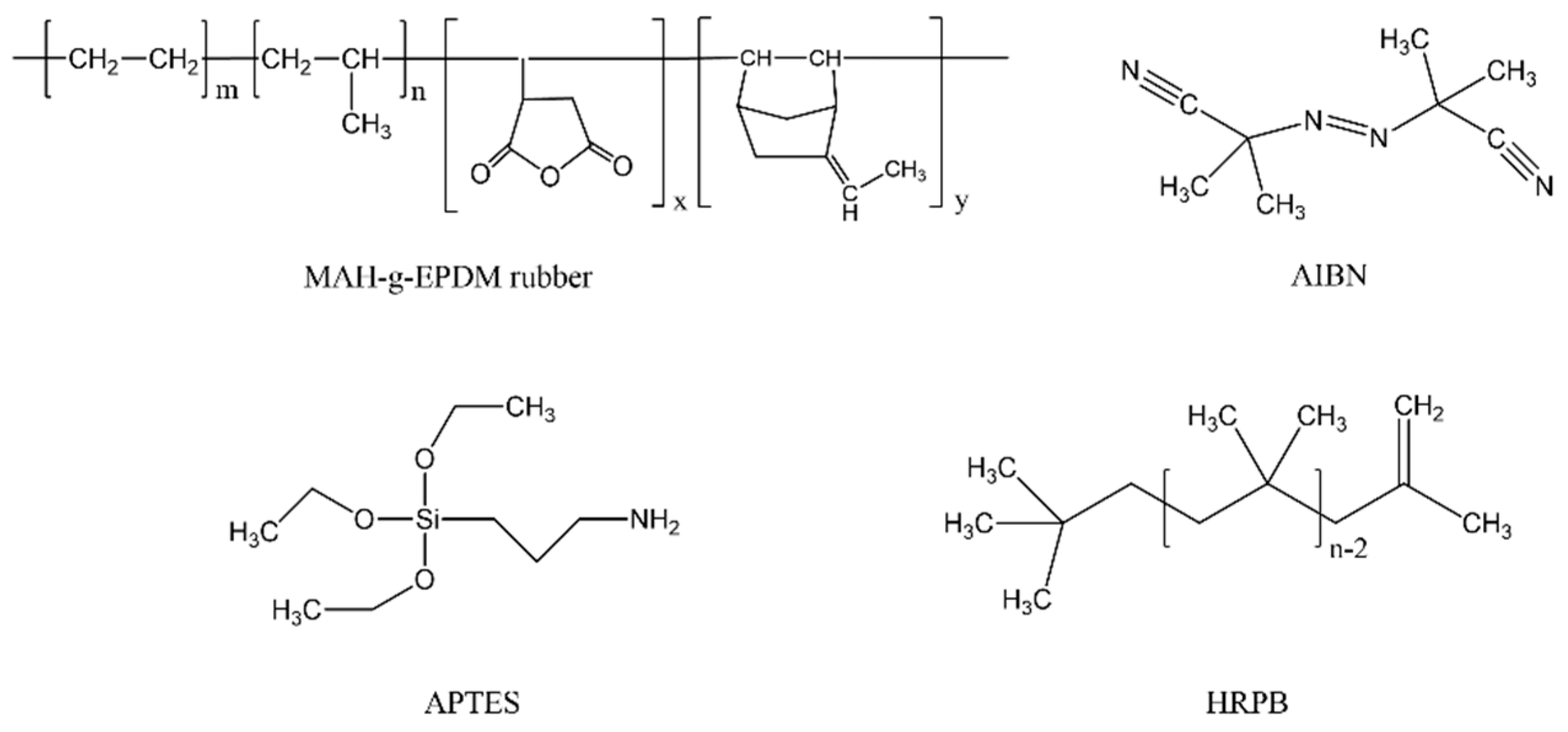
2.1. Materials
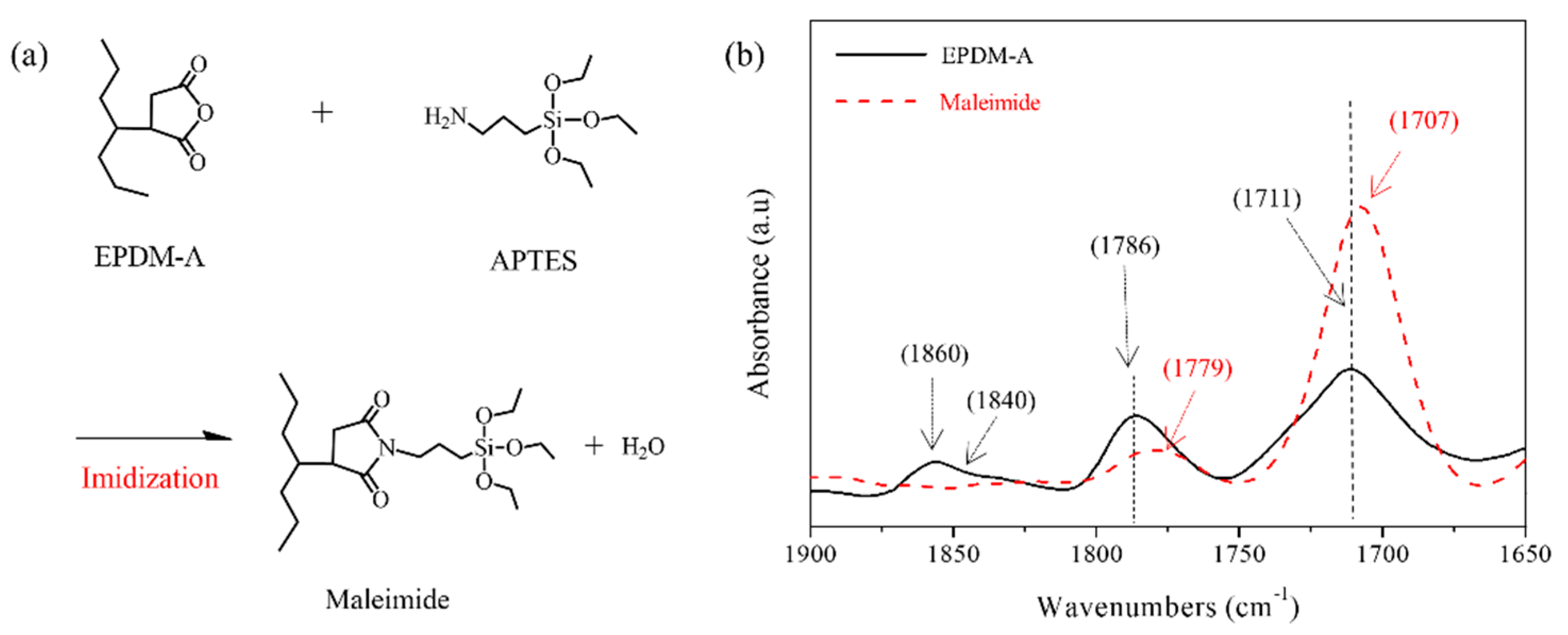
2.2. Adhesive Preparation
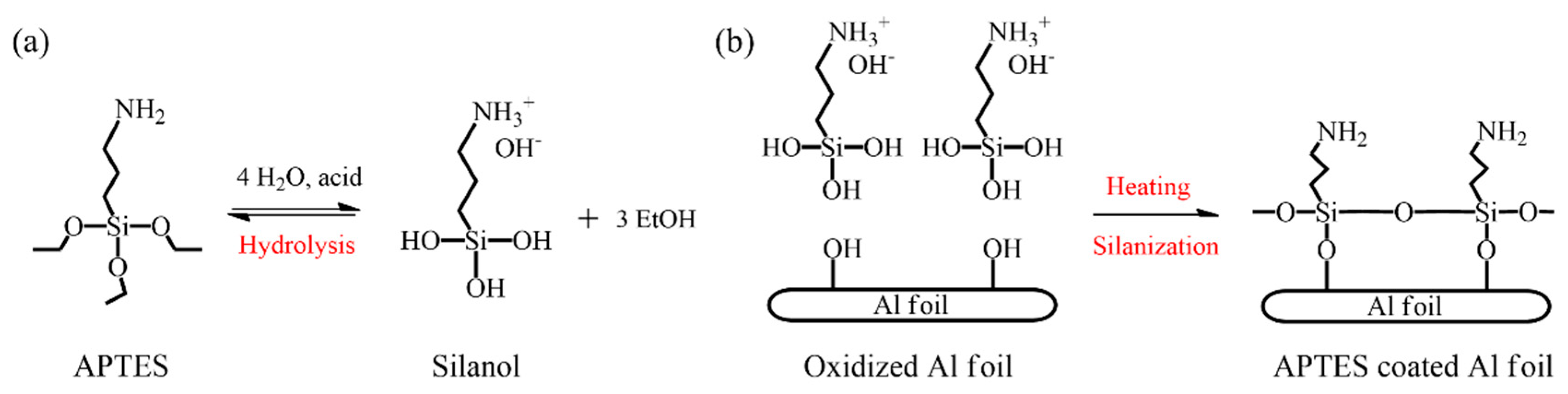
2.3. Modification of Al Surface
2.4. Adhesive Coating on Al Foil and Lamination with CPP
2.5. Characterization
3. Results and Discussion
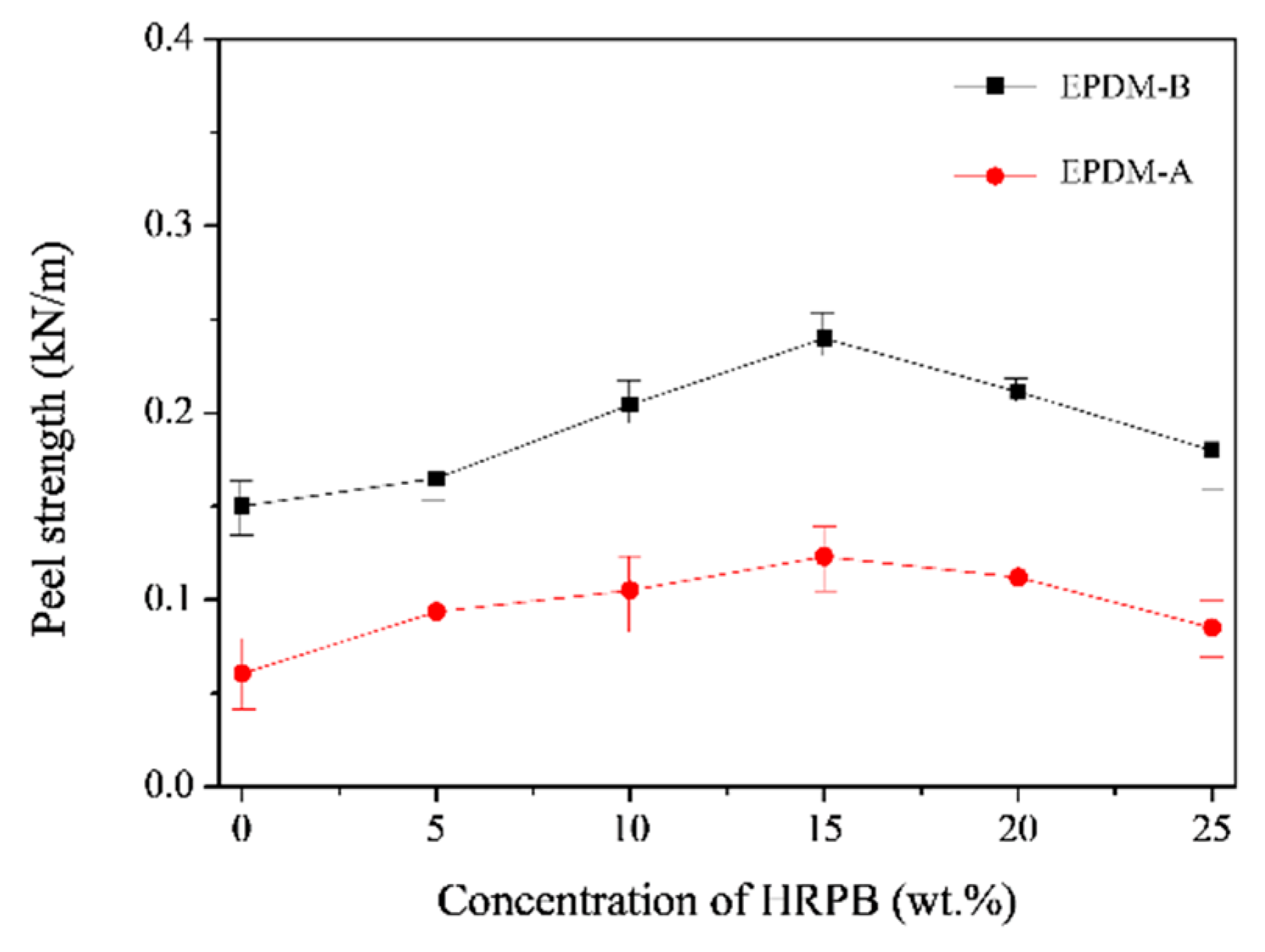
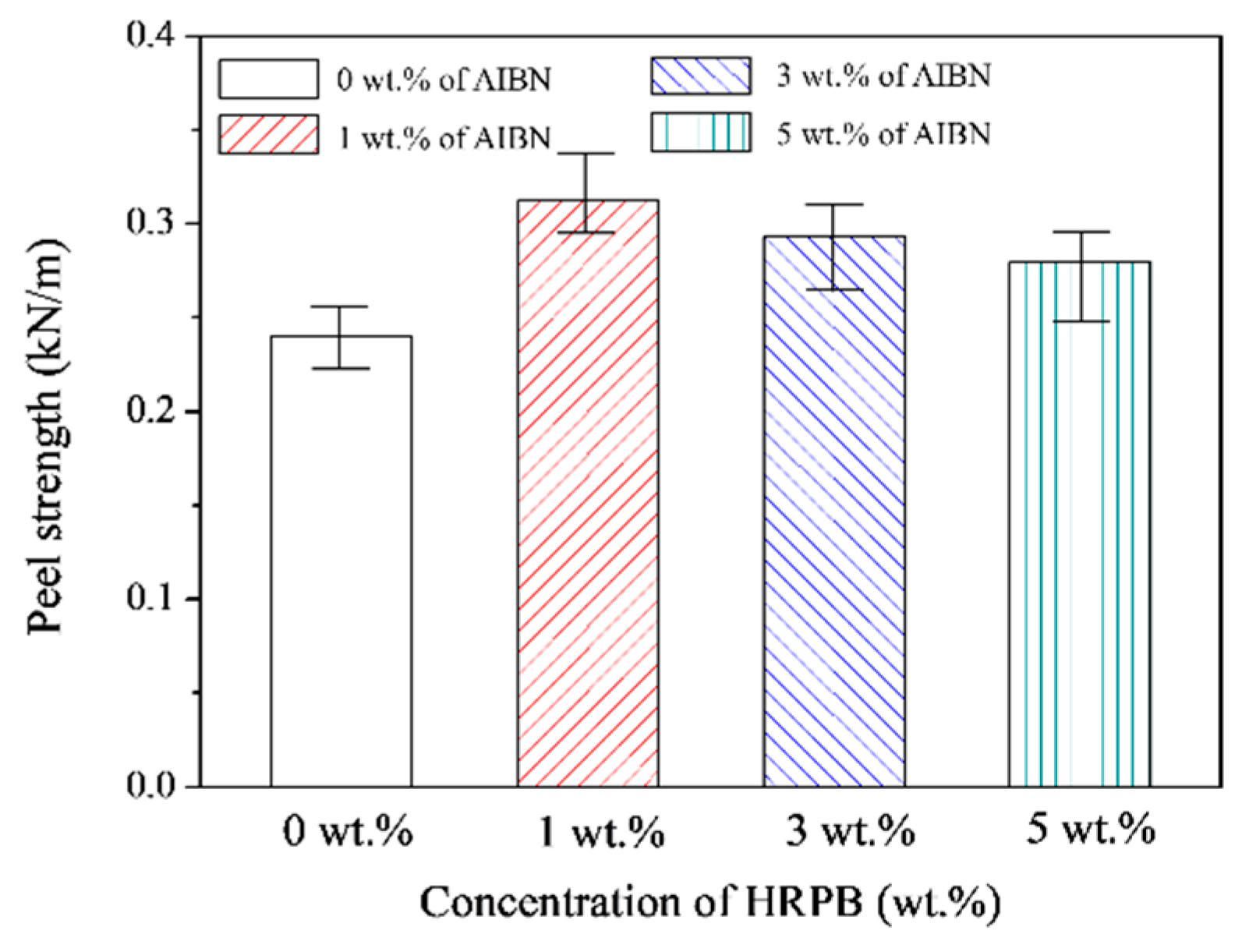
3.1. Adhesion Based on EPDM-A, EPDM-C and HRPB
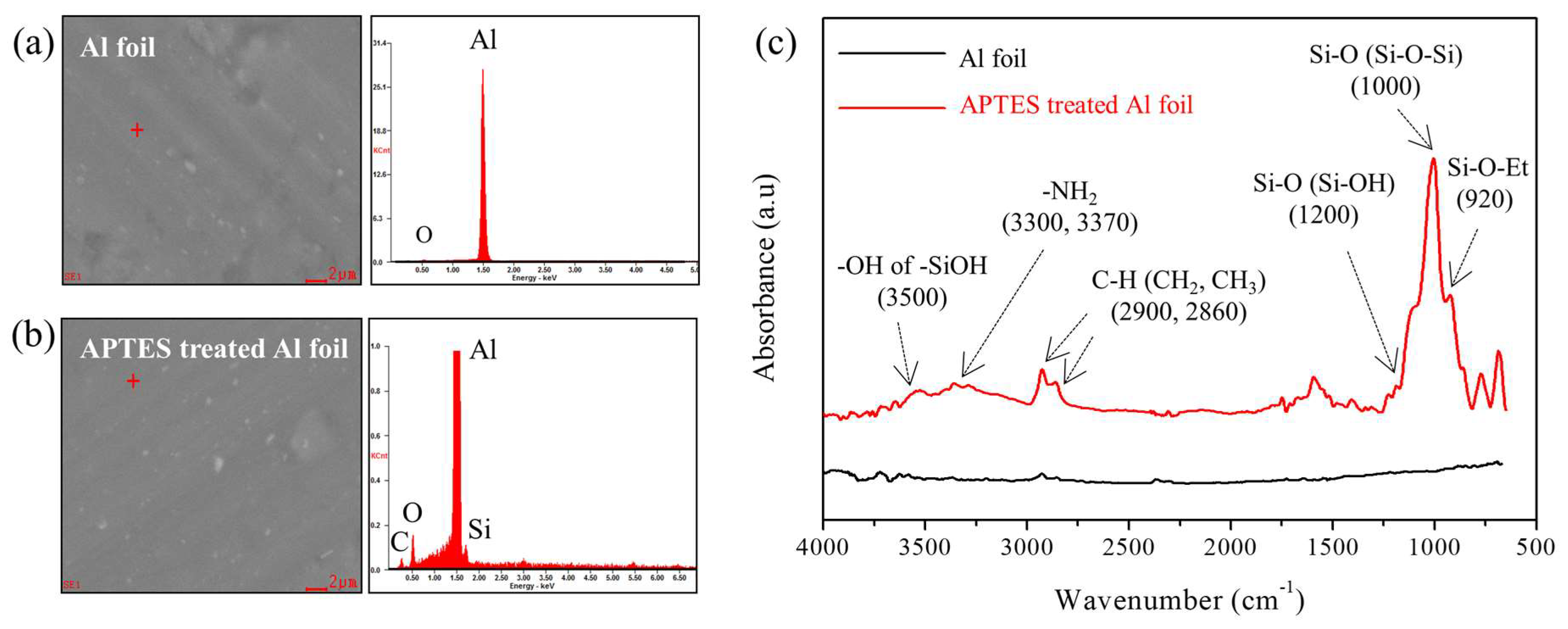
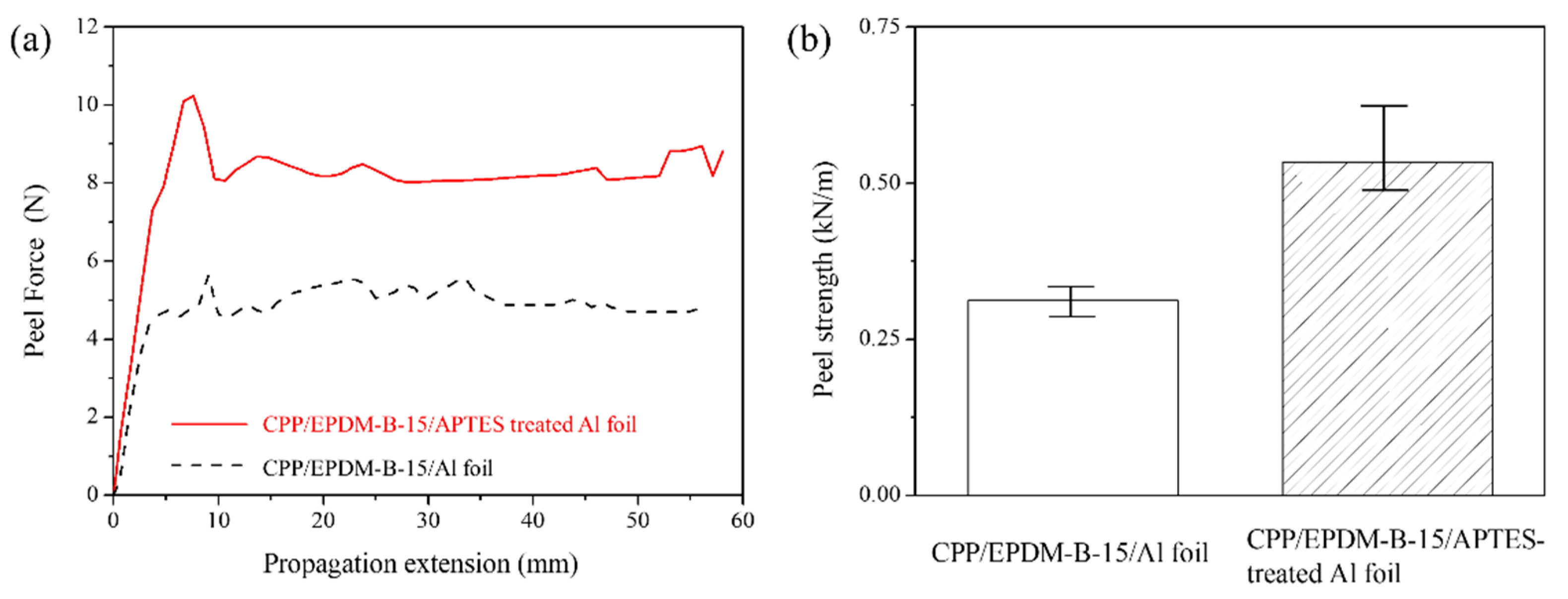
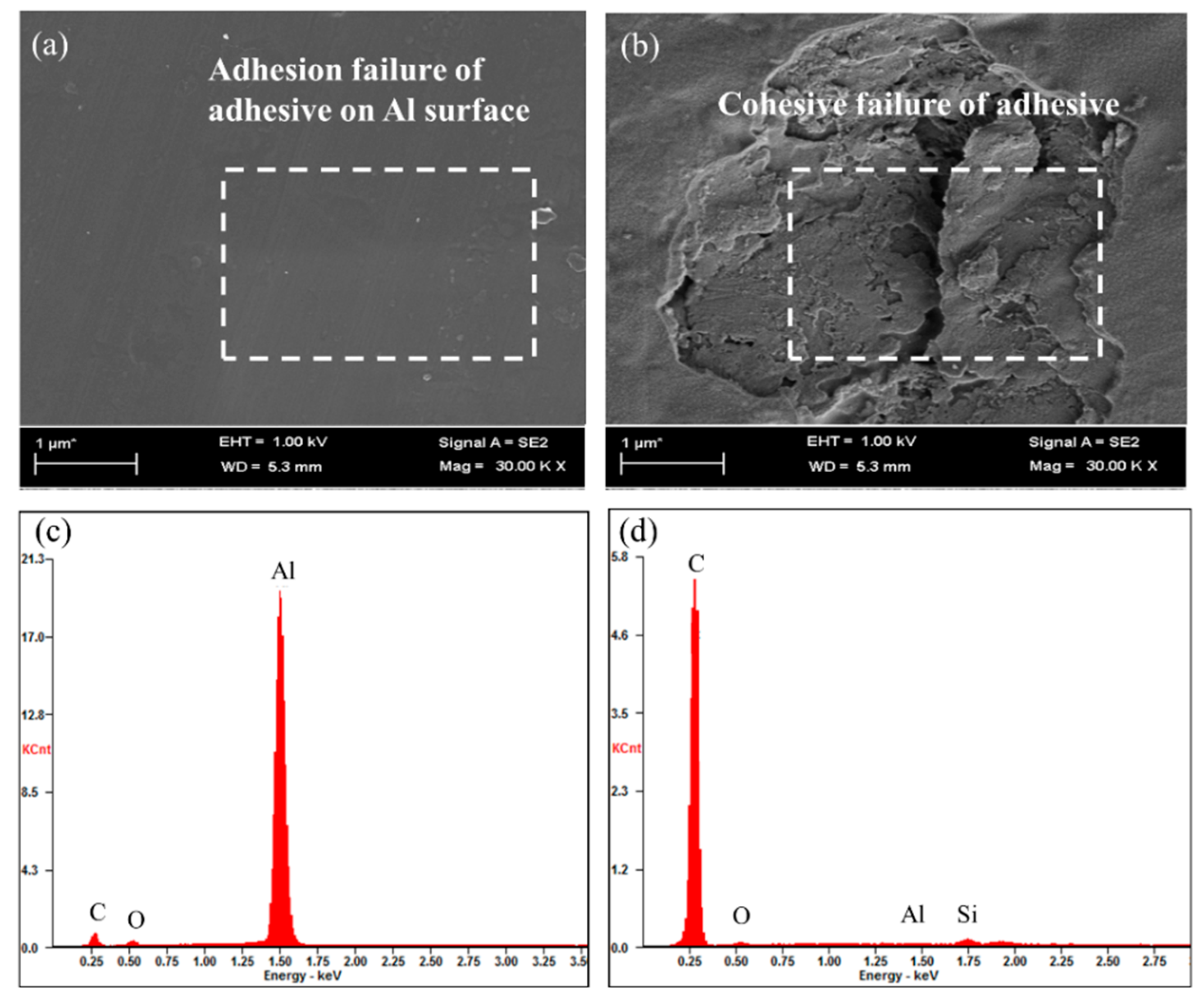
3.2. Surface Treatment of Al Foil with APTES
3.3. Adhesive Strength of CPP/APTES-Treated Al Foil Laminates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xia, F.; Xu, S. Effect of surface pre-treatment on the hydrophilicity and adhesive properties of multilayered laminate used for lithium battery packaging. Appl. Surf. Sci. 2013, 268, 337–342. [Google Scholar] [CrossRef]
- Nakai, M.; Eto, T. New aspects of development of high strength aluminum alloys for aerospace applications. Mater. Sci. Eng. A 2000, 285, 62–68. [Google Scholar] [CrossRef]
- Subasri, R.; Jyothirmayi, A.; Reddy, D.S. Effect of plasma surface treatment and heat treatment ambience on mechanical and corrosion protection properties of hybrid sol-gel coatings on aluminum. Surf. Coat. Technol. 2010, 205, 806–813. [Google Scholar] [CrossRef]
- Joshi, S.P.; Toma, R.B.; Medora, N.; O’Connor, K. Detection of aluminium residue in sauces packaged in aluminium pouches. Food Chem. 2003, 83, 383–386. [Google Scholar] [CrossRef]
- Keles, O.; Dundar, M. Aluminum foil: Its typical quality problems and their causes. J. Mater. Process. Technol. 2007, 186, 125–137. [Google Scholar] [CrossRef]
- Byun, Y.; Bae, H.J.; Cooksey, K.; Whiteside, S. Comparison of the quality and storage stability of salmon packaged in various retort pouches. LWT Food Sci. Technol. 2010, 43, 551–555. [Google Scholar] [CrossRef]
- Ashley, R.J.; Cochran, M.A.; Allen, K.W. Adhesives in packaging. Int. J. Adhes. Adhes. 1995, 15, 101–108. [Google Scholar] [CrossRef]
- Zand, B.N.; Mahdavian, M. Corrosion and adhesion study of polyurethane coating on silane pretreated aluminum. Surf. Coat. Technol. 2009, 203, 1677–1681. [Google Scholar] [CrossRef]
- Zand, B.N.; Mahdavian, M. Evaluation of the effect of vinyltrimethoxysilane on corrosion resistance and adhesion strength of epoxy coated AA1050. Electrochim. Acta 2007, 52, 6438–6442. [Google Scholar] [CrossRef]
- Niknahad, M.; Moradian, S.; Mirabedini, S.M. The adhesion properties and corrosion performance of differently pretreated epoxy coatings on an aluminium alloy. Corros. Sci. 2010, 52, 1948–1957. [Google Scholar] [CrossRef]
- Leena, K.; Athira, K.K.; Bhuvaneswari, S.; Suraj, S.; Rao, V.L. Effect of surface pre-treatment on surface characteristics and adhesive bond strength of aluminium alloy. Int. J. Adhes. Adhes. 2016, 70, 265–270. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Shen, Y.; Liu, S.; Wang, W.; Tao, J. Improvement of adhesion performance between aluminum alloy sheet and epoxy based on anodizing technique. Int. J. Adhes. Adhes. 2016, 70, 74–80. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Huo, Q. Self-assembled monolayer of 3-aminopropyltrimethoxysilane for improved adhesion between aluminum alloy substrate and polyurethane coating. Thin Solid Films 2007, 515, 7181–7189. [Google Scholar] [CrossRef]
- Taniguchi, T. Packaging Material for Lithium Ion Battery. U.S. Patent 9,601,724, 21 March 2017. [Google Scholar]
- Taniguchi, T. Outer Package Material for Lithium-on Battery and Method for Producing Lithium-on Battery Using the Outer Package Materal. U.S. Patent 9,627,660, 18 April 2017. [Google Scholar]
- Liang, C.; Lv, Z.; Bo, Y.; Cui, J.; Xu, S. Effect of modified polypropylene on the interfacial bonding of polymer—Aluminum laminated films. J. Mater. 2015, 81, 141–148. [Google Scholar] [CrossRef]
- Shin, K.S.; Kim, K.J.; Choi, S.-W.; Rhee, M.H. Mechanical properties of aluminum/polypropylene/aluminum sandwich sheets. Met. Mater. 1999, 5, 613–618. [Google Scholar] [CrossRef]
- Nielsen, A.S.; Batchelder, D.N.; Pyrz, R. Estimation of crystallinity of isotactic polypropylene using raman spectroscopy. Polymer 2002, 43, 2671–2676. [Google Scholar] [CrossRef]
- Parenteau, T.; Ausias, G.; Grohens, Y.; Pilvin, P. Structure, mechanical properties and modelling of polypropylene for different degrees of crystallinity. Polymer 2012, 53, 5873–5884. [Google Scholar] [CrossRef]
- Tall, S.; Albertsson, A.C.; Karlsson, S. Enhanced rigidity of recycled polypropylen from packaging waste by compounding with talc and high crystallinity polypropylene. Polym. Adv. Technol. 2001, 12, 279–284. [Google Scholar] [CrossRef]
- Wang, K.H.; Choi, M.H.; Koo, C.M.; Choi, Y.S.; Chung, I.J. Synthesis and characterization of maleated polyethylene/clays nanocomposites. Polymer 2001, 42, 9819–9826. [Google Scholar] [CrossRef]
- Baldwin, F.P.; Strate, G.V. Polyolefin elastomers based on ethylene and propylene. Rubber Chem. Technol. 1972, 45, 709–881. [Google Scholar] [CrossRef]
- Cesca, S. The chemistry of unsaturated ethylene-propylene based terpolymers. J. Polym. Sci. Macromol. Rev. 1975, 10, 1–230. [Google Scholar] [CrossRef]
- Grestenberger, G.; Potter, G.D.; Grein, C. Polypropylene/ethylene-propylene rubber (PP/EPR) blends for the automotive industry: Basic correlations between EPR-design and shrinkage. Express Polym. Lett. 2014, 8, 282–292. [Google Scholar] [CrossRef]
- Danesi, S.; Porter, R.S. Blends of isotactic polypropylene and ethylene-propylene rubbers: Rheology, morphology and mechanics. Polymer 1978, 19, 448–457. [Google Scholar] [CrossRef]
- Greco, R.; Mancarella, C.; Martuscelli, E.; Ragosta, G.; Yin, J. Polyolefin blends: 2. Effect of EPR composition on structure, morphology and mechanical properties of IPP/EPR alloys. Polymer 1987, 28, 1929–1936. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Kalló, A.; Kuleznev, V.N. Phase structure of impact-modified polypropylene blends. Polymer 1984, 25, 279–286. [Google Scholar] [CrossRef]
- Pukanszky, B.; Tüdös, F.; Kallo, A.; Bodor, G. Effect of multiple morphology on the properties of polypropylene/ethylene-propylene-diene terpolymer blends. Polymer 1987, 30, 1407–1413. [Google Scholar] [CrossRef]
- Van Der Wal, A.; Gaymans, R.J. Polypropylene-rubber blends: 5. Deformation mechanism during fracture. Polymer 1999, 40, 6067–6075. [Google Scholar] [CrossRef]
- Gaca, M.; Zaborski, M. The Properties of ethylene–propylene elastomers obtained with the use of a new cross-linking substance. J. Therm. Anal. Calorim. 2016, 125, 1105–1113. [Google Scholar] [CrossRef]
- Polgar, L.M.; Kingma, A.; Roelfs, M.; van Essen, M.; van Duin, M.; Picchioni, F. Kinetics of cross-linking and de-cross-linking of EPM rubber with thermoreversible Diels-Alder chemistry. Eur. Polym. J. 2017, 90, 150–161. [Google Scholar] [CrossRef]
- Zhang, H.; Datta, R.N.; Talma, A.G.; Noordermeer, J.W.M. Maleic-anhydride grafted EPM as compatibilising agent in NR/BR/EPDM blends. Eur. Polym. J. 2010, 46, 754–766. [Google Scholar] [CrossRef]
- Grigoryeva, O.P.; Karger-Kocsis, J. Melt grafting of maleic anhydride onto an ethylene-propylene-diene terpolymer (EPDM). Eur. Polym. J. 2000, 36, 1419–1429. [Google Scholar] [CrossRef]
- Manjhi, S.; Sarkhel, G. Effect of maleic anhydride grafted ethylene propylene diene monomer (MAH-g-EPDM) on the properties of kaolin reinforced EPDM rubber. J. Appl. Polym. Sci. 2011, 119, 2268–2274. [Google Scholar] [CrossRef]
- Pasbakhsh, P.; Ismail, H.; Fauzi, M.N.A.; Bakar, A.A. Influence of maleic anhydride grafted ethylene propylene diene monomer (MAH-g-EPDM) on the properties of EPDM nanocomposites reinforced by halloysite nanotubes. Polym. Test. 2009, 28, 548–559. [Google Scholar] [CrossRef]
- Salmah, H.; Ruzaidi, C.M.; Supri, A.G. Compatibilisation of polypropylene/ethylene propylene diene terpolymer/kaolin composites: The effect of maleic anhydride-grafted-polypropylene. J. Phys. Sci. 2009, 20, 99–107. [Google Scholar]
- López Manchado, M.A.; Arroyo, M.; Biagiotti, J.; Kenny, J.M. Enhancement of mechanical properties and interfacial adhesion of PP/EPDM/flax fiber composites using maleic anhydride as a compatibilizer. J. Appl. Polym. Sci. 2003, 90, 2170–2178. [Google Scholar] [CrossRef]
- Barra, G.M.O.; Crespo, J.S.; Bertolino, J.R.; Soldi, V.; Pires, A.T.N. Maleic anhydride grafting on EPDM: Qualitative and quantitative determination. J. Braz. Chem. Soc. 1999, 10, 31–34. [Google Scholar] [CrossRef]
- Jiang, Z.; Du, Z.; Xue, J.; Liu, W.; Li, M.; Tang, T. Hierarchical structure and properties of rigid PVC foam crosslinked by the reaction between anhydride and diisocyanate. J. Appl. Polym. Sci. 2018, 135, 46141. [Google Scholar] [CrossRef]
- Zhou, M.; He, Y.; Chen, Y.; Yang, Y.; Lin, H.; Han, S. Synthesis and evaluation of terpolymers consist of methacrylates with maleic anhydride and methacrylic morpholine and their amine compound as pour point depressants in diesel fuels. Energy Fuels 2015, 29, 5618–5624. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, Z.C.; Ma, N.; Liu, H.L.; Zhan, X.Q.; Li, B.; Gao, W.; Tsai, F.C.; Jiang, T.; Chang, C.J.; et al. Effect of surface modified kaolin on properties of polypropylene grafted maleic anhydride. Results Phys. 2017, 7, 969–974. [Google Scholar] [CrossRef]
- Guo, H.; Meador, M.A.; McCorkle, L.S.; Scheiman, D.A.; McCrone, J.D.; Wilkewitz, B. Poly(maleic anhydride) cross-linked polyimide aerogels: synthesis and properties. RSC Adv. 2016, 6, 26055–26065. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Y.; Chen, J.; Li, H.; Ji, J.; Liu, D. Chemical modification of palygorskite with maleic anhydride modified polypropylene: mechanical properties, morphology, and crystal structure of palygorskite/polypropylene nanocomposites. Appl. Clay Sci. 2015, 115, 230–237. [Google Scholar] [CrossRef]
- Heravi, M.M.; Hashemi, E.; Beheshtiha, Y.S.; Ahmadi, S.; Hosseinnejad, T. PdCl2 on modified poly(styrene-co-maleic anhydride): A highly active and recyclable catalyst for the Suzuki-Miyaura and Sonogashira reactions. J. Mol. Catal. A Chem. 2014, 394, 74–82. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.H.; Hyun, T.O.; Jeon, H.K. The effect of weld-lines on the morphology and mechanical properties of amorphous polyamide/poly(ethylene-ran-propylene) blend with various amounts of an in situ compatibilizer. Polymer 2001, 42, 2209–2221. [Google Scholar] [CrossRef]
- Koriyama, H.; Oyama, H.T.; Ougizawa, T.; Inoue, T.; Weber, M.; Koch, E. Studies on the reactive polysulfone-polyamide interface: Interfacial thickness and adhesion. Polymer 1999, 40, 6381–6393. [Google Scholar] [CrossRef]
- Cecchetto, L.; Denoyelle, A.; Delabouglise, D.; Petit, J.P. A silane pre-treatment for improving corrosion resistance performances of emeraldine base-coated aluminium samples in neutral environment. Appl. Surf. Sci. 2008, 254, 1736–1743. [Google Scholar] [CrossRef]
- Van der Wal, A.; Mulder, J.J.; Oderkerk, J.; Gaymans, R.J. Polypropylène-rubber blends: 1. The effect of the matrix properties on the impact behaviour. Polymer 1998, 39, 6781–6787. [Google Scholar] [CrossRef]
- Chatterjee, K.; Naskar, K. Development of thermoplastic elastomers based on maleated ethylene propylene rubber (m-EPM) and polypropylene (PP) by dynamic vulcanization. Express Polym. Lett. 2007, 1, 527–534. [Google Scholar] [CrossRef]
- Loan, L.D. Mechanism of peroxide vulcanization of elastomers. Rubber Chem. Technol. 1967, 40, 149–176. [Google Scholar] [CrossRef]
- Baldwin, F.P.; Borzel, P.; Cohen, C.A.; Makowski, H.S.; Van de Castle, J.F. The influence of residual olefin structure on EPDM vulcanization. Rubber Chem. Technol. 1970, 43, 522–548. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Reminiscing on silane coupling agents. J. Adhes. Sci. Technol. 1991, 5, 261–277. [Google Scholar] [CrossRef]
- Kunst, S.R.; Beltrami, L.V.; Cardoso, H.R.; Santana, J.A.; Sarmento, V.H.; Müller, I.L.; Malfatti, C.D. Characterization of siloxane-poly(methyl methacrylate) hybrid films obtained on a tinplate substrate modified by the addition of organic and inorganic acids. Mater. Res. 2015, 18, 151–163. [Google Scholar] [CrossRef]
- Cui, L.; Paul, D.R. Evaluation of amine functionalized polypropylenes as compatibilizers for polypropylene nanocomposites. Polymer 2007, 48, 1632–1640. [Google Scholar] [CrossRef]
- Song, P.; Yu, Y.; Wu, Q.; Fu, S. Facile fabrication of HDPE-g-MA/nanodiamond nanocomposites via one-step reactive blending. Nanoscale Res. Lett. 2012, 7, 2–18. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Peijs, T.; Camino, G. POSS grafting on PPgMA by one-step reactive blending. Polymer 2009, 50, 218–226. [Google Scholar] [CrossRef]
- Perrier, S.; Takolpuckdee, P.; Mars, C.A. Reversible addition—Fragmentation chain transfer polymerization: End group modification for functionalized polymers and chain transfer agent recovery. Macromolecules 2005, 38, 2033–2036. [Google Scholar] [CrossRef]

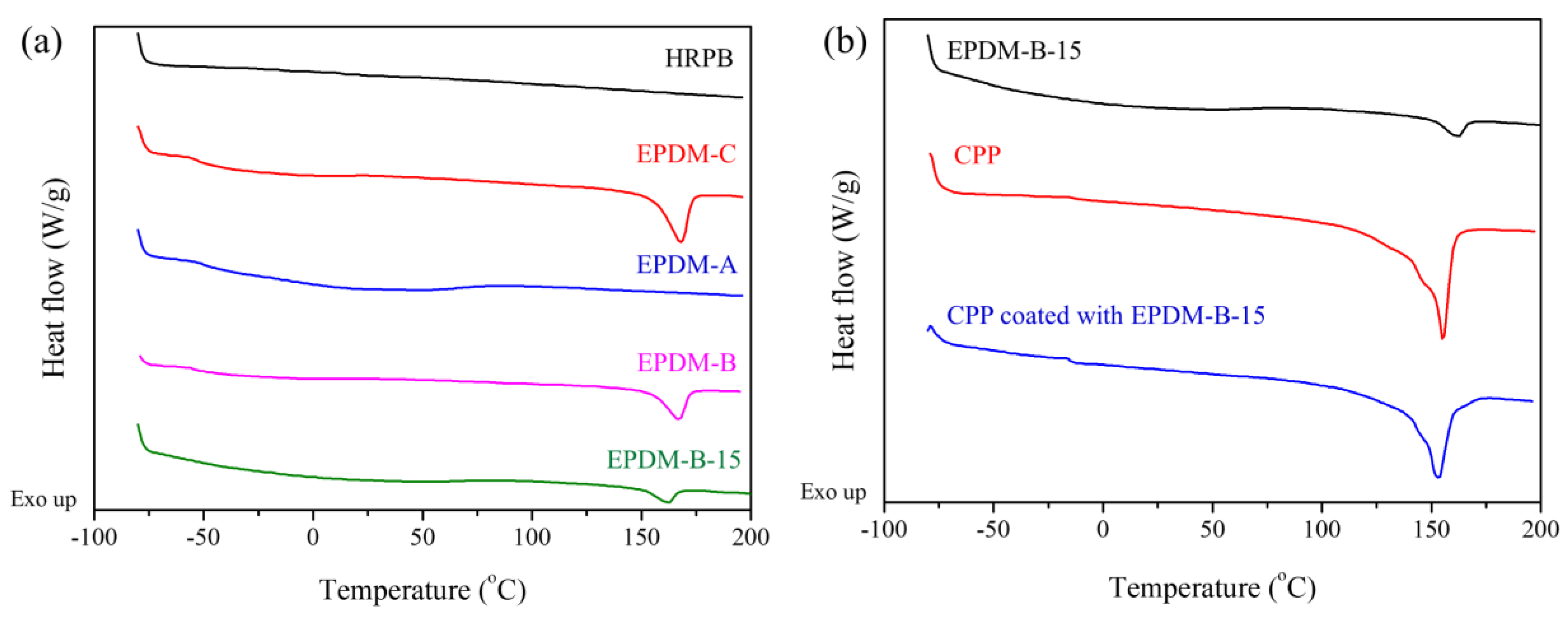

| Thermal Properties | Samples | ||||
|---|---|---|---|---|---|
| EPDM-C | EPDM-B | EPDM-B-15 | CPP | CPP Coated with EPDM-B-15 | |
| Tm (°C) | 168 | 167 | 162 | 155 | 152 |
| ΔHm (J/g) | 24 | 16 | 5 | 50 | 38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-H.; Yang, S.-W.; Park, E.-S.; Hwang, J.-Y.; Lee, D.-S. High-Performance Adhesives Based on Maleic Anhydride-g-EPDM Rubbers and Polybutene for Laminating Cast Polypropylene Film and Aluminum Foil. Coatings 2019, 9, 61. https://doi.org/10.3390/coatings9010061
Lee S-H, Yang S-W, Park E-S, Hwang J-Y, Lee D-S. High-Performance Adhesives Based on Maleic Anhydride-g-EPDM Rubbers and Polybutene for Laminating Cast Polypropylene Film and Aluminum Foil. Coatings. 2019; 9(1):61. https://doi.org/10.3390/coatings9010061
Chicago/Turabian StyleLee, Sang-Hyub, Se-Woo Yang, Eun-Suk Park, Ji-Young Hwang, and Dai-Soo Lee. 2019. "High-Performance Adhesives Based on Maleic Anhydride-g-EPDM Rubbers and Polybutene for Laminating Cast Polypropylene Film and Aluminum Foil" Coatings 9, no. 1: 61. https://doi.org/10.3390/coatings9010061
APA StyleLee, S.-H., Yang, S.-W., Park, E.-S., Hwang, J.-Y., & Lee, D.-S. (2019). High-Performance Adhesives Based on Maleic Anhydride-g-EPDM Rubbers and Polybutene for Laminating Cast Polypropylene Film and Aluminum Foil. Coatings, 9(1), 61. https://doi.org/10.3390/coatings9010061