Effect of Synthesis Conditions on the Controlled Growth of MgAl–LDH Corrosion Resistance Film: Structure and Corrosion Resistance Properties
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Mg–Al Layered Double Hydroxide Film
3. Characterization
4. Results and Discussion
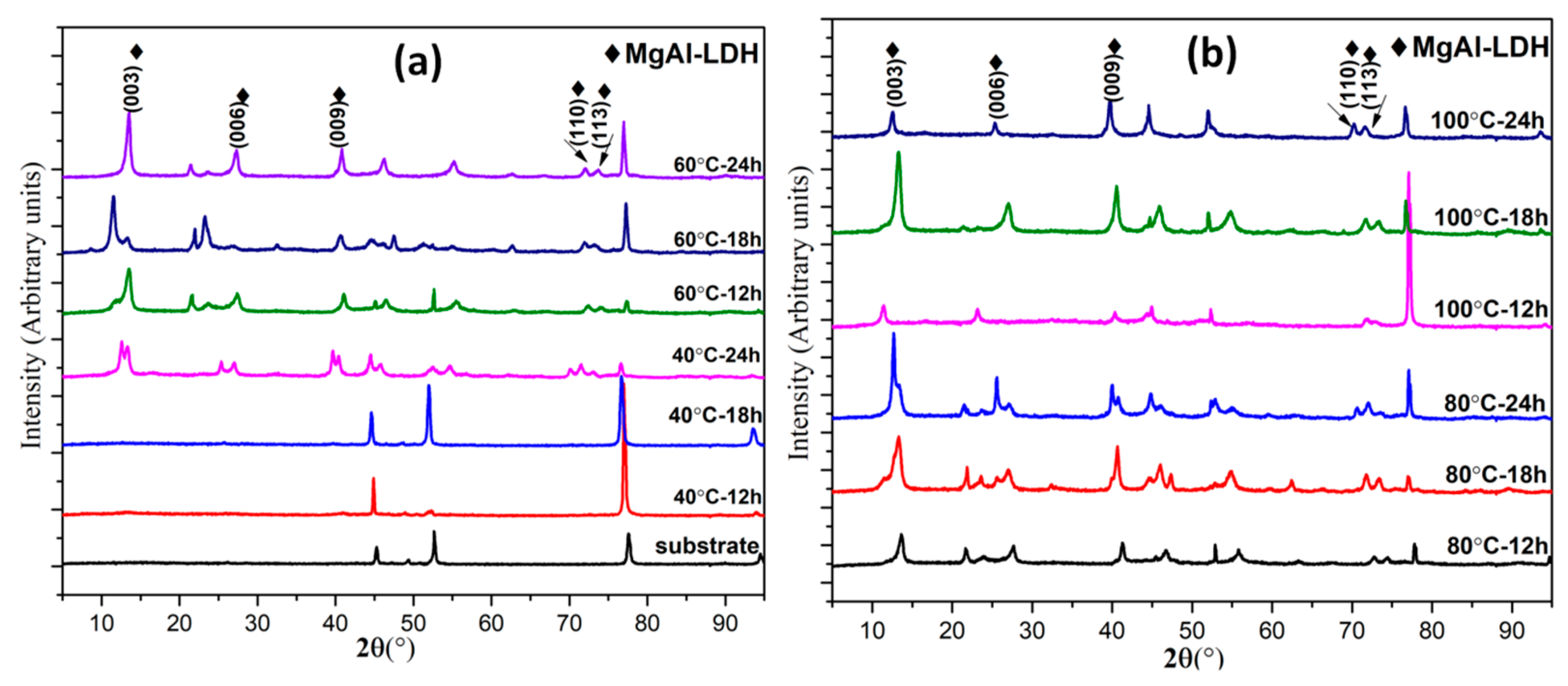
4.1. XRD Analysis
4.2. Fourier Transform Infrared Spectroscopy (FTIR)
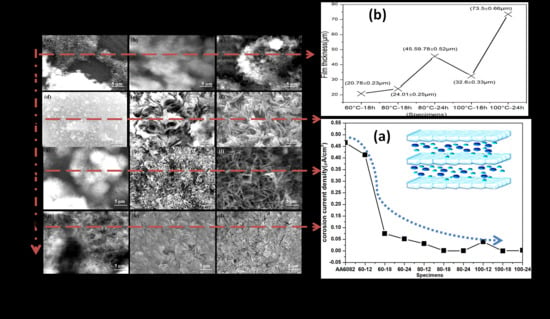
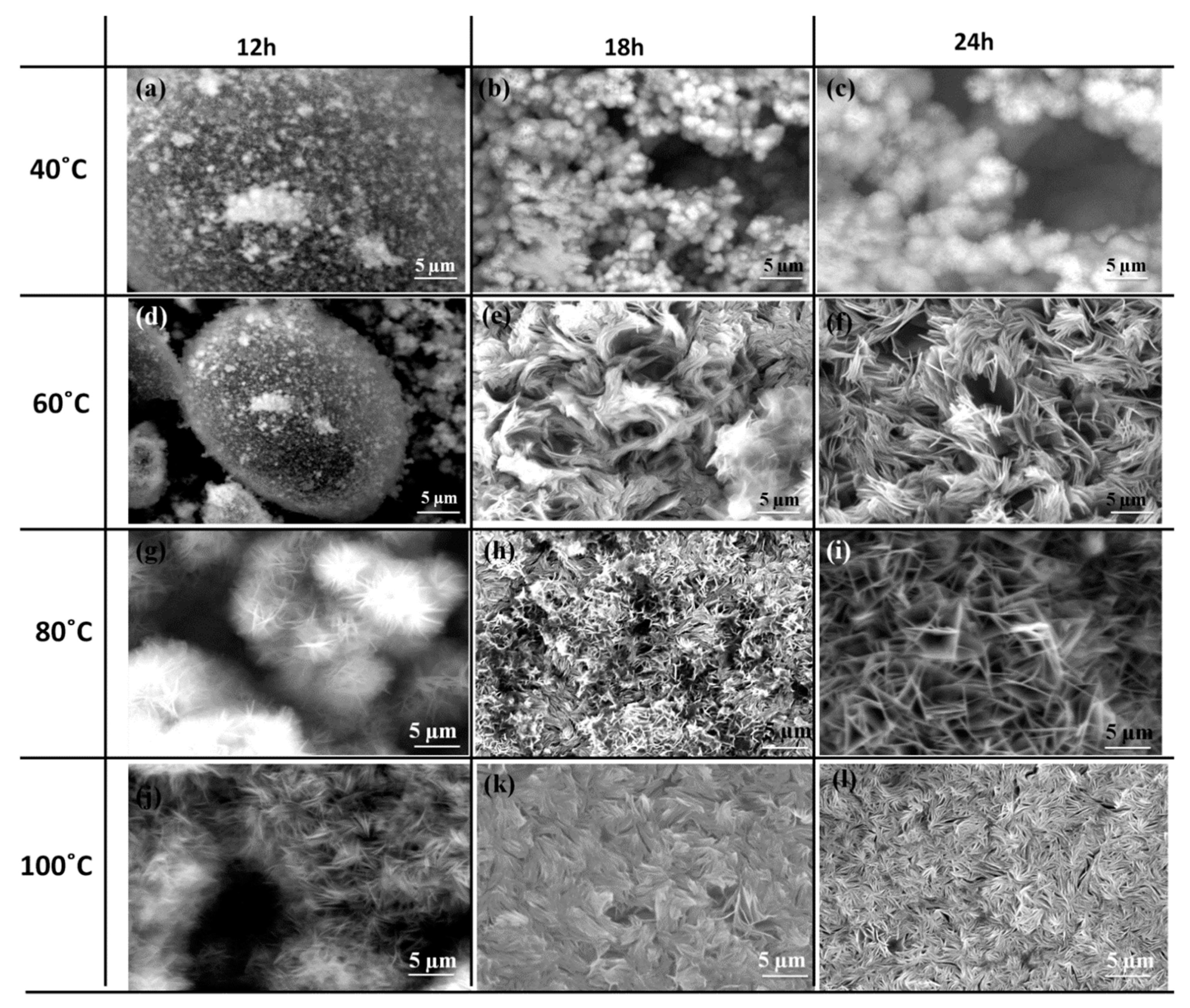
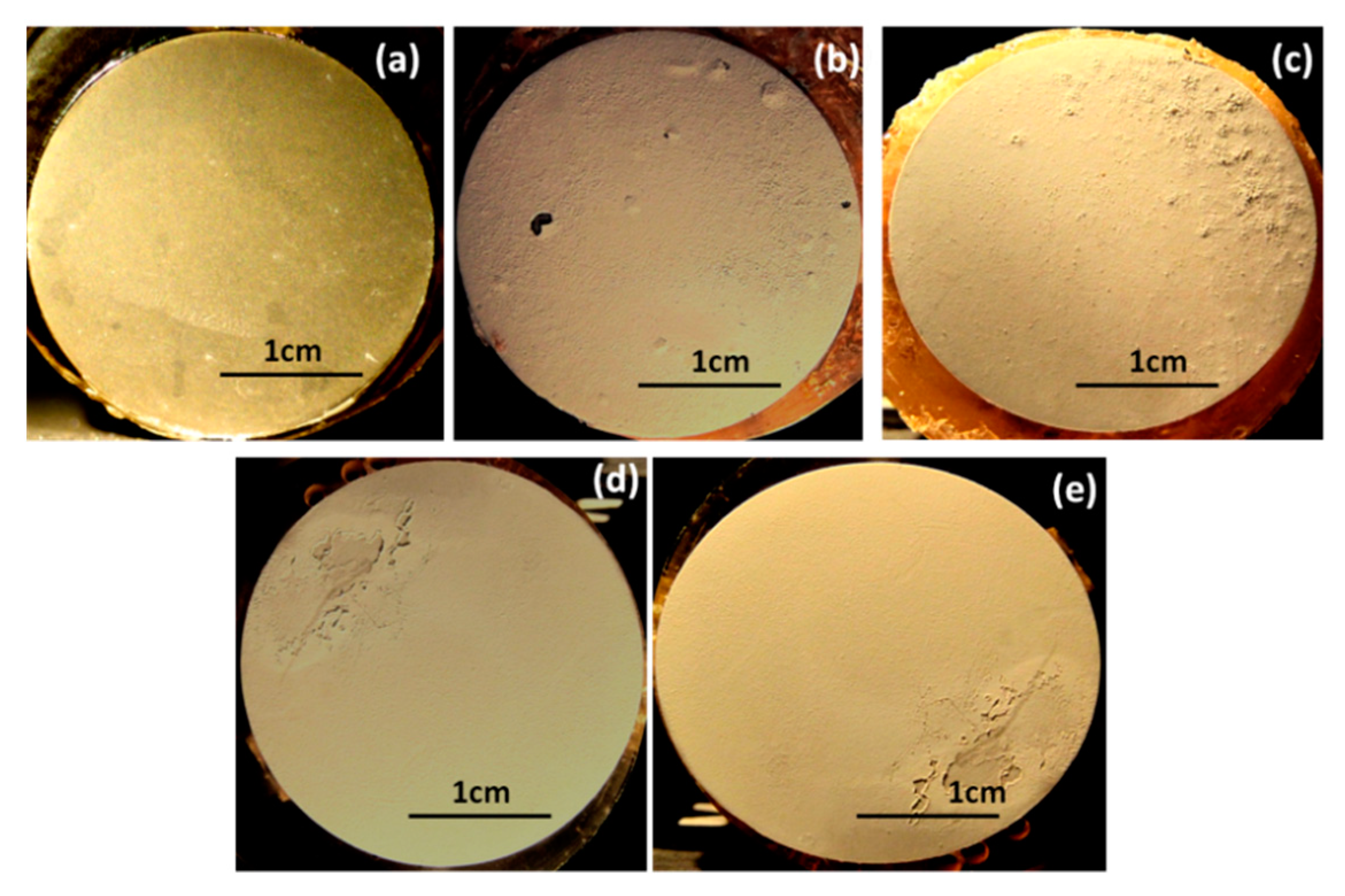
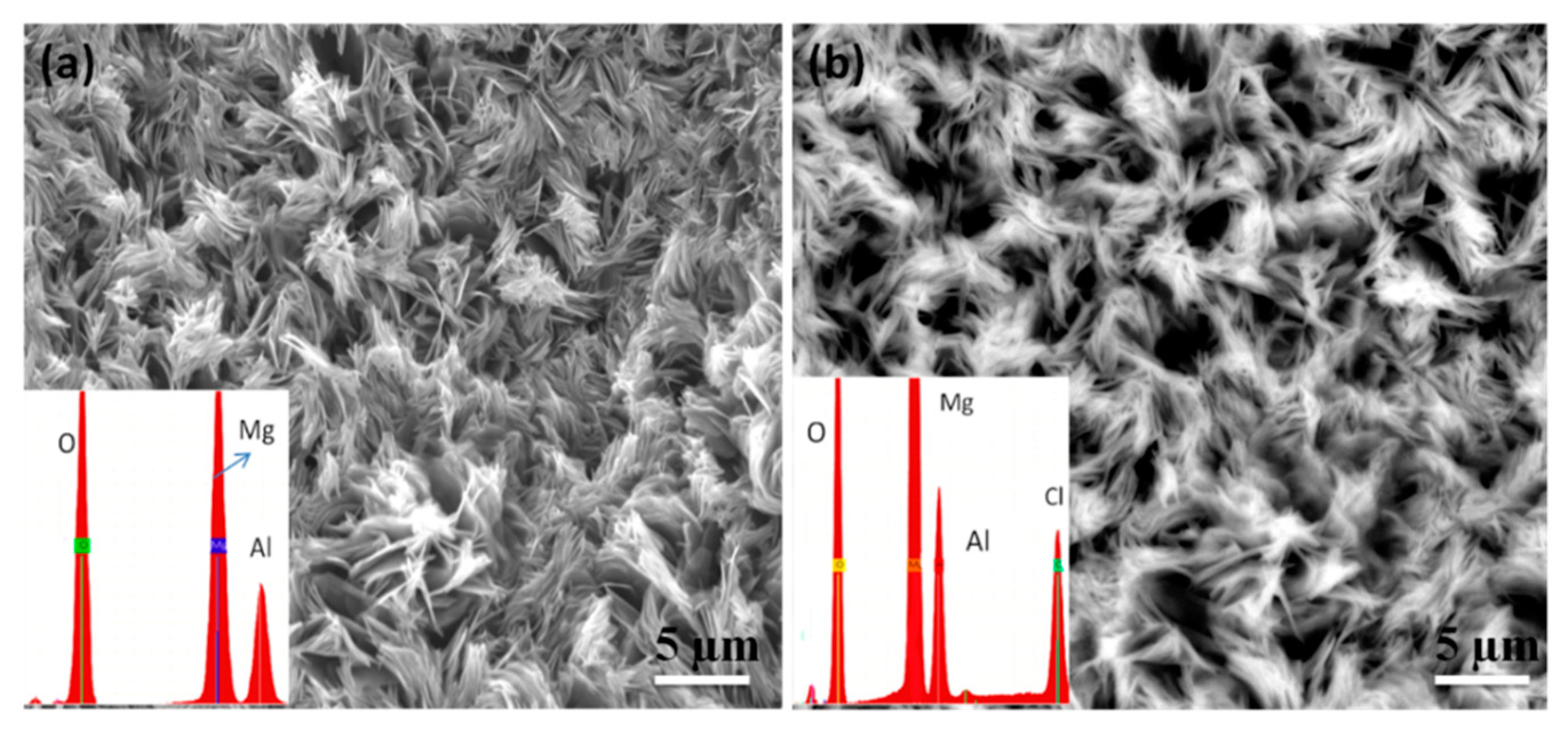
4.3. Scanning Electron Microscopy (SEM)
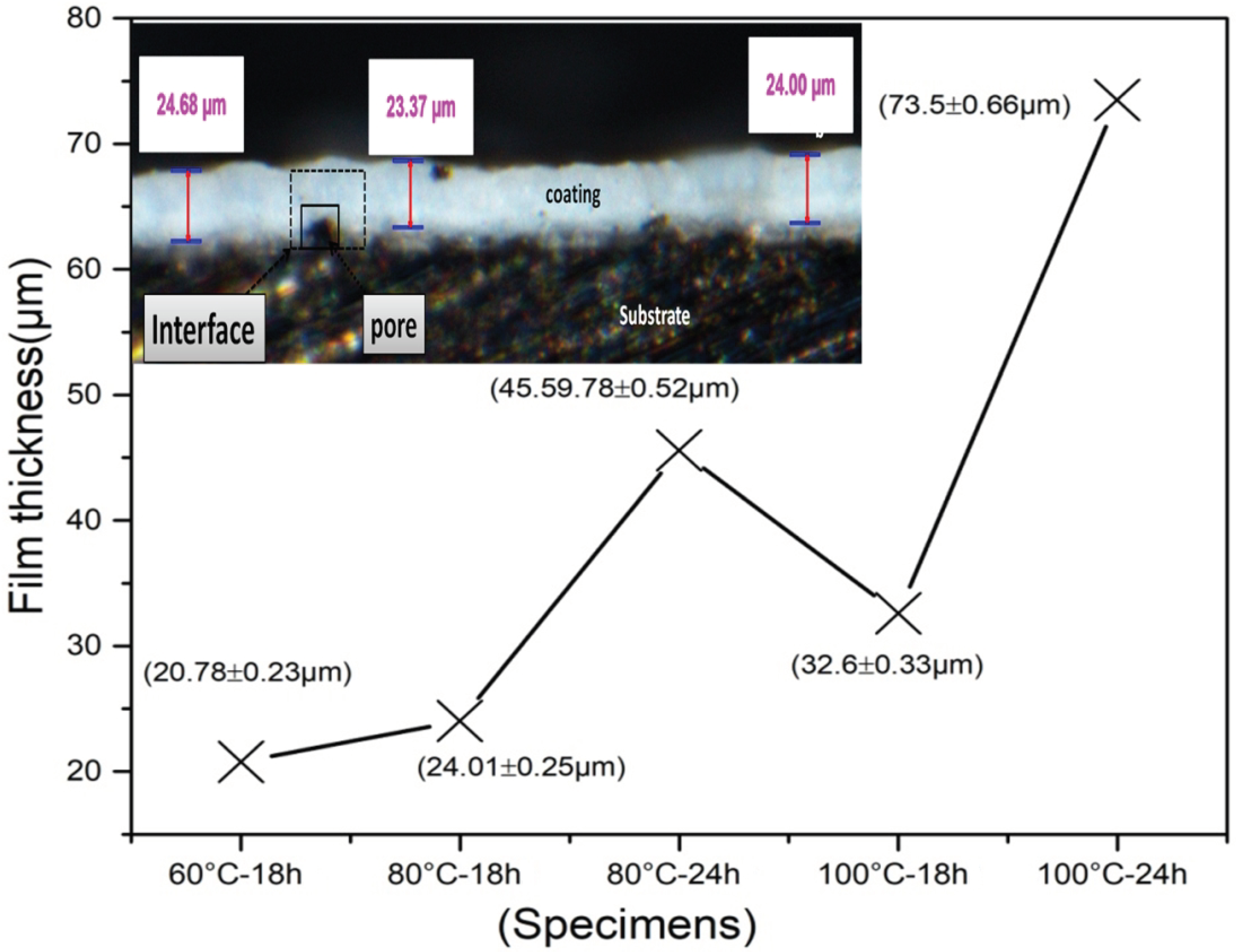
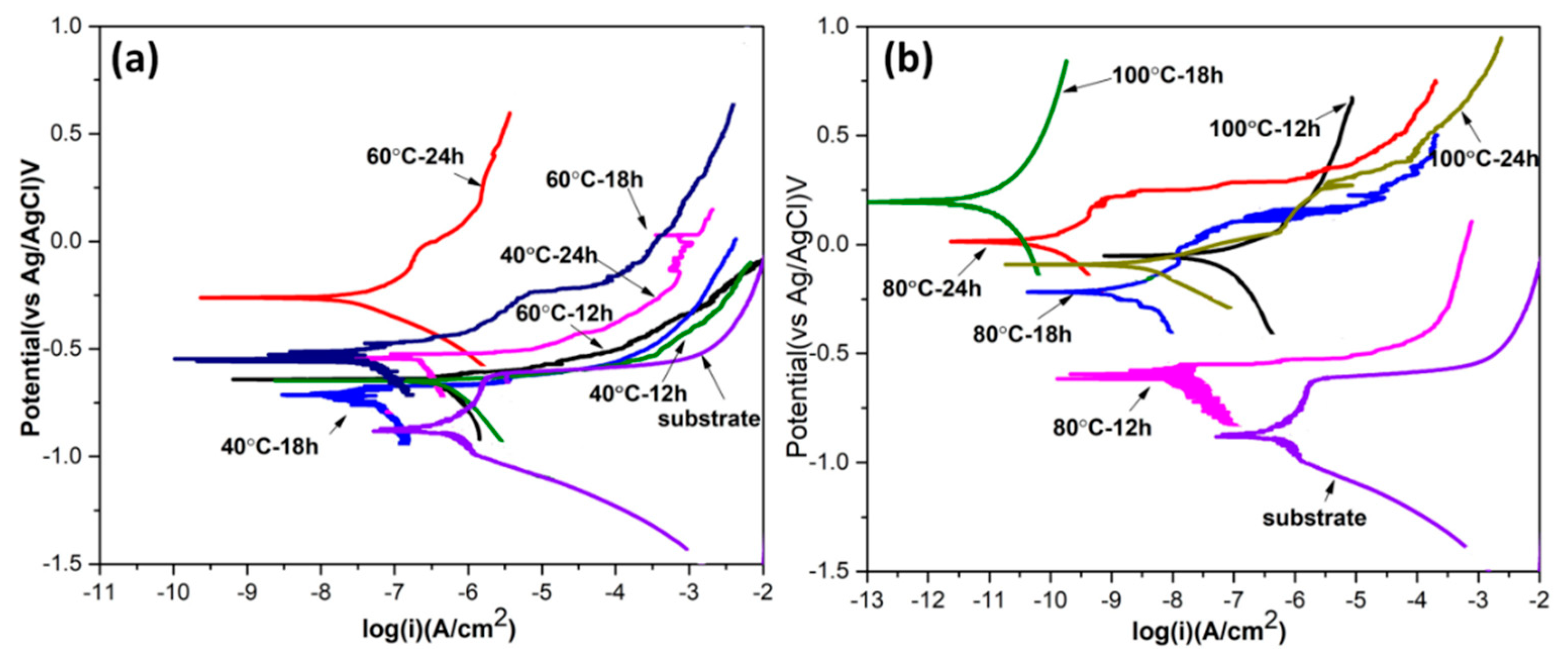
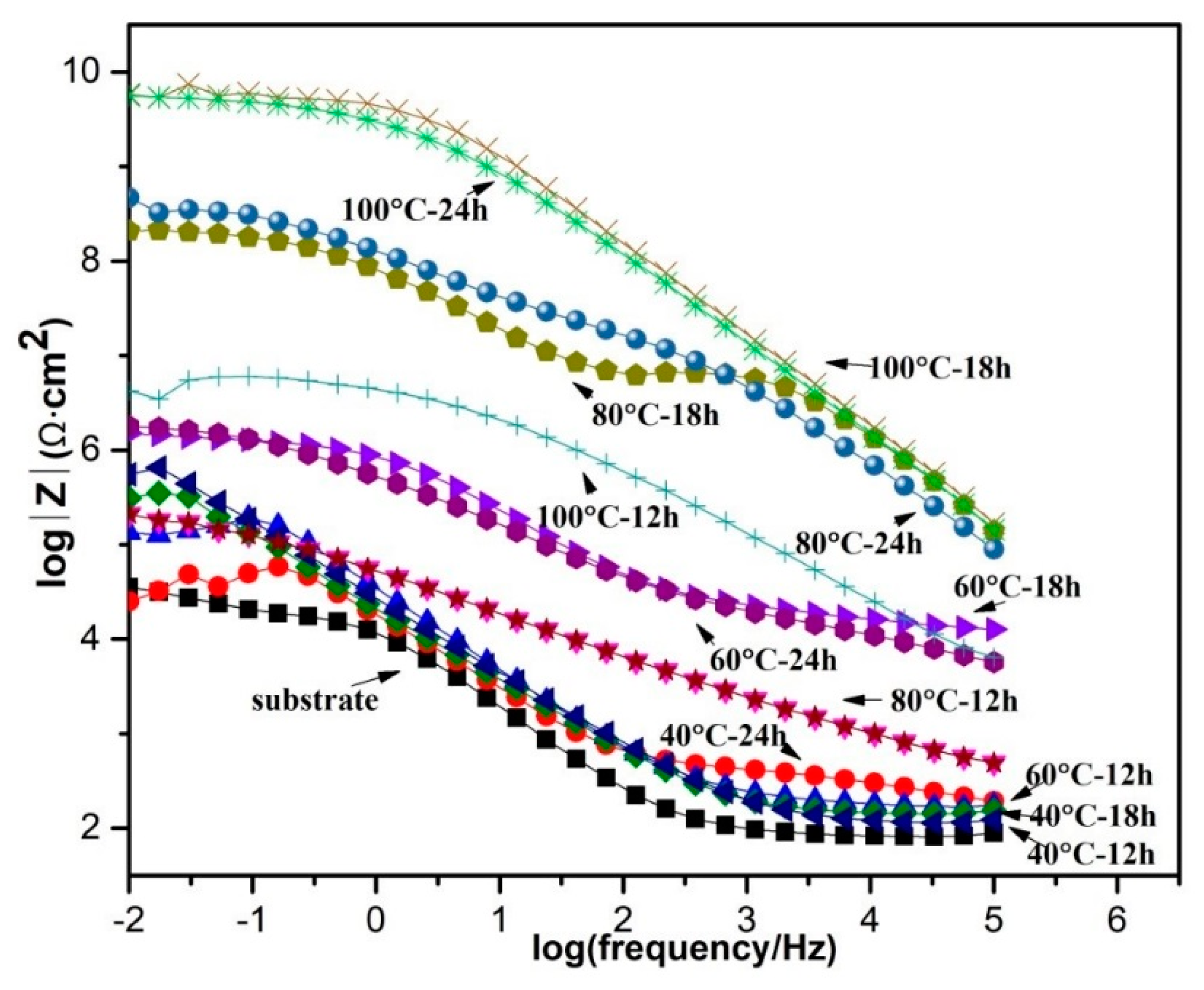
4.4. Electrochemical Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davis, J.R. Corrosion of Aluminium and Aluminium Alloys; ASM International: Novelty, OH, USA, 1999. [Google Scholar]
- Revie, R.W. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, 4th ed.; John Wiley & Sons: New York, NY, USA, 2008. [Google Scholar]
- Xhanari, K.; Finšgar, M. Organic corrosion inhibitors for aluminum and its alloys in chloride and alkaline solutions: A review. Arab. J. Chem. 2016, in press. [Google Scholar] [CrossRef]
- Kumar, N.; Rao, P.N.; Jayaganthan, R.; Brokmeier, H.G. Effect of cryorolling and annealing on recovery, recrystallisation, grain growth and their influence on mechanical and corrosion behaviour of 6082 Al alloy. Mater. Chem. Phys. 2015, 165, 177–187. [Google Scholar] [CrossRef]
- Liang, W.J.; Rometsch, P.A.; Cao, L.F.; Birbilis, N. General aspects related to the corrosion of 6xxx series aluminium alloys: Exploring the influence of Mg/Si ratio and Cu. Corros. Sci. 2013, 76, 119–128. [Google Scholar] [CrossRef]
- Larsen, M.H.; Walmsley, J.C.; Lunder, O.; Mathiesen, R.H.; Nisancioglu, K. Intergranular corrosion of copper-containing AA6xxx AlMgSi aluminum alloys. J. Electrochem. Soc. 2008, 155, C550–C556. [Google Scholar] [CrossRef]
- Zubillaga, O.; Cano, F.J.; Azkarate, I.; Molchan, I.S.; Thompson, G.E.; Skeldon, P. Anodic films containing polyaniline and nanoparticles for corrosion protection of AA2024T3 aluminium alloy. Surf. Coat. Technol. 2009, 203, 1494–1501. [Google Scholar] [CrossRef]
- Boisier, G.; Lamure, A.; Pébère, N.; Portail, N.; Villatte, M. Corrosion protection of AA2024 sealed anodic layers using the hydrophobic properties of carboxylic acids. Surf. Coat. Technol. 2009, 203, 3420–3426. [Google Scholar] [CrossRef]
- Johansen, H.D.; Brett, C.M.; Motheo, A.J. Corrosion protection of aluminium alloy by cerium conversion and conducting polymer duplex coatings. Corros. Sci. 2012, 63, 342–350. [Google Scholar] [CrossRef]
- Shi, H.; Han, E.H.; Liu, F.; Kallip, S. Protection of 2024-T3 aluminium alloy by corrosion resistant phytic acid conversion coating. Appl. Surf. Sci. 2013, 280, 325–331. [Google Scholar] [CrossRef]
- Schäfer, H.; Stock, H.R. Improving the corrosion protection of aluminium alloys using reactive magnetron sputtering. Corros. Sci. 2005, 47, 953–964. [Google Scholar] [CrossRef]
- Diesselberg, M.; Stock, H.R.; Mayr, P. Corrosion protection of magnetron sputtered TiN coatings deposited on high strength aluminium alloys. Surf. Coat. Technol. 2004, 177−178, 399–403. [Google Scholar] [CrossRef]
- Arenas, M.A.; Conde, A.; de Damborenea, J.J. Effect of acid traces on hydrothermal sealing of anodising layers on 2024 aluminium alloy. Electrochim. Acta 2010, 55, 8704–8708. [Google Scholar] [CrossRef]
- Venugopal, A.; Panda, R.; Manwatkar, S.; Sreekumar, K.; Krishna, L.R.; Sundararajan, G. Effect of micro arc oxidation treatment on localized corrosion behaviour of AA7075 aluminum alloy in 3.5% NaCl solution. Trans. Nonferrous Met. Soc. China 2012, 22, 700–710. [Google Scholar] [CrossRef]
- Dalmoro, V.; dos Santos, J.H.; Armelin, E.; Alemán, C.; Azambuja, D.S. A synergistic combination of tetraethylorthosilicate and multiphosphonic acid offers excellent corrosion protection to AA1100 aluminum alloy. Appl. Surf. Sci. 2013, 273, 758–768. [Google Scholar] [CrossRef]
- Voevodin, N.N.; Kurdziel, J.W.; Mantz, R. Corrosion protection for aerospace aluminum alloys by modified selfassembled nanophase particle (MSNAP) sol–gel. Surf. Coat. Technol. 2006, 201, 1080–1084. [Google Scholar] [CrossRef]
- Qu, J.E.; Geng, C.H.E.N.; Wang, H.R.; Nie, D.J. Effect of water content on corrosion inhibition behavior of self-assembled TDPA on aluminum alloy surface. Trans. Nonferrous Met. Soc. China 2013, 23, 3137–3144. [Google Scholar] [CrossRef]
- Zou, K.; Wang, X.; Liu, W.; Zhao, Y. Preparation and characterization of Ce−silane−ZrO2 composite coatings on 1060 aluminum. Trans. Nonferrous Met. Soc. China 2014, 24, 1474–1480. [Google Scholar]
- Lutz, A.; van den Berg, O.; Van Damme, J.; Verheyen, K.; Bauters, E.; De Graeve, I.; Du Prez, F.; Terryn, H. A shape-recovery polymer coating for the corrosion protection of metallic surfaces. ACS Appl. Mater. Interfaces 2014, 7, 175–183. [Google Scholar] [CrossRef]
- Ates, M. A review on conducting polymer coatings for corrosion protection. J. Adhes. Sci. Technol. 2016, 30, 1510–1536. [Google Scholar] [CrossRef]
- Leroux, F.; Taviot-Guého, C. Fine tuning between organic and inorganic host structure: New trends in layered double hydroxide hybrid assemblies. J. Mater. Chem. 2005, 15, 3628–3642. [Google Scholar] [CrossRef]
- Evans, D.G.; Duan, X. Preparation of layered double hydroxides and their applications as additives in polymers, as precursors to magnetic materials and in biology and medicine. Chem. Commun. 2006, 5, 485–496. [Google Scholar] [CrossRef]
- Williams, G.R.; O’Hare, D. Towards understanding, control and application of layered double hydroxide chemistry. J. Mater. Chem. 2006, 16, 3065–3074. [Google Scholar] [CrossRef]
- Lei, X.D.; Yang, L.; Zhang, F.Z.; Evans, D.G.; Duan, X. Synthesis of oriented layered double hydroxide thin films on sulfonated polystyrene substrates. Chem. Lett. 2005, 34, 1610–1611. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, F.; Fu, S.; Duan, X. In situ microstructure control of oriented layered double hydroxide monolayer films with curved hexagonal crystals as superhydrophobic materials. Adv. Mater. 2006, 18, 3089–3093. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Liu, M.; Evans, D.G.; Duan, X. Large continuous, transparent and oriented self-supporting films of layered double hydroxides with tunable chemical composition. Chem. Commun. 2007, 2, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, C.; Song, L.; Zeng, R.; Liu, Z.; Cui, H. Corrosion of in-situ grown MgAl–LDH coating on aluminum alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 3498–3504. [Google Scholar] [CrossRef]
- Lin, J.-K.; Jeng, K.-L.; Uan, J.-Y. Crystallization of a chemical conversion layer that forms on AZ91D magnesium alloy in carbonic acid. Corros. Sci. 2011, 53, 3832–3839. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Xie, Z.-H.; Yu, G. Duplex coating combining layered double hydroxide and 8-quinolinol layers on Mg alloy for corrosion protection. Electrochim. Acta 2018, 283, 1845–1857. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; He, H.; Sun, W.; Zong, Q.; Liu, G. Enhanced corrosion resistance of MgAl hydrotalcite conversion coating on aluminum by chemical conversion treatment. Surf. Coat. Technol. 2013, 235, 484–488. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, R.; Osada, M.; Iyi, N.; Ebina, Y.; Takada, K.; Sasaki, T. Synthesis, anion exchange, and delamination of Co–Al layered double hydroxide: Assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies. J. Am. Chem. Soc. 2006, 128, 4872–4880. [Google Scholar] [CrossRef]
- Okamoto, K.; Iyi, N.; Sasaki, T. Factors affecting the crystal size of the MgAl–LDH (layered double hydroxide) prepared by using ammonia-releasing reagents. Appl. Clay Sci. 2007, 37, 23–31. [Google Scholar] [CrossRef]
- Wu, L.; Zheng, Z.; Pan, F.; Tang, A.; Zhang, G.; Liu, L. Influence of Reaction Temperature on the Controlled Growth of Mg–Al LDH Film. Int. J. Electrochem. Sci. 2017, 12, 6352–6364. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Pearson Education: London, UK, 2014. [Google Scholar]
- Iqbal, M.A.; Fedel, M. The effect of the surface morphologies on the corrosion resistance of in situ growth MgAl–LDH based conversion film on AA6082. Surf. Coat. Technol. 2018, 352, 166–174. [Google Scholar] [CrossRef]
- Aisawa, S.; Hirahara, H.; Uchiyama, H.; Takahashi, S.; Narita, E. Synthesis and thermal decomposition of Mn–Al layered double hydroxides. J. Solid State Chem. 2002, 167, 152–159. [Google Scholar] [CrossRef]
- Wu, Q.; Olafsen, A.; Vistad, Ø.B.; Roots, J.; Norby, P. Delamination and restacking of a layered double hydroxide with nitrate as counter anion. J. Mater. Chem. 2005, 15, 4695–4700. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L. Fourier transform infrared and Raman spectroscopic study of the local structure of Mg-, Ni-, and Co-hydrotalcites. J. Solid State Chem. 1999, 146, 506–515. [Google Scholar] [CrossRef]
- Ni, Z.M.; Xia, S.J.; Fang, C.P.; Wang, L.G.; Hu, J. Synthesis, characterization and thermal property of Cu/Co/Mg/Al hydrotalcite like compounds. Rare Met. Mater. Eng. 2008, 37, 634–637. [Google Scholar]
- Beving, D.E.; McDonnell, A.M.; Yang, W.; Yan, Y. Corrosion resistant high-silica-zeolite MFI coating one general solution formulation for aluminum alloy AA-2024-T3, AA-5052-H32, AA-6061-T4, and AA-7075-T6. J. Electrochem. Soc. 2006, 153, B325–B329. [Google Scholar] [CrossRef]
- Tedim, J.; Kuznetsova, A.; Salak, A.N.; Montemor, F.; Snihirova, D.; Pilz, M.; Zheludkevich, M.L.; Ferreira, M.G.S. Zn–Al layered double hydroxides as chloride nanotraps in active protective coatings. Corros. Sci. 2012, 55, 1–4. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.G.; Zeng, R.C.; Li, S.Q.; Cui, H.Z.; Song, L.; Han, E.H. Corrosion resistance of Mg–Al–LDH coating on magnesium alloy AZ31. Surf. Coat. Technol. 2014, 258, 1152–1158. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, X.; Pan, X.; Liao, L.; Wu, X.; Liu, Y. Self-healing Li–Al layered double hydroxide conversion coating modified with aspartic acid for 6N01 Al alloy. Appl. Surf. Sci. 2017, 394, 275–281. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Z. In situ growth of durable superhydrophobic Mg–Al layered double hydroxides nanoplatelets on aluminum alloys for corrosion resistance. J. Alloy. Compd. 2018, 767, 382–391. [Google Scholar] [CrossRef]
- Lin, K.; Luo, X.; Pan, X.; Zhang, C.; Liu, Y. Enhanced corrosion resistance of LiAl–layered double hydroxide (LDH) coating modified with a Schiff base salt on aluminum alloy by one step in situ synthesis at low temperature. Appl. Surf. Sci. 2019, 463, 1085–1096. [Google Scholar] [CrossRef]
- Tedim, J.; Zheludkevich, M.L.; Bastos, A.C.; Salak, A.N.; Lisenkov, A.D.; Ferreira, M.G.S. Influence of preparation conditions of layered double hydroxide conversion films on corrosion protection. Electrochim. Acta 2014, 117, 164–171. [Google Scholar] [CrossRef]




| Sample | Cell Parameter, a (nm) | Cell Parameter, c (nm) | Interlayer Distance, d003 (nm) | Interlayer Distance, d110 (nm) | Crystallite Size, D (nm) |
|---|---|---|---|---|---|
| 40 °C–24 h | 0.308 | 2.329 | 0.776 | 0.154 | 11.214 |
| 60 °C–12 h | 0.306 | 2.296 | 0.765 | 0.153 | 12.908 |
| 60 °C–18 h | 0.305 | 2.668 | 0.889 | 0.152 | 7.608 |
| 60 °C–24 h | 0.305 | 2.294 | 0.765 | 0.152 | 11.657 |
| 80 °C–12 h | 0.303 | 2.275 | 0.759 | 0.151 | 6.685 |
| 80 °C–18 h | 0.306 | 2.461 | 0.820 | 0.153 | 6.987 |
| 80 °C–24 h | 0.311 | 2.412 | 0.804 | 0.155 | 8.465 |
| 100 °C–12 h | 0.311 | 2.691 | 0.897 | 0.155 | 5.929 |
| 100 °C–18 h | 0.311 | 2.391 | 0.797 | 0.156 | 6.165 |
| 100 °C–24 h | 0.311 | 2.463 | 0.821 | 0.157 | 6.411 |
| Sample | Mg (at.%) | Al (at.%) | N (at.%) | O (at.%) | Mg/Al |
|---|---|---|---|---|---|
| 40 °C–24 h | 17.6 | 6.8 | 3.1 | 64.7 | 2.6 |
| 60 °C–12 h | 18.1 | 7.2 | 3.0 | 60.7 | 2.5 |
| 60 °C–18 h | 26.2 | 7.5 | 3.3 | 57.4 | 3.5 |
| 60 °C–24 h | 27.5 | 7.5 | 4.2 | 57.0 | 3.7 |
| 80 °C–12 h | 19.3 | 7.3 | 3.1 | 58.7 | 2.6 |
| 80 °C–18 h | 27.2 | 7.6 | 4.1 | 53.8 | 3.6 |
| 80 °C–24 h | 24.7 | 6.6 | 4.7 | 56.2 | 3.8 |
| 100°C–12 h | 24.5 | 7.6 | 4.2 | 53.9 | 3.2 |
| 100 °C–18 h | 28.6 | 7.5 | 4.7 | 53.9 | 3.8 |
| 100 °C–24 h | 28.7 | 7.4 | 4.3 | 63.1 | 3.9 |
| Specimens | E (vs. Ag/AgCl) (V) | I (μA/cm2) |
|---|---|---|
| AA6082 | −0.879 | 0.46556 |
| 40 °C–12 h | −0.679 | 0.33138 |
| 40 °C–18 h | −0.721 | 0.07629 |
| 40 °C–24 h | −0.564 | 0.28947 |
| 60 °C–12 h | −0.683 | 0.40345 |
| 60 °C–18 h | −0.519 | 0.07524 |
| 60 °C–24 h | −0.254 | 0.05214 |
| 80 °C–12 h | −0.623 | 0.03121 |
| 80 °C–18 h | −0.243 | 0.00156 |
| 80 °C–24 h | +0.016 | 0.00024 |
| 100 °C–12 h | −0.099 | 0.03903 |
| 100 °C–18 h | +0.241 | 0.00001 |
| 100 °C–24 h | −0.122 | 0.00263 |
| Sample | Immersion Time | RLDH (kΩ cm2) | QLDH (Ω−1 cm−2 sα) | αLDH | Rct (kΩ cm2) | Qdl (Ω−1 cm−2 sα) | αdl |
|---|---|---|---|---|---|---|---|
| 60 °C–24 h | 1 day | 13.0 | 4.2 × 10−7 | 0.51 | 420.0 | 1.4 × 10−6 | 0.72 |
| 3 days | 96.30 | 1.7 × 10−6 | 0.31 | 828.7 | 1.1 × 10−6 | 0.71 | |
| 7 days | 98.3 | 1.1 ×10−5 | 0.85 | 0.5 | 7.8 × 10−6 | 0.46 | |
| 80 °C–18 h | 1 day | 5705.0 | 5.1 × 10−6 | 0.91 | 0.9 | 7.2 × 10−5 | 0.46 |
| 3 days | 50.1 | 7.2 × 10−6 | 0.51 | 270.2 | 9.9 × 10−6 | 0.65 | |
| 7 days | 118.1 | 1.7 × 10−5 | 0.85 | 2.2 | 7.9 × 10−5 | 0.19 | |
| 80 °C–24 h | 1 day | 41.0 | 4.6 × 10−7 | 0.54 | 2095.0 | 8.1 × 10−8 | 0.77 |
| 3 days | 11.2 | 2.0 × 10−6 | 0.43 | 372.9 | 1.8 × 10−6 | 0.69 | |
| 7 days | 7.8 | 2.5 × 10−5 | 0.71 | 354.8 | 1.0 × 10−5 | 0.93 | |
| 100 °C–18 h | 1 day | 6135.0 | 2.0 × 10−8 | 0.69 | 18420 | 7.8 × 10−8 | 0.68 |
| 3 days | 13.0 | 4.2 × 10−7 | 0.51 | 418.4 | 1.4 × 10−6 | 0.72 | |
| 7 days | 104.2 | 8.8 × 10−6 | 0.46 | 97.4 | 1.2 × 10−4 | 1.0 |
| LDH | NaCl Concen. | Time | RLDH (kΩ cm2) | Rct (kΩ cm2) | Ref. |
|---|---|---|---|---|---|
| Li/Al | 3.5 wt % | 0 h | 2.2 | 6.49 × 103 | [43] |
| Mg/Al | 3.5 wt % | 1 day | n.p. | 5.88 | [44] |
| Li/Al | 3.5 wt % | 1 day | 0.8 | 0.18 × 103 | [45] |
| Zn/Al (+VOx) | 0.05 M | 1 day | 18.2 | 7.96 × 108 | [46] |
| Mg/Al 80 °C–24 h | 0.1 M | 1 day | 41.0 | 2.09 × 103 | This work |
| Mg/Al 100 °C–18 h | 6135.0 | 18.4 × 103 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, M.A.; Fedel, M. Effect of Synthesis Conditions on the Controlled Growth of MgAl–LDH Corrosion Resistance Film: Structure and Corrosion Resistance Properties. Coatings 2019, 9, 30. https://doi.org/10.3390/coatings9010030
Iqbal MA, Fedel M. Effect of Synthesis Conditions on the Controlled Growth of MgAl–LDH Corrosion Resistance Film: Structure and Corrosion Resistance Properties. Coatings. 2019; 9(1):30. https://doi.org/10.3390/coatings9010030
Chicago/Turabian StyleIqbal, Muhammad Ahsan, and Michele Fedel. 2019. "Effect of Synthesis Conditions on the Controlled Growth of MgAl–LDH Corrosion Resistance Film: Structure and Corrosion Resistance Properties" Coatings 9, no. 1: 30. https://doi.org/10.3390/coatings9010030
APA StyleIqbal, M. A., & Fedel, M. (2019). Effect of Synthesis Conditions on the Controlled Growth of MgAl–LDH Corrosion Resistance Film: Structure and Corrosion Resistance Properties. Coatings, 9(1), 30. https://doi.org/10.3390/coatings9010030