Nanochannelar Topography Positively Modulates Osteoblast Differentiation and Inhibits Osteoclastogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation and Characterization
2.2. Cell Culture Model
2.3. Cell Morphology
2.4. Cell Viability and Proliferation
2.5. MC3T3-E1 Cell Differentiation
2.6. Osteoclast Differentiation
2.7. Statistical Analysis
3. Results and Discussion
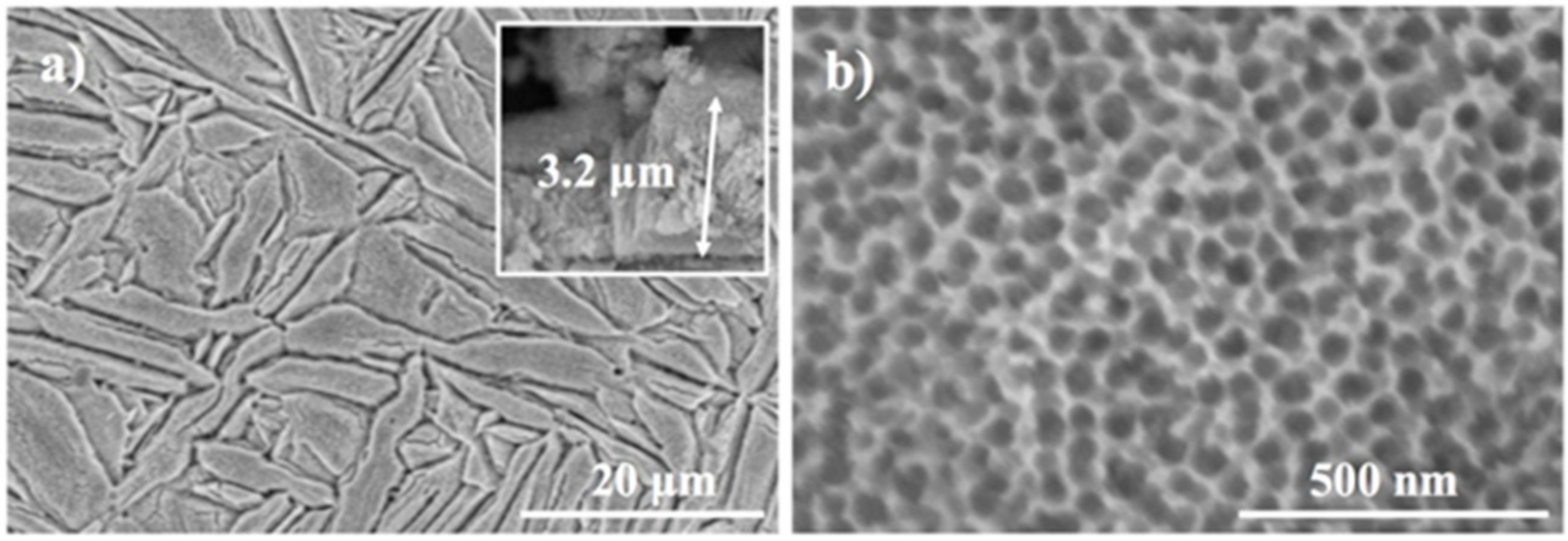
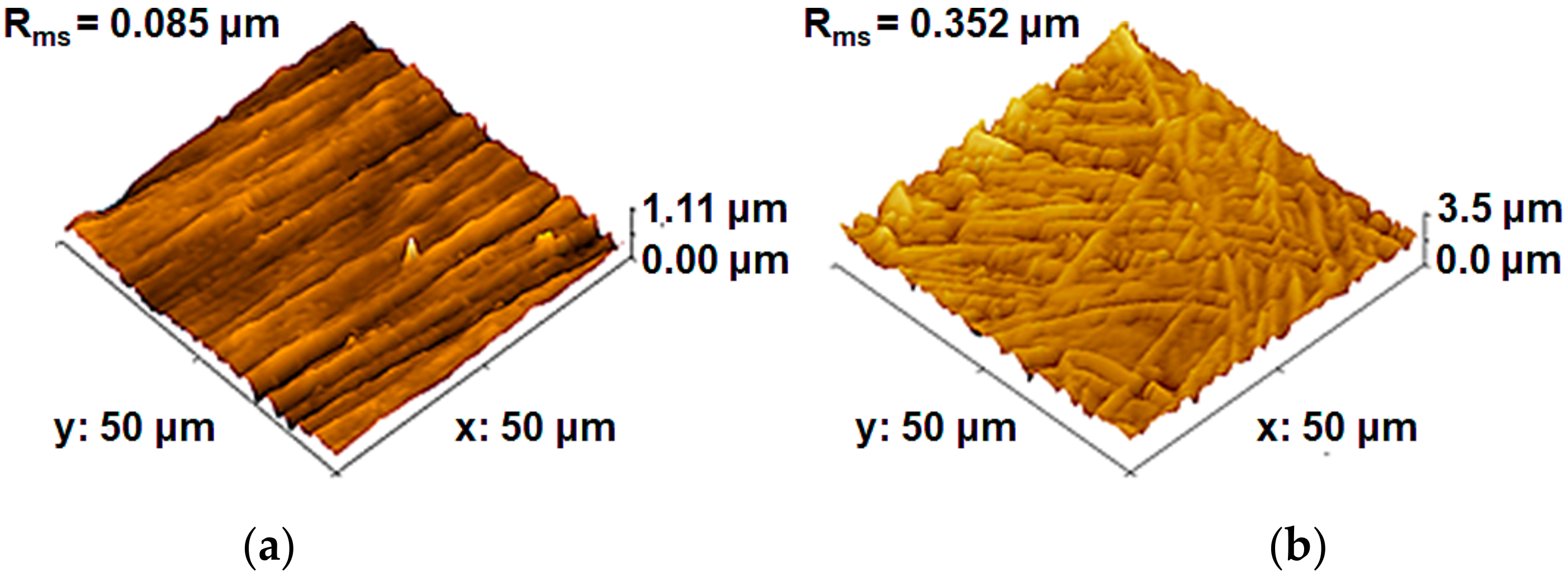
3.1. Materials Characterization
3.2. In Vitro Osteoblast Behavior
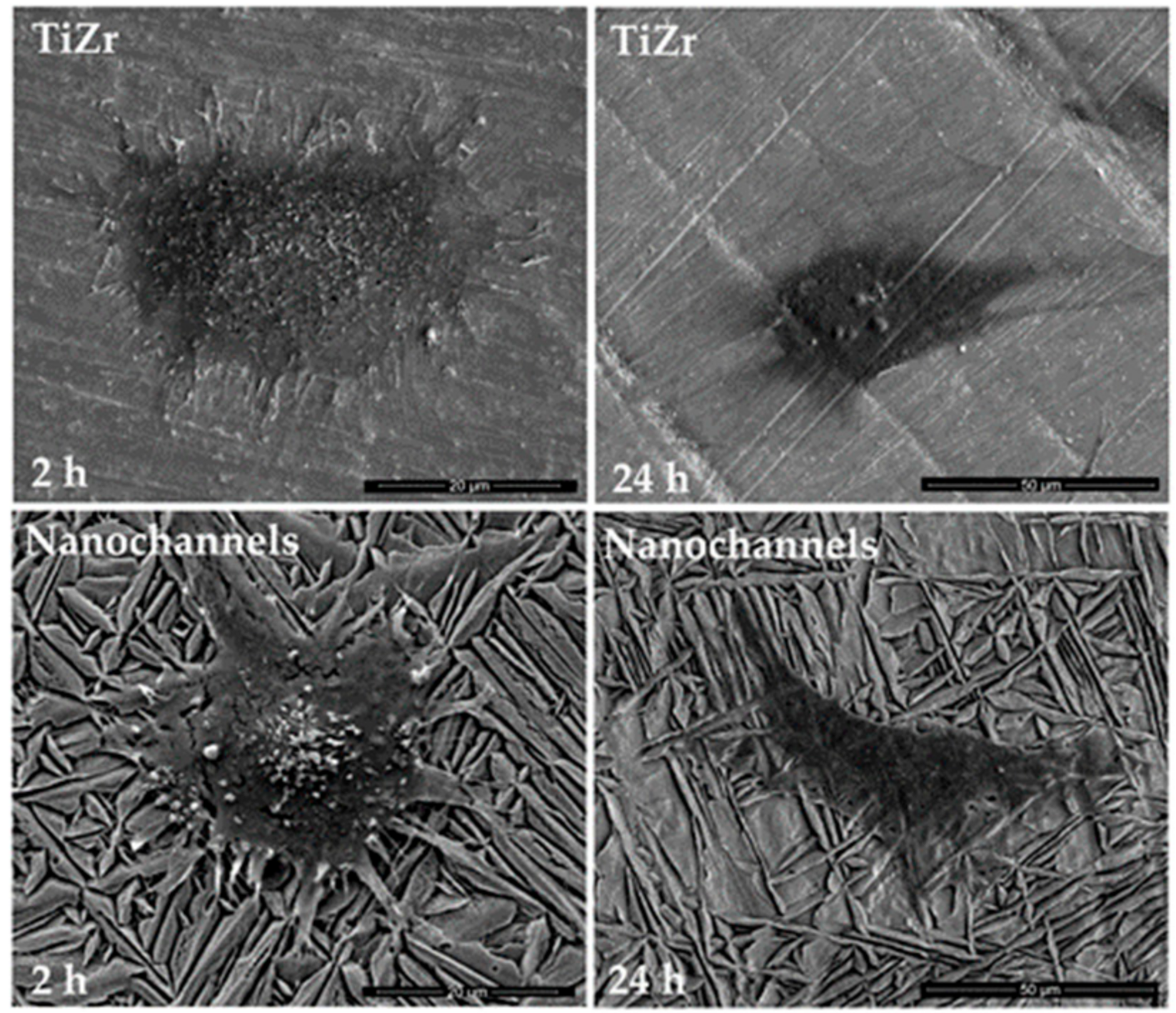
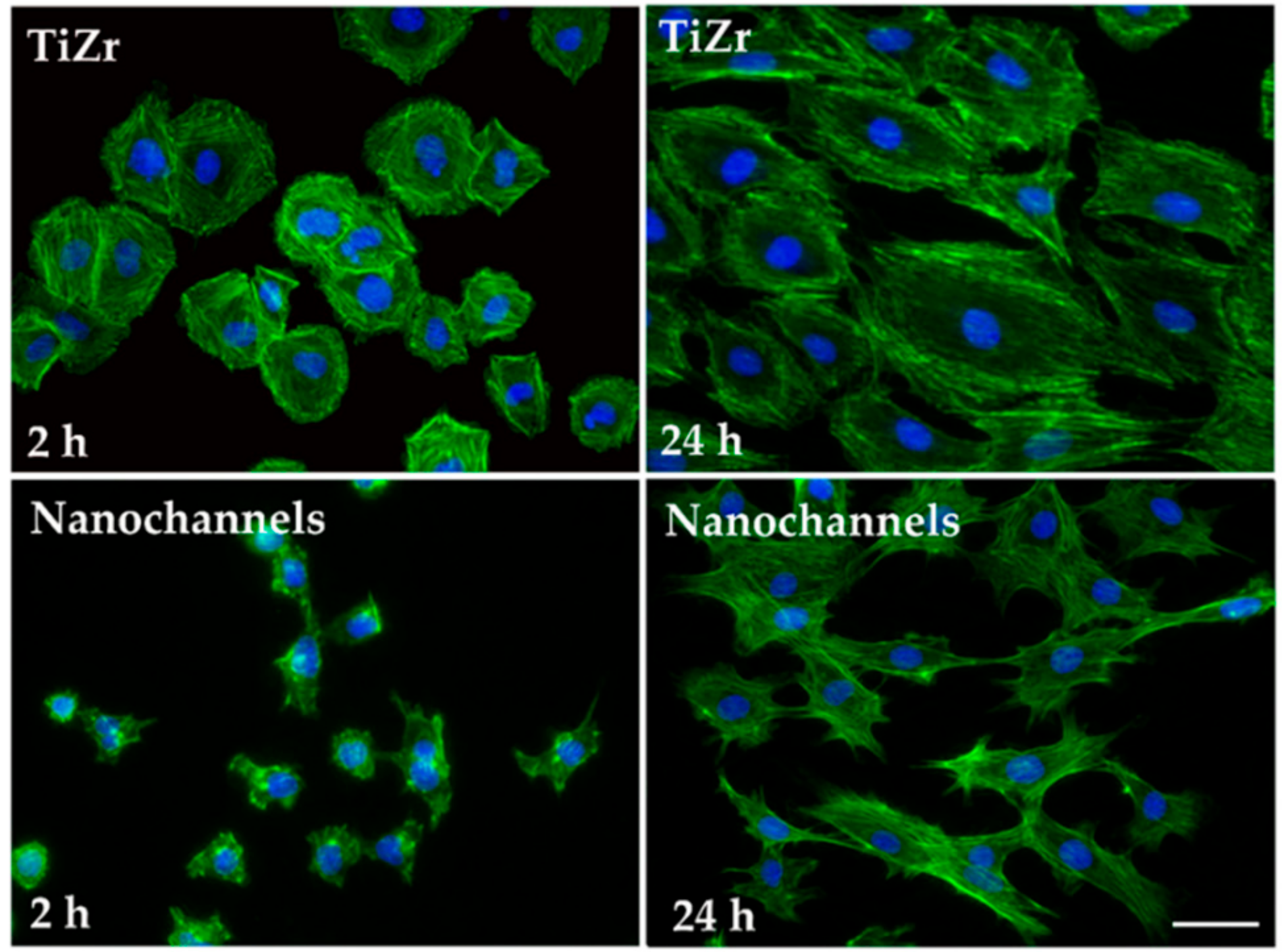
3.2.1. Cell Morphology
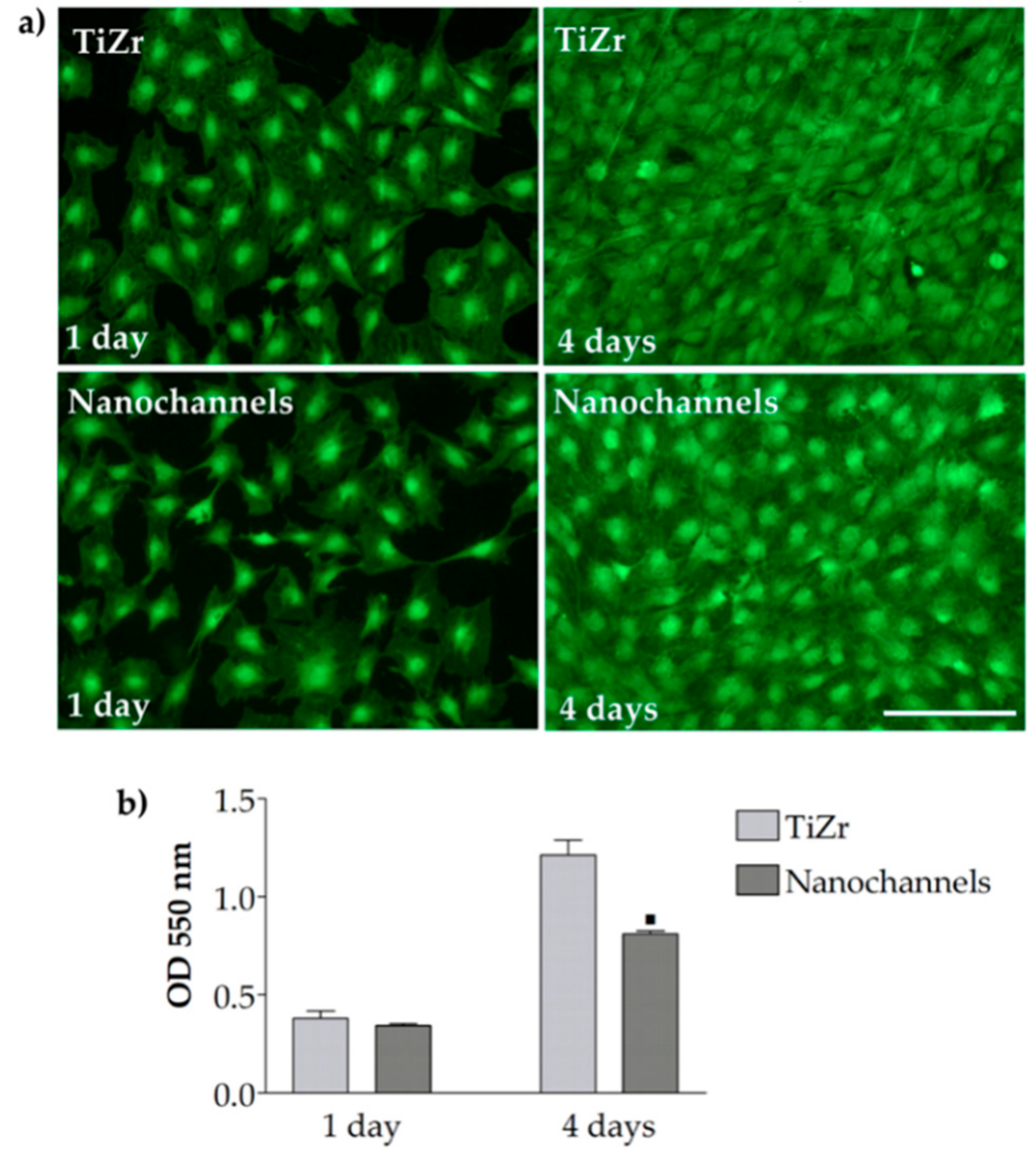
3.2.2. Cell Viability and Proliferation
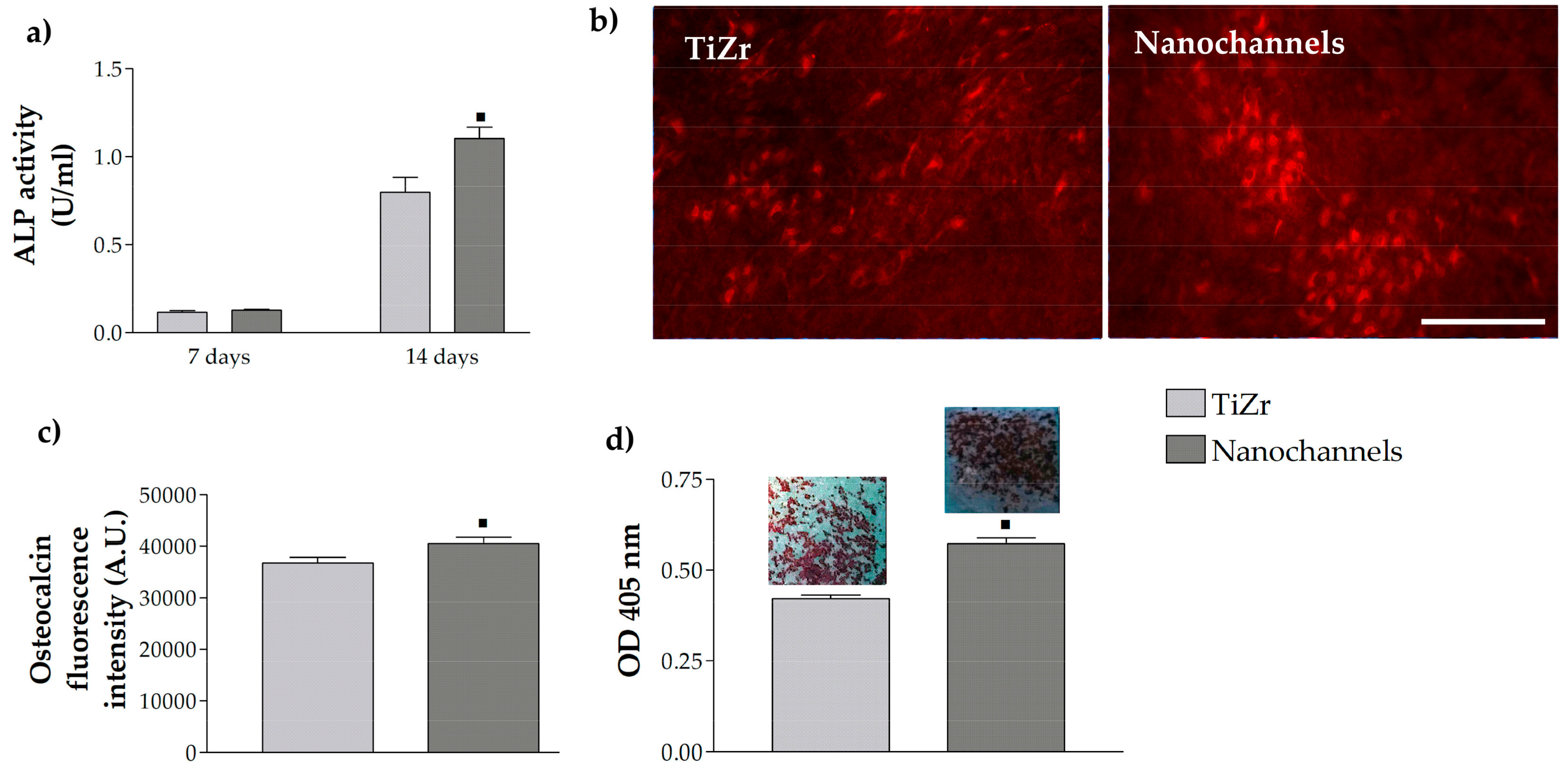
3.2.3. Pre-Osteoblast Cell Differentiation
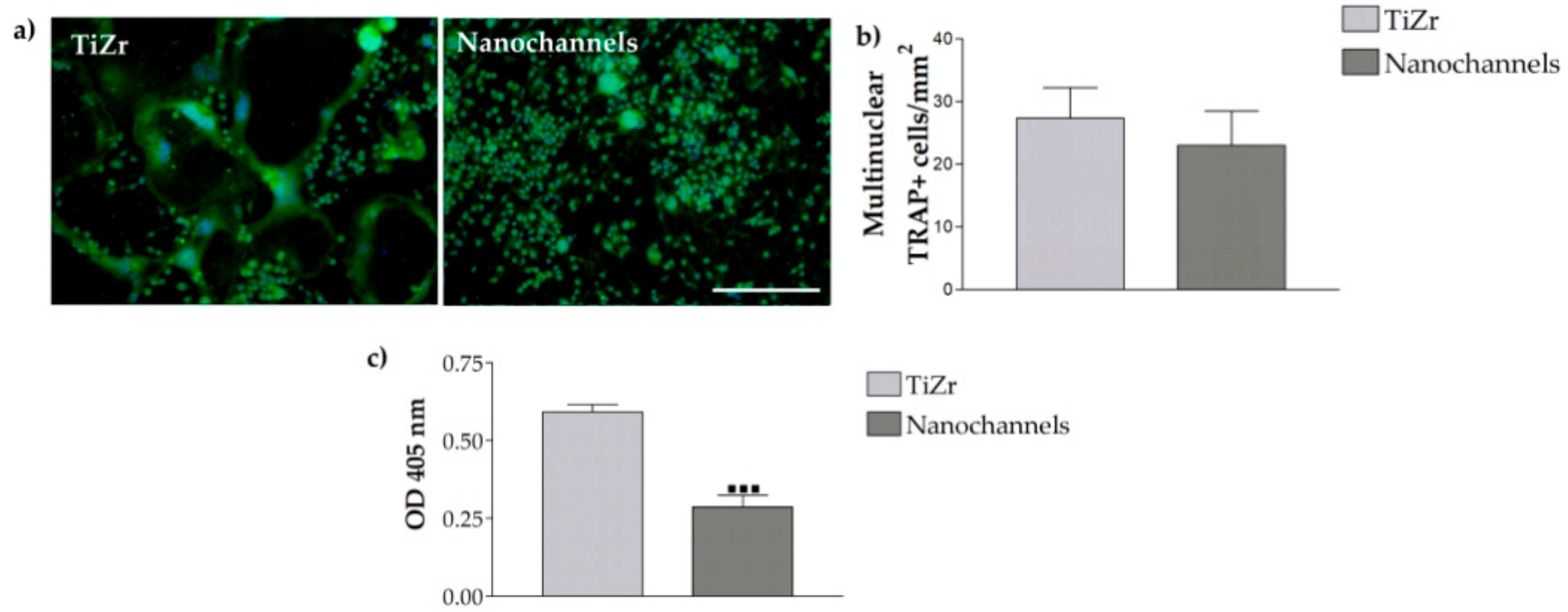
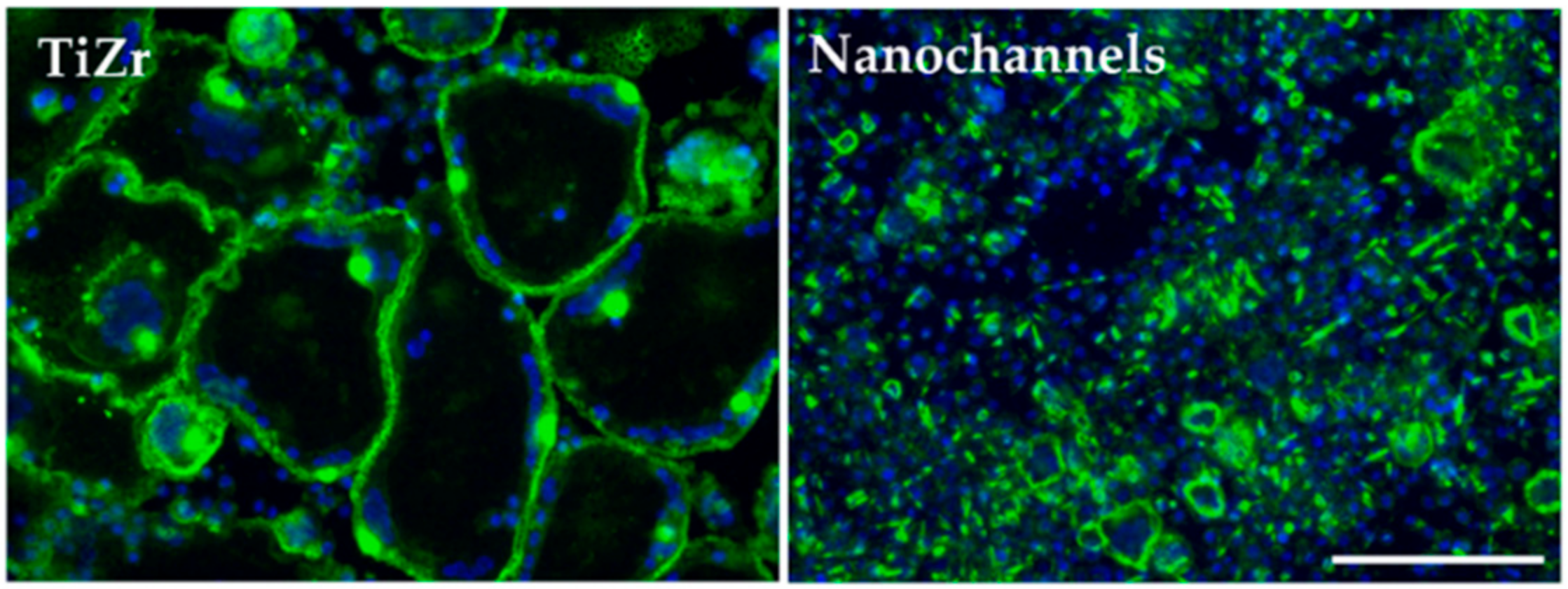
3.2.4. Osteoclast Differentiation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Karazisis, D.; Ballo, A.M.; Petronis, S.; Agheli, H.; Emanuelsson, L.; Thomsen, P.; Omar, O. The role of well-defined nanotopography of titanium implants on osseointegration: Cellular and molecular events in vivo. Int. J. Nanomed. 2016, 11, 1367–1382. [Google Scholar]
- Kulkarni, M.; Mazare, A.; Park, J.; Gongadze, E.; Killian, M.S.; Kralj, S.; von der Mark, K.; Iglic, A.; Schmuki, P. Protein interactions with layers of TiO2 nanotube and nanopore arrays: Morphology and surface charge influence. Acta Biomater. 2016, 45, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, R.; Tan, J.; Wang, J.; Lu, X.; Qu, S.; Weng, J.; Feng, B. Osteoblast behaviors on titania nanotube and mesopore layers. Regen. Biomater. 2017, 4, 81–87. [Google Scholar] [CrossRef]
- Lv, L.; Liu, Y.; Zhang, P.; Zhang, X.; Liu, J.; Chen, T.; Su, P.; Li, H.; Zhou, Y. The nanoscale geometry of TiO2 nanotubes influences the osteogenic differentiation of human adipose-derived stem cells by modulating h3k4 trimethylation. Biomaterials 2014, 39, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Son, W.W.; Zhu, X.; Shin, H.I.; Ong, J.L.; Kim, K.H. In vivo histological response to anodized and anodized/hydrothermally treated titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 66, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; Schlegel, K.A.; Neukam, F.W.; von der Mark, K.; Schmuki, P. TiO2 nanotube surfaces: 15 nm—An optimal length scale of surface topography for cell adhesion and differentiation. Small 2009, 5, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; von der Mark, K.; Schmuki, P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Su, E.P.; Justin, D.F.; Pratt, C.R.; Sarin, V.K.; Nguyen, V.S.; Oh, S.; Jin, S. Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces. Bone Jt. J. 2018, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, L.M.; Brammer, K.S.; Johnston, G.W.; Chien, S.; Jin, S. Macrophage inflammatory response to TiO2 nanotube surfaces. J. Biomater. Nanotechnol. 2011, 2, 293–300. [Google Scholar] [CrossRef]
- Ma, Q.L.; Zhao, L.Z.; Liu, R.R.; Jin, B.Q.; Song, W.; Wang, Y.; Zhang, Y.S.; Chen, L.H.; Zhang, Y.M. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials 2014, 35, 9853–9867. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, P.; Mazare, A.; Cimpean, A.; Park, J.; Costache, M.; Schmuki, P.; Demetrescu, I. Reduced inflammatory activity of RAW 264.7 macrophages on titania nanotube modified Ti surface. Int. J. Biochem. Cell Biol. 2014, 55, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Feng, X.; Li, W.; Wang, L.-N.; Wang, X. Regulation of RAW 264.7 macrophages behavior on anodic TiO2 nanotubular arrays. Front. Mater. Sci. 2017, 11, 318–327. [Google Scholar] [CrossRef]
- Ercan, B.; Taylor, E.; Alpaslan, E.; Webster, T.J. Diameter of titanium nanotubes influences anti-bacterial efficacy. Nanotechnology 2011, 22, 295102. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, S.; Nguyen, N.T.; Mazare, A.; Hahn, R.; Cerri, I.; Schmuki, P. Fast growth of TiO2 nanotube arrays with controlled tube spacing based on a self-ordering process at two different scales. Electrochem. Commun. 2017, 77, 98–102. [Google Scholar] [CrossRef]
- Lu, H.; Fan, H.; Jin, R.; Chong, B.; Shen, X.; Yan, S.; Zhu, X. Formation and morphology evolution of anodic TiO2 nanotubes under negative pressure. Electrochim. Acta 2016, 215, 380–387. [Google Scholar] [CrossRef]
- Kapusta-Kołodziej, J.; Syrek, K.; Pawlik, A.; Jarosz, M.; Tynkevych, O.; Sulka, G.D. Effects of anodizing potential and temperature on the growth of anodic TiO2 and its photoelectrochemical properties. Appl. Surf. Sci. 2017, 396, 1119–1129. [Google Scholar] [CrossRef]
- Kowalski, D.; Kim, D.; Schmuki, P. TiO2 nanotubes, nanochannels and mesosponge: Self-organized formation and applications. Nano Today 2013, 8, 235–264. [Google Scholar] [CrossRef]
- Ion, R.; Stoian, A.B.; Dumitriu, C.; Grigorescu, S.; Mazare, A.; Cimpean, A.; Demetrescu, I.; Schmuki, P. Nanochannels formed on TiZr alloy improve biological response. Acta Biomater. 2015, 24, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Ion, R.; Vizireanu, S.; Stancu, C.E.; Luculescu, C.; Cimpean, A.; Dinescu, G. Surface plasma functionalization influences macrophage behavior on carbon nanowalls. Mater. Sci. Eng. C 2015, 48, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Ion, R.; Drob, S.I.; Ijaz, M.F.; Vasilescu, C.; Osiceanu, P.; Gordin, D.M.; Cimpean, A.; Gloriant, T. Surface characterization, corrosion resistance and in vitro biocompatibility of a new Ti-Hf-Mo-Sn alloy. Materials 2016, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, P.; Staras, A.I.; Voicu, S.I.; Ionascu, I.; Soare, T.; Uzun, S.; Cojocaru, V.D.; Pandele, A.M.; Croitoru, S.M.; Miculescu, F. Characterization and in vitro and in vivo assessment of a novel cellulose acetate-coated Mg-based alloy for orthopedic applications. Materials 2017, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Mitran, V.; Dinca, V.; Ion, R.; Cojocaru, V.D.; Neacsu, P.; Dinu, C.Z.; Rusen, L.; Brajnicov, S.; Bonciu, A.; Dinescu, M.; et al. Graphene nanoplatelets-sericin surface-modified gum alloy for improved biological response. RSC Adv. 2018, 8, 18492–18501. [Google Scholar] [CrossRef]
- Ion, R.; Gordin, D.M.; Mitran, V.; Osiceanu, P.; Dinescu, S.; Gloriant, T.; Cimpean, A. In vitro bio-functional performances of the novel superelastic beta-type Ti–23Nb–0.7Ta–2Zr–0.5N alloy. Mater. Sci. Eng. C 2014, 35, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Owen, T.A.; Aronow, M.; Shalhoub, V.; Barone, L.M.; Wilming, L.; Tassinari, M.S.; Kennedy, M.B.; Pockwinse, S.; Lian, J.B.; Stein, G.S. Progressive development of the rat osteoblast phenotype in vitro: Reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J. Cell Physiol. 1990, 143, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Biggs, M.J.; Richards, R.G.; Gadegaard, N.; McMurray, R.J.; Affrossman, S.; Wilkinson, C.D.; Oreffo, R.O.; Dalby, M.J. Interactions with nanoscale topography: Adhesion quantification and signal transduction in cells of osteogenic and multipotent lineage. J. Biomed. Mater. Res. A 2008, 91, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Biggs, M.J.P.; Dalby, M.J. Focal adhesions in osteoneogenesis. Proc. Inst. Mech. Eng. H 2010, 224, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.K.F.; Darling, E.M.; Kulangara, K.; Guilak, F.; Leong, K.W. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials 2010, 31, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Provenzano, P.P.; Smith, C.L.; Levchenko, A. Matrix nanotopography as a regulator of cell function. J. Cell Biol. 2012, 197, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.L.; McDevitt, T.C.; Engler, A.J. Materials as stem cell regulators. Nat. Mater. 2014, 13, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Abagnale, G.; Steger, M.; Nguyen, V.H.; Hersch, N.; Sechi, A.; Joussen, S.; Denecke, B.; Merkel, R.; Hoffmann, B.; Dreser, A.; et al. Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages. Biomaterials 2015, 61, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Shi, Y.; Cao, Y.; Liu, W. Recent progress in stem cell differentiation directed by material and mechanical cues. Biomed. Mater. 2016, 11, 014109. [Google Scholar] [CrossRef] [PubMed]
- Newman, P.; Galeano Niño, J.L.; Graney, P.; Razal, J.M.; Minett, A.I.; Ribas, J.; Ovalle-Robles, R.; Biro, M.; Zreiqat, H. Relationship between nanotopographical alignment and stem cell fate with live imaging and shape analysis. Sci. Rep. 2016, 6, 37909. [Google Scholar] [CrossRef] [PubMed]
- Isaac, J.; Galtayries, A.; Kizuki, T.; Kokubo, T.; Berda, A.; Sautier, J.M. Bioengineered titanium surfaces affect the gene-expression and phenotypic response of osteoprogenitor cells derived from mouse calvarial bones. Eur. Cells Mater. 2010, 20, 178–196. [Google Scholar] [CrossRef]
- Andrukhov, O.; Huber, R.; Shi, B.; Berner, S.; Rausch-Fan, X.; Moritz, A.; Spencer, N.D.; Schedle, A. Proliferation, behavior, and differentiation of osteoblasts on surfaces of different microroughness. Dent. Mater. 2016, 32, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Jäger, M.; Urselmann, F.; Witte, F.; Zanger, K.; Li, X.; Ayers, D.C.; Krauspe, R. Osteoblast differentiation onto different biometals with an endoprosthetic surface topography in vitro. J. Biomed. Mater. Res. A 2007, 86, 61–75. [Google Scholar]
- Velasco-Ortega, E.; Alfonso-Rodríguez, C.A.; Monsalve-Guil, L.; España-López, A.; Jiménez-Guerra, A.; Garzón, I.; Alaminos, M.; Gil, F.J. Relevant aspects in the surface properties in titanium dental implants for the cellular viability. Mater. Sci. Eng. C 2016, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.; Oreffo, R.O. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Brammer, K.S.; Li, Y.S.J.; Teng, D.; Engler, A.J.; Chien, S.; Jin, S. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Lavenus, S.; Pilet, P.; Guicheux, J.; Weiss, P.; Louarn, G.; Layrolle, P. Behaviour of mesenchymal stem cells, fibroblasts and osteoblasts on smooth surfaces. Acta Biomater. 2011, 7, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Alonso, J.L.; Ostuni, E.; Whitesides, G.M.; Ingber, D.E. Cell shape provides global control of focal adhesion assembly. Biochem. Biophys. Res. Commun. 2003, 307, 355–361. [Google Scholar] [CrossRef]
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.G.; Kojima, S.-I.; Goldman, R.D. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010, 24, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Damania, D.; Subramanian, H.; Tiwari, A.K.; Stypula, Y.; Kunte, D.; Pradhan, P.; Roy, H.K.; Backman, V. Role of cytoskeleton in controlling the disorder strength of cellular nanoscale architecture. Biophys. J. 2010, 99, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, E.; Cervinka, M.; Cerman, J.; Schroterova, L. Hexavalent chromium disrupts the actin cytoskeleton and induces mitochondria-dependent apoptosis in human dermal fibroblasts. Toxicol. In Vitro 2005, 19, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Vetterkind, S.; Illenberger, S.; Kubicek, J.; Boosen, M.; Appel, S.; Naim, H.Y.; Scheidtmann, K.-H.; Preuss, U. Binding of par-4 to the actin cytoskeleton is essential for Par-4/Dlk-mediated apoptosis. Exp. Cell Res. 2005, 305, 392–408. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, C.; Nakamura, N.; Yoshikawa, H.; Itoh, K. Transient dynamic actin cytoskeletal change stimulates the osteoblastic differentiation. J. Bone Miner. Metab. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, B.; Chambers, T.J.; Fuller, K. Secretion of tartrate-resistant acid phosphatase by osteoclasts correlates with resorptive behavior. J. Cell. Biochem. 2006, 98, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Halleen, J.M.; Alatalo, S.L.; Suominen, H.; Cheng, S.; Janckila, A.J.; Vaananen, H.K. Tartrate-resistant acid phosphatase 5b: A novel serum marker of bone resorption. J. Bone Miner. Res. 2000, 15, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Takito, J.; Otsuka, H.; Yanagisawa, N.; Arai, H.; Shiga, M.; Inoue, M.; Nonaka, N.; Nakamura, M. Regulation of osteoclast multinucleation by the actin cytoskeleton signaling network. J. Cell. Physiol. 2014, 230, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, F.; Zhang, C.; Gao, B.; Tan, P.; Mi, B.; Zhang, Y.; Cheng, H.; Liao, H.; Huo, K.; et al. The dimension of titania nanotubes influences implant success for osteoclastogenesis and osteogenesis patients. J. Nanosci. Nanotechnol. 2015, 15, 4136–4142. [Google Scholar] [CrossRef] [PubMed]
- Silverwood, R.K.; Fairhurst, P.G.; Sjöström, T.; Welsh, F.; Sun, Y.; Li, G.; Yu, B.; Young, P.S.; Su, B.; Meek, R.D.M.; et al. Analysis of osteoclastogenesis/osteoblastogenesis on nanotopographicaltitania surfaces. Adv. Healthc. Mater. 2016, 5, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Sommer, B.; Felix, R.; Sprecher, C.; Leunig, M.; Ganz, R.; Hofstetter, W. Wear particles and surface topographies are modulators of osteoclastogenesis in vitro. J. Biomed. Mater. Res. A 2004, 72, 67–76. [Google Scholar]
- Brinkmann, J.; Hefti, T.; Schlottig, F.; Spencer, N.D.; Hall, H. Response of osteoclasts to titanium surfaces with increasing surface roughness: An in vitro study. Biointerphases 2012, 7, 34. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ion, R.; Mazare, A.; Dumitriu, C.; Pirvu, C.; Schmuki, P.; Cimpean, A. Nanochannelar Topography Positively Modulates Osteoblast Differentiation and Inhibits Osteoclastogenesis. Coatings 2018, 8, 294. https://doi.org/10.3390/coatings8090294
Ion R, Mazare A, Dumitriu C, Pirvu C, Schmuki P, Cimpean A. Nanochannelar Topography Positively Modulates Osteoblast Differentiation and Inhibits Osteoclastogenesis. Coatings. 2018; 8(9):294. https://doi.org/10.3390/coatings8090294
Chicago/Turabian StyleIon, Raluca, Anca Mazare, Cristina Dumitriu, Cristian Pirvu, Patrick Schmuki, and Anisoara Cimpean. 2018. "Nanochannelar Topography Positively Modulates Osteoblast Differentiation and Inhibits Osteoclastogenesis" Coatings 8, no. 9: 294. https://doi.org/10.3390/coatings8090294
APA StyleIon, R., Mazare, A., Dumitriu, C., Pirvu, C., Schmuki, P., & Cimpean, A. (2018). Nanochannelar Topography Positively Modulates Osteoblast Differentiation and Inhibits Osteoclastogenesis. Coatings, 8(9), 294. https://doi.org/10.3390/coatings8090294








